化工学报 ›› 2019, Vol. 70 ›› Issue (3): 830-839.DOI: 10.11949/j.issn.0438-1157.20181154
于旭东1,2,3( ),黄琴1,王林1,李茂兰1,郑洪1,曾英1,3
),黄琴1,王林1,李茂兰1,郑洪1,曾英1,3
收稿日期:2018-10-08
修回日期:2018-12-18
出版日期:2019-03-05
发布日期:2019-03-05
通讯作者:
于旭东
作者简介:于旭东(1985—),男,博士,副教授,<email>xwdlyxd@126.com</email>
基金资助:
Xudong YU1,2,3( ),Qin HUANG1,Lin WANG1,Maolan LI1,Hong ZHENG1,Ying ZENG1,3
),Qin HUANG1,Lin WANG1,Maolan LI1,Hong ZHENG1,Ying ZENG1,3
Received:2018-10-08
Revised:2018-12-18
Online:2019-03-05
Published:2019-03-05
Contact:
Xudong YU
摘要:
采用等温溶解平衡法研究了288、298、308 K下三元体系KCl-PEG4000-H2O的相平衡关系,绘制了相应的相图、密度-组成图和折射率-组成图。研究发现:288、298、308 K下,三元体系KCl-PEG4000-H2O无分层现象,只存在固液相平衡关系,其平衡相图由不饱和液相区(L)、一固一液区(S+L)和两固一液区(2S+L)构成,且两固一液区随温度升高减小。KCl溶解度随着溶液中PEG4000含量不断增加而减小。溶液中PEG4000含量小于0.50时,盐析率受温度影响较小;含量大于0.50时,盐析率随着温度升高呈减小趋势。采用修正后的Pitzer方程进行了三元体系KCl-PEG4000-H2O (288、298、308 K)溶解度计算,对比发现,理论计算值与实验值吻合较好。
中图分类号:
于旭东, 黄琴, 王林, 李茂兰, 郑洪, 曾英. KCl-PEG4000-H2O三元体系288、298、308 K相平衡测定及计算[J]. 化工学报, 2019, 70(3): 830-839.
Xudong YU, Qin HUANG, Lin WANG, Maolan LI, Hong ZHENG, Ying ZENG. Measurements and simulation for ternary system KCl-PEG4000-H2O at 288, 298 and 308 K[J]. CIESC Journal, 2019, 70(3): 830-839.
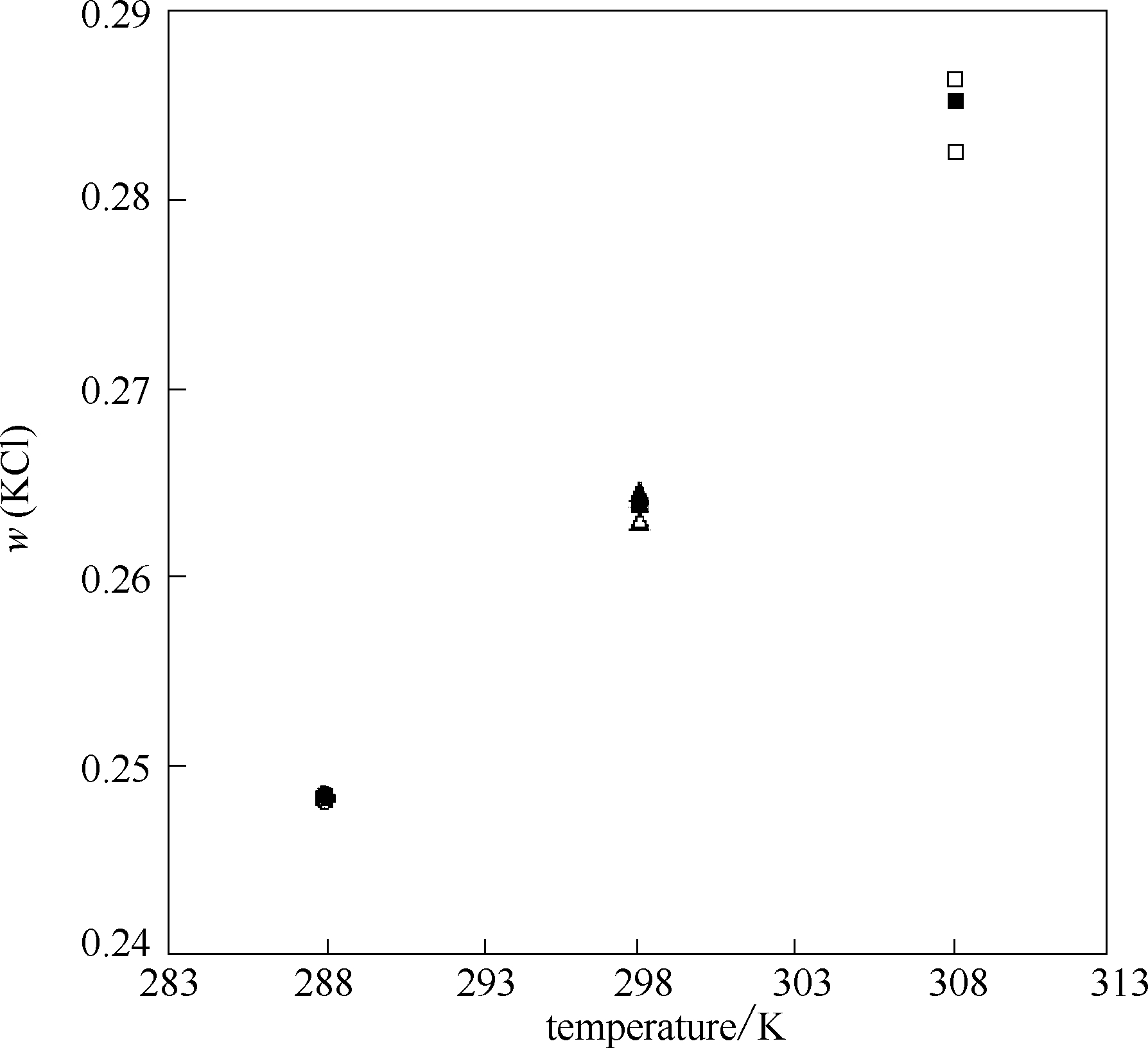
图1 288、298、308 K时KCl在水中的溶解度对比
Fig.1 Solubility for KCl in pure water at 288,298 and 308 K: ●,▲,■ solubility for KCl at 288,298 and 308 K by this work,○,Δ,□ solubility for KCl at 288,298 and 308 K from literatures[23,24,25,26,27,28]
| T/K | Solubility/(g/g) | Relative deviation(RD)① | |
|---|---|---|---|
| This work | Ref. | ||
288 | 0.2485 | 0.2482[ | –0.0012 |
| 0.2484[ | –0.0004 | ||
298 | 0.2639 | 0.2642[ | 0.0011 |
| 0.2645[ | 0.0023 | ||
| 0.2630[ | –0.0034 | ||
308 | 0.2851 | 0.2824[ | –0.0095 |
| 0.2863[ | 0.0042 | ||
表1 288、298、308 K时KCl在水中的溶解度
Table 1 Solubility of KCl in pure water at 288,298 and 308 K
| T/K | Solubility/(g/g) | Relative deviation(RD)① | |
|---|---|---|---|
| This work | Ref. | ||
288 | 0.2485 | 0.2482[ | –0.0012 |
| 0.2484[ | –0.0004 | ||
298 | 0.2639 | 0.2642[ | 0.0011 |
| 0.2645[ | 0.0023 | ||
| 0.2630[ | –0.0034 | ||
308 | 0.2851 | 0.2824[ | –0.0095 |
| 0.2863[ | 0.0042 | ||
| No. | Density,ρ/ (g?cm–3) | Refractive index,nD | Composition of equilibrated solution | R | Composition of wet solid phase | Equilibrated solid phase | |||
|---|---|---|---|---|---|---|---|---|---|
| w(KCl) | w(PEG4000) | w(KCl) | w(PEG4000) | ||||||
| T=288 K | |||||||||
| 1A | 1.1671 | 1.3680 | 0.2485 | 0.0000 | 0.0000 | - | - | KCl | |
| 2 | 1.1649 | 1.3723 | 0.2387 | 0.0381 | 0.0396 | 0.8875 | 0.0056 | KCl | |
| 3 | 1.1622 | 1.3764 | 0.2219 | 0.0778 | 0.1070 | 0.8694 | 0.0131 | KCl | |
| 4 | 1.1592 | 1.3807 | 0.2073 | 0.1188 | 0.1656 | 0.8615 | 0.0208 | KCl | |
| 5 | 1.1569 | 1.3852 | 0.1899 | 0.1619 | 0.2357 | 0.7081 | 0.0583 | KCl | |
| 6 | 1.1548 | 1.3898 | 0.1758 | 0.2061 | 0.2924 | 0.7530 | 0.0618 | KCl | |
| 7 | 1.1520 | 1.3950 | 0.1613 | 0.2517 | 0.3507 | 0.6919 | 0.0925 | KCl | |
| 8 | 1.1510 | 1.4003 | 0.1448 | 0.2993 | 0.4174 | 0.6924 | 0.1077 | KCl | |
| 9 | 1.1507 | 1.4061 | 0.1300 | 0.3478 | 0.4768 | 0.6974 | 0.1210 | KCl | |
| 10 | 1.1509 | 1.4122 | 0.1188 | 0.3966 | 0.5221 | 0.7025 | 0.1339 | KCl | |
| 11 | 1.1516 | 1.4174 | 0.1056 | 0.4468 | 0.5751 | 0.6464 | 0.1766 | KCl | |
| 12 | 1.1496 | 1.4240 | 0.0893 | 0.5010 | 0.6406 | 0.6672 | 0.1831 | KCl | |
| 13 | 1.1364 | 1.4282 | 0.0389 | 0.5767 | 0.8434 | 0.7232 | 0.1661 | KCl | |
| 14B | 1.1071 | 1.4281 | 0.0000 | 0.6485 | 1.0000 | - | - | - | |
| T=298 K | |||||||||
| 15C | 1.1798 | 1.3704 | 0.2639 | 0.0000 | 0.0000 | - | - | KCl | |
| 16 | 1.1746 | 1.3735 | 0.2532 | 0.0374 | 0.0446 | 0.8688 | 0.0066 | KCl | |
| 17 | 1.1712 | 1.3775 | 0.2382 | 0.0762 | 0.1013 | 0.9442 | 0.0056 | KCl | |
| 18 | 1.1675 | 1.3820 | 0.2238 | 0.1164 | 0.1555 | 0.8957 | 0.0156 | KCl | |
| 19 | 1.1641 | 1.3861 | 0.2073 | 0.1584 | 0.2178 | 0.8541 | 0.0292 | KCl | |
| 20 | 1.1612 | 1.3906 | 0.1903 | 0.2025 | 0.2818 | 0.7349 | 0.0663 | KCl | |
| 21 | 1.1587 | 1.3956 | 0.1744 | 0.2478 | 0.3418 | 0.7347 | 0.0796 | KCl | |
| 22 | 1.1561 | 1.4002 | 0.1581 | 0.2947 | 0.4033 | 0.7318 | 0.0939 | KCl | |
| 23 | 1.1539 | 1.4059 | 0.1409 | 0.3435 | 0.4684 | 0.7328 | 0.1068 | KCl | |
| 24 | 1.1523 | 1.4114 | 0.1258 | 0.3934 | 0.5254 | 0.7340 | 0.1197 | KCl | |
| 25 | 1.1516 | 1.4173 | 0.1087 | 0.4453 | 0.5896 | 0.7189 | 0.1404 | KCl | |
| 26 | 1.1490 | 1.4229 | 0.0929 | 0.4990 | 0.6496 | 0.6976 | 0.1664 | KCl | |
| 27 | 1.1431 | 1.4282 | 0.0669 | 0.5600 | 0.7477 | 0.6789 | 0.1927 | KCl | |
| 28 | 1.1428 | 1.4340 | 0.0548 | 0.6130 | 0.7933 | 0.7376 | 0.1702 | KCl | |
| 29D | 1.1118 | 1.4314 | 0.0000 | 0.6990 | 1.0000 | - | - | - | |
| T=308 K | |||||||||
| 30E | 1.1847 | 1.3692 | 0.2851 | 0.0000 | 0.0000 | - | - | KCl | |
| 31 | 1.1794 | 1.3744 | 0.2680 | 0.0367 | 0.0598 | 0.9781 | 0.0011 | KCl | |
| 32 | 1.1748 | 1.3780 | 0.2510 | 0.0749 | 0.1196 | 0.9224 | 0.0078 | KCl | |
| 33 | 1.1707 | 1.3829 | 0.2371 | 0.1144 | 0.1685 | 0.9498 | 0.0075 | KCl | |
| 34 | 1.1658 | 1.3861 | 0.2185 | 0.1562 | 0.2336 | 0.9496 | 0.0101 | KCl | |
| 35 | 1.1629 | 1.3905 | 0.2030 | 0.1993 | 0.2880 | 0.8288 | 0.0428 | KCl | |
| 36 | 1.1589 | 1.3945 | 0.1841 | 0.2449 | 0.3544 | 0.8756 | 0.0373 | KCl | |
| 37 | 1.1559 | 1.4000 | 0.1680 | 0.2912 | 0.4109 | 0.7442 | 0.0895 | KCl | |
| 38 | 1.1522 | 1.4050 | 0.1490 | 0.3402 | 0.4772 | 0.7634 | 0.0946 | KCl | |
| 39 | 1.1497 | 1.4101 | 0.1323 | 0.3905 | 0.5360 | 0.7283 | 0.1222 | KCl | |
| 40 | 1.1464 | 1.4159 | 0.1153 | 0.4420 | 0.5957 | 0.7034 | 0.1482 | KCl | |
| 41 | 1.1441 | 1.4215 | 0.0973 | 0.4966 | 0.6586 | 0.7191 | 0.1545 | KCl | |
| 42 | 1.1421 | 1.4272 | 0.0796 | 0.5523 | 0.7208 | 0.7530 | 0.1482 | KCl | |
| 43 | 1.1356 | 1.4321 | 0.0592 | 0.6101 | 0.7923 | 0.7134 | 0.1859 | KCl | |
| 44 | 1.1308 | 1.4337 | 0.0441 | 0.6689 | 0.8453 | 0.1845 | 0.5707 | KCl | |
| 45 | 1.1274 | 1.4376 | 0.0400 | 0.7191 | 0.8599 | 0.1704 | 0.6214 | KCl | |
| 46F | 1.1089 | 1.4445 | 0.0000 | 0.7800 | 1.0000 | - | - | - | |
表2 三元体系KCl-PEG4000-H2O 在288、298和308 K下的溶解度、密度、折射率和盐析率数据
| No. | Density,ρ/ (g?cm–3) | Refractive index,nD | Composition of equilibrated solution | R | Composition of wet solid phase | Equilibrated solid phase | |||
|---|---|---|---|---|---|---|---|---|---|
| w(KCl) | w(PEG4000) | w(KCl) | w(PEG4000) | ||||||
| T=288 K | |||||||||
| 1A | 1.1671 | 1.3680 | 0.2485 | 0.0000 | 0.0000 | - | - | KCl | |
| 2 | 1.1649 | 1.3723 | 0.2387 | 0.0381 | 0.0396 | 0.8875 | 0.0056 | KCl | |
| 3 | 1.1622 | 1.3764 | 0.2219 | 0.0778 | 0.1070 | 0.8694 | 0.0131 | KCl | |
| 4 | 1.1592 | 1.3807 | 0.2073 | 0.1188 | 0.1656 | 0.8615 | 0.0208 | KCl | |
| 5 | 1.1569 | 1.3852 | 0.1899 | 0.1619 | 0.2357 | 0.7081 | 0.0583 | KCl | |
| 6 | 1.1548 | 1.3898 | 0.1758 | 0.2061 | 0.2924 | 0.7530 | 0.0618 | KCl | |
| 7 | 1.1520 | 1.3950 | 0.1613 | 0.2517 | 0.3507 | 0.6919 | 0.0925 | KCl | |
| 8 | 1.1510 | 1.4003 | 0.1448 | 0.2993 | 0.4174 | 0.6924 | 0.1077 | KCl | |
| 9 | 1.1507 | 1.4061 | 0.1300 | 0.3478 | 0.4768 | 0.6974 | 0.1210 | KCl | |
| 10 | 1.1509 | 1.4122 | 0.1188 | 0.3966 | 0.5221 | 0.7025 | 0.1339 | KCl | |
| 11 | 1.1516 | 1.4174 | 0.1056 | 0.4468 | 0.5751 | 0.6464 | 0.1766 | KCl | |
| 12 | 1.1496 | 1.4240 | 0.0893 | 0.5010 | 0.6406 | 0.6672 | 0.1831 | KCl | |
| 13 | 1.1364 | 1.4282 | 0.0389 | 0.5767 | 0.8434 | 0.7232 | 0.1661 | KCl | |
| 14B | 1.1071 | 1.4281 | 0.0000 | 0.6485 | 1.0000 | - | - | - | |
| T=298 K | |||||||||
| 15C | 1.1798 | 1.3704 | 0.2639 | 0.0000 | 0.0000 | - | - | KCl | |
| 16 | 1.1746 | 1.3735 | 0.2532 | 0.0374 | 0.0446 | 0.8688 | 0.0066 | KCl | |
| 17 | 1.1712 | 1.3775 | 0.2382 | 0.0762 | 0.1013 | 0.9442 | 0.0056 | KCl | |
| 18 | 1.1675 | 1.3820 | 0.2238 | 0.1164 | 0.1555 | 0.8957 | 0.0156 | KCl | |
| 19 | 1.1641 | 1.3861 | 0.2073 | 0.1584 | 0.2178 | 0.8541 | 0.0292 | KCl | |
| 20 | 1.1612 | 1.3906 | 0.1903 | 0.2025 | 0.2818 | 0.7349 | 0.0663 | KCl | |
| 21 | 1.1587 | 1.3956 | 0.1744 | 0.2478 | 0.3418 | 0.7347 | 0.0796 | KCl | |
| 22 | 1.1561 | 1.4002 | 0.1581 | 0.2947 | 0.4033 | 0.7318 | 0.0939 | KCl | |
| 23 | 1.1539 | 1.4059 | 0.1409 | 0.3435 | 0.4684 | 0.7328 | 0.1068 | KCl | |
| 24 | 1.1523 | 1.4114 | 0.1258 | 0.3934 | 0.5254 | 0.7340 | 0.1197 | KCl | |
| 25 | 1.1516 | 1.4173 | 0.1087 | 0.4453 | 0.5896 | 0.7189 | 0.1404 | KCl | |
| 26 | 1.1490 | 1.4229 | 0.0929 | 0.4990 | 0.6496 | 0.6976 | 0.1664 | KCl | |
| 27 | 1.1431 | 1.4282 | 0.0669 | 0.5600 | 0.7477 | 0.6789 | 0.1927 | KCl | |
| 28 | 1.1428 | 1.4340 | 0.0548 | 0.6130 | 0.7933 | 0.7376 | 0.1702 | KCl | |
| 29D | 1.1118 | 1.4314 | 0.0000 | 0.6990 | 1.0000 | - | - | - | |
| T=308 K | |||||||||
| 30E | 1.1847 | 1.3692 | 0.2851 | 0.0000 | 0.0000 | - | - | KCl | |
| 31 | 1.1794 | 1.3744 | 0.2680 | 0.0367 | 0.0598 | 0.9781 | 0.0011 | KCl | |
| 32 | 1.1748 | 1.3780 | 0.2510 | 0.0749 | 0.1196 | 0.9224 | 0.0078 | KCl | |
| 33 | 1.1707 | 1.3829 | 0.2371 | 0.1144 | 0.1685 | 0.9498 | 0.0075 | KCl | |
| 34 | 1.1658 | 1.3861 | 0.2185 | 0.1562 | 0.2336 | 0.9496 | 0.0101 | KCl | |
| 35 | 1.1629 | 1.3905 | 0.2030 | 0.1993 | 0.2880 | 0.8288 | 0.0428 | KCl | |
| 36 | 1.1589 | 1.3945 | 0.1841 | 0.2449 | 0.3544 | 0.8756 | 0.0373 | KCl | |
| 37 | 1.1559 | 1.4000 | 0.1680 | 0.2912 | 0.4109 | 0.7442 | 0.0895 | KCl | |
| 38 | 1.1522 | 1.4050 | 0.1490 | 0.3402 | 0.4772 | 0.7634 | 0.0946 | KCl | |
| 39 | 1.1497 | 1.4101 | 0.1323 | 0.3905 | 0.5360 | 0.7283 | 0.1222 | KCl | |
| 40 | 1.1464 | 1.4159 | 0.1153 | 0.4420 | 0.5957 | 0.7034 | 0.1482 | KCl | |
| 41 | 1.1441 | 1.4215 | 0.0973 | 0.4966 | 0.6586 | 0.7191 | 0.1545 | KCl | |
| 42 | 1.1421 | 1.4272 | 0.0796 | 0.5523 | 0.7208 | 0.7530 | 0.1482 | KCl | |
| 43 | 1.1356 | 1.4321 | 0.0592 | 0.6101 | 0.7923 | 0.7134 | 0.1859 | KCl | |
| 44 | 1.1308 | 1.4337 | 0.0441 | 0.6689 | 0.8453 | 0.1845 | 0.5707 | KCl | |
| 45 | 1.1274 | 1.4376 | 0.0400 | 0.7191 | 0.8599 | 0.1704 | 0.6214 | KCl | |
| 46F | 1.1089 | 1.4445 | 0.0000 | 0.7800 | 1.0000 | - | - | - | |

图6 KCl-PEG4000-H2O三元体系288、298、308 K密度/折射率-组成图
Fig.6 Diagrams of density vs composition and refractive index vs composition for system KCl-PEG4000-H2O at 288,298 and 308 K
| 二元参数 | |||||||
|---|---|---|---|---|---|---|---|
| T/K | B11×102 | C111×105 | Np | σ×102 | |||
| 288 | 0.7088[ | 1.296[ | –0.4398 | –1.095 | 0.7345 | 11 | 0.3129 |
| 298 | 0.7088[ | 1.296[ | 0.7523 | –0.8338 | 0.9664 | 16 | 0.4356 |
| 308 | 0.7088[ | 1.296[ | 0.9950 | –1.053 | 1.981 | 14 | 0.2434 |
| 交互作用参数 | |||||||
| T/K | C112 | C122 | Np | σ | |||
| 288 | 0.101683 | –0.09897 | 0.000586 | –0.00325 | 13 | 0.26 | |
| 298 | 0.114476 | –0.10325 | –0.00015 | –0.00294 | 13 | 0.09 | |
| 308 | 0.110781 | 0.056002 | –0.0007 | –0.00233 | 15 | 0.20 | |
表3 三元体系KCl-PEG4000-H2O在288,298和308 K下的二元参数和交互作用参数
Table 3 Binary parameters and cross parameters for ternary system KCl-PEG4000-H2O at 288,298 and 308 K
| 二元参数 | |||||||
|---|---|---|---|---|---|---|---|
| T/K | B11×102 | C111×105 | Np | σ×102 | |||
| 288 | 0.7088[ | 1.296[ | –0.4398 | –1.095 | 0.7345 | 11 | 0.3129 |
| 298 | 0.7088[ | 1.296[ | 0.7523 | –0.8338 | 0.9664 | 16 | 0.4356 |
| 308 | 0.7088[ | 1.296[ | 0.9950 | –1.053 | 1.981 | 14 | 0.2434 |
| 交互作用参数 | |||||||
| T/K | C112 | C122 | Np | σ | |||
| 288 | 0.101683 | –0.09897 | 0.000586 | –0.00325 | 13 | 0.26 | |
| 298 | 0.114476 | –0.10325 | –0.00015 | –0.00294 | 13 | 0.09 | |
| 308 | 0.110781 | 0.056002 | –0.0007 | –0.00233 | 15 | 0.20 | |
| 1 | 侯献华, 樊馥, 郑绵平, 等. 青海盐湖钾盐资源开发利用及产业发展[J]. 科技导报, 2017, 35(12): 67-71. |
| HouX H, FanF, ZhengM P, et al. Development and utilization of potash resources of saline lakes in Qinghai province[J]. Sci. Technol. Rev. 2017, 35(12): 67-71. | |
| 2 | FareloF, FernandesC, AvelinoA. Solubilities for six ternary systems: NaCl + NH4Cl + H2O, KCl + NH4Cl + H2O, NaCl + LiCl + H2O, KCl + LiCl + H2O, NaCl + AlCl3 + H2O, NaCl + AlCl3 + H2O and KCl + AlCl3 + H2O at T = (298 to 333) K[J]. J. Chem. Eng. Data., 2005, 50(4): 1470-1477. |
| 3 | YangH T, LiangT Y, ZengD W, et al. Phase diagram of the quaternary system LiCl + MgCl2 + KCl +H2O at 323.15 K[J]. CALPHAD: Comput. Coupling Phase Diagrams Thermochem., 2017, 57: 126-133. |
| 4 | MengL Z, LiD, DengT L, et al. Solubility calculation for the brine system Na+, K+//Cl–, Br–-H2O using Pitzer thermodynamic model[J]. J. Chem. Eng. Jpn., 2018, 51(3): 185-189. |
| 5 | 桑世华, 张婷婷, 傅超, 等. 四元体系Li+, K+, Mg2+//B4O72–-H2O 273 K相平衡[J]. 化工学报, 2017, 68(9): 3343-3349. |
| SangS H, ZhangT T, FuC, et al. Phase equilibria in quaternary system Li+, K+, Mg2+//B4O72–-H2O at 273 K[J]. CIESC Journal, 2017, 68(9): 3343-3349. | |
| 6 | ZhangX, SangS H, ZhongS Y, et al. Equilibria in the ternary system SrCl2-KCl-H2O and the quaternary system SrCl2-KCl-NaCl-H2O at 323 K[J]. Russ. J. Phys. Chem. A, 2015, 89(12): 2322-2326. |
| 7 | 于旭东, 刘敏, 王林, 等. 三元体系硼酸钾+硼酸铷+水和硼酸铷+硼酸镁+水323 K相平衡[J]. 高校化学工程学报, 2018, 32(3): 514-521. |
| YuX D, LiuM, WangL, et al. Phase equilibria of potassium borate + rubidium borate + H2O and rubidium borate + magnesium borate + H2O aqueoue ternary systems at 323 K[J]. J. Chem. Eng. Chin. Univ., 2018, 32(3): 514-521. | |
| 8 | GuoS S, YuX D, ZengY. Phase equilibria for the aqueous reciprocal quaternary system K+, Mg2+//Cl-, Borate-H2O at 298 K[J]. J. Chem. Eng. Data, 2016, 61(4): 1566-1572. |
| 9 | YuX D, ZengY, GuoS S, et al. Stable phase equilibrium and phase diagram of the quinary system Li+, K+, Rb+, Mg2+//borate-H2O at T=348.15 K[J]. J.Chem. Eng. Data, 2016, 61(3): 1246-1253. |
| 10 | YuX D, ZengY, ChenP J, et al. Solid-liquid equilibrium of the quaternary system lithium, potassium, rubidium, and borate at T = 323 K[J]. J. Chem. Eng. Data, 2018, 63(8): 3125-3129. |
| 11 | YuX D, ZengY, MuP T, et al. Solid-liquid equilibria in the quinary system LiCl-KCl-RbCl-MgCl2-H2O at T = 323 K[J]. Fluid Phase Equilib., 2015, 387: 88-94. |
| 12 | LiZ Q, YuX D, YinQ H, et al. Thermodynamics metastable phase equilibria of aqueous quaternary system LiCl + KCl + RbCl+ H2O at 323.15 K[J]. Fluid Phase Equilib., 2013, 358: 131-136. |
| 13 | 任永胜, 何婷婷, 谢娟, 等. 333.15 K K+, NH4+//Cl–, SO42–-H2O和K+, NH4+//Cl–, SO42–-(CH2OH)2-H2O体系固液相平衡[J]. 化工学报, 2018, 69(7): 2838-2850. |
| RenY S, HeT T, XieJ, et al. Phase equilibria in systems K+, NH4+//Cl–, SO42–-H2O and K+, NH4+//Cl–, SO42--(CH2OH)2-H2O at 333.15 K[J]. CIESC Journal, 2018, 69(7): 2838-2850. | |
| 14 | SampaioV S, BonomoR C F, Monteiro FilhoE S, et al. Phyical properties and liquid-liquid equilibrium of aqueous two-phase systems containing poly(ethylene glycol) + potassium chloride + sodium polyacrylate [J]. J. Chem. Eng. Data, 2012, 57(12): 3651-3657. |
| 15 | 雷红, 李淑妮, 翟全国, 等. 298.15和308.15 K时1,2-丙二醇 + MCl (M=Na, K, Rb, Cs) + H2O三元体系的溶解度、密度和折射率[J]. 物理化学学报, 2012, 28(7): 1599-1607. |
| LeiH, LiS N, ZhaiQ G, et al. Solibility, density and refractive index for the ternary systems of 1,2-propanediol, MCl (M=Na, K, Rb, Cs) and H2O at 298.15 and 308.15 K [J]. Acta Phys. -Chim. Sin., 2012, 28(7): 1599-1607. | |
| 16 | TaboadaM E, GalleguillosH R, GraberT A. Compositions, densities, conductivities, and refractive indices of potassium chloride or/and sodium chloride +PEG4000 + water at 298.15 and liquid-liquid equilibrium of potassium chloride or sodium chloride +PEG4000 + water at 333.15 K[J]. J. Chem. Eng. Data, 2005, 50 (1): 264-269. |
| 17 | Hernandes-LuisF, Rodriguez-RaposoR, GalleguillosH, et al. Acivity coefficients of KCl in PEG4000 + water mixtures at 288.15, 298.15 and 308.15 K[J]. Fluid Phase Equilib., 2010, 295: 163-171. |
| 18 | LoveraJ A, PadillaA P, GalleguillosH, et al. Correlation of the solubilities of alkali chlorides in mixed solvents: polyethylene glycol + H2O and ethanol + H2O[J]. CALPHAD: Comput. Coupling Phase Diagrams Thermochem., 2012, 38: 35-42. |
| 19 | WuY T, LinD Q, ZhuZ Q, et al. Prediction of liquid-liquid equilibria of polymer-salt aqueous two-phase systms by modified Pitzer’s virial equation[J]. Fluid Phase Equilib., 1996, 124: 67-79. |
| 20 | FosbolP L, ThomsenK, StenbyE H. Reverse schreinemakers method for experimental analysis of mixed-solvent electrolyte systems [J]. J. Solution Chem., 2009, 38(1): 1-14. |
| 21 | 中国科学院青海盐湖研究所. 卤水和盐的分析方法(第二版)[M]. 北京:科学出版社, 1988: 69-72. |
| Institute of Qinghai Salt-Lake of Chinese Academy of Sciences. Analytical Methods of Brines and Salts (2nd ed) [M]. Beijing: Chinese Science Press, 1988: 69-72. | |
| 22 | ChelugetE L, GelinasS, VeraJ H, et al. Liquid-liquid equilibrium of aqueous mixtures of poly(propylene glycol) with NaCl [J]. J. Chem. Eng. Data, 1994, 39(1): 127-130. |
| 23 | DengT L, LiD C, WangS Q. Metastable phase equilibrium in the aqueous ternary system (KCl-CaCl2-H2O) at (288.15 and 308.15) K[J]. J. Chem. Eng. Data, 2008, 53(4): 1007-1011. |
| 24 | LongJ, TangJ H, YouY K, et al. Phase equilibrium in the aqueous ternary system KH2PO4+KCl+H2O at (288.15 and 303.15) K[J]. J. Chem. Eng. Data, 2015, 60(6): 1906-1909. |
| 25 | ShenW, RenY S, ZhangX R, et al. Solid-liquid phase equilibrium for the ternary system (potassium chloride + potassium dihydrogen phosphate + water) at (298.15 and 313.15) K[J]. J. Chem. Eng. Data, 2015, 60(7): 2070-2078. |
| 26 | LinS Q, TangJ H, TengJ, et al. Phase equilibrium in the system KH2PO4 + KCl +H3PO4 at 298.15 K and 308.15 K[J]. J. Chem. Eng. Data, 2017, 62(12): 4169-4173. |
| 27 | JiaX Y, LiJ, JinY, et al. Solid-liquid equilibria in the quaternary system Na+, K+//HPO42–, Cl–-H2O and its subsystems Na+//HPO42–, Cl–-H2O, K+//HPO42–, Cl–-H2O, and Na+, K+//HPO42–-H2O at 298.2 K[J]. J. Chem. Eng. Data, 2017, 62(11): 3679-3686. |
| 28 | MengR, LiS N, ZhaiQ G, et al. Solubilities, densities, and refractive indices for the ternary systems glycerin + MCl + H2O (M=Na, K, Rb, Cs) at (298.15 and 308.15) K[J]. J. Chem. Eng. Data, 2011, 56(12): 4643-4650. |
| 29 | PitzerK S. Thermodynamics of electrolytes. I. Theoretical basis and general equations[J]. J. Phys. Chem., 1973, 77(2): 268-277. |
| 30 | HariveC E, EugsterH P, WeareJ H. Mineral equilibria in the six-component seawater system, Na-K-Mg-Ca-SO4-Cl-H2O at 25℃. II: Compositions of the saturated solutions[J]. Geochim. Cosmochim. Acta., 1982, 46(9): 1603-1618. |
| 31 | CleggS L, RardJ A, MillerD G. Isopiestic determination of the osmotic and activity coefficients of NaCl+SrCl2+H2O at 298.15K and representation with an extended ion-interaction model. J. Chem. Eng. Data, 2005, 50(4): 1162-1170. |
| 32 | KrevelenD W V, NijehuisK T. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions[M].(4th ed). Amsterdam: Elsevier, 2009: 319-321. |
| 33 | GuoL J, HanH J, DongO Y, et al. Thermodynamics and phase equilibrium of the high concentration solid solution-aqueous solution system KCl-RbCl-H2O from T = 298.15 K to T = 323.15 K[J]. J. Chem. Thermodyn., 2016, 106: 285-294. |
| [1] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [2] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [3] | 姚晓宇, 沈俊, 李健, 李振兴, 康慧芳, 唐博, 董学强, 公茂琼. 流体气液临界参数测量方法研究进展[J]. 化工学报, 2023, 74(5): 1847-1861. |
| [4] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [5] | 毛元敬, 杨智, 莫松平, 郭浩, 陈颖, 罗向龙, 陈健勇, 梁颖宗. C6~C10烷醇的SAFT-VR Mie状态方程参数回归及其热物性研究[J]. 化工学报, 2023, 74(3): 1033-1041. |
| [6] | 高靖博, 孙强, 李青, 王逸伟, 郭绪强. 考虑水合物结构转变的含氢气体水合物相平衡模型[J]. 化工学报, 2023, 74(2): 666-673. |
| [7] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [8] | 蔡进, 王晓辉, 汤涵, 陈光进, 孙长宇. TBAB水溶液体系中半笼型水合物的相平衡预测模型[J]. 化工学报, 2023, 74(1): 408-415. |
| [9] | 杨松涛, 李东洋, 牛玉清, 李鑫钢, 康绍辉, 李洪, 叶开凯, 周志全, 高鑫. 氟化物势能函数和热力学性质的分子模拟研究进展[J]. 化工学报, 2022, 73(9): 3828-3840. |
| [10] | 周桓, 张梦丽, 郝晴, 吴思, 李杰, 徐存兵. 硫酸镁型光卤石转化钾盐镁矾的过程机制与动态规律[J]. 化工学报, 2022, 73(9): 3841-3850. |
| [11] | 刘潜, 张香兰, 李志平, 李玉龙, 韩梦醒. 油酚分离过程低共熔溶剂的筛选及萃取性能研究[J]. 化工学报, 2022, 73(9): 3915-3928. |
| [12] | 孙哲, 金华强, 李康, 顾江萍, 黄跃进, 沈希. 基于知识数据化表达的制冷空调系统故障诊断方法[J]. 化工学报, 2022, 73(7): 3131-3144. |
| [13] | 任玉鑫, 徐润峰, 王婉颖, 陈鹏忠, 彭孝军. 彩色光刻胶用蒽醌染料的合成及稳定性研究[J]. 化工学报, 2022, 73(5): 2251-2261. |
| [14] | 张家仁, 刘海超. 大豆油与甲醇酯交换反应体系的相平衡研究[J]. 化工学报, 2022, 73(5): 1920-1929. |
| [15] | 任嘉辉, 刘豫, 刘朝, 刘浪, 李莹. 基于分子指纹和拓扑指数的工质临界温度理论预测[J]. 化工学报, 2022, 73(4): 1493-1500. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号