化工学报 ›› 2022, Vol. 73 ›› Issue (1): 255-265.DOI: 10.11949/0438-1157.20211149
收稿日期:2021-08-12
修回日期:2021-11-10
出版日期:2022-01-05
发布日期:2022-01-18
通讯作者:
赵璐
作者简介:王乾浩(1994—),男,硕士研究生,基金资助:
Qianhao WANG1,2( ),Lu ZHAO1(
),Lu ZHAO1( ),Fulin SUN1,3,Kegong FANG1
),Fulin SUN1,3,Kegong FANG1
Received:2021-08-12
Revised:2021-11-10
Online:2022-01-05
Published:2022-01-18
Contact:
Lu ZHAO
摘要:
将H2S和CO2混合酸气一步转化制合成气,既实现了二者无害化处理,又生产出合成气,是一条理想的废气资源化利用新路线。由于分子结构稳定,在常规条件下因受热力学平衡限制,二者转化率极低。而在低温等离子体中,H2S和CO2可被激发为高活性物种来参与反应。研究了具有不同Si/Al摩尔比的ZSM-5催化剂与低温等离子体结合实现H2S-CO2一步高选择性制合成气,显著提高了H2S-CO2转化性能。考察了ZSM-5催化剂中Si/Al比和低温等离子体放电条件等对反应的影响。其中,当Si/Al比为80时表现出最优催化性能,最高H2和CO产率分别达到56.1%和10.0%。对常规条件和低温等离子体氛围下的不同ZSM-5催化剂上CO2、H2S、CO、H2等化学吸脱附行为进行了对比研究,发现低温等离子体促进了催化剂对CO2、H2及CO分子的吸附活化,进而明显提升了H2S和CO2转化。
中图分类号:
王乾浩, 赵璐, 孙付琳, 房克功. ZSM-5催化剂与低温等离子体协同转化H2S-CO2制合成气[J]. 化工学报, 2022, 73(1): 255-265.
Qianhao WANG, Lu ZHAO, Fulin SUN, Kegong FANG. Production of syngas derived from H2S-CO2via synergy of ZSM-5 catalyst and non-thermal plasma[J]. CIESC Journal, 2022, 73(1): 255-265.

图1 低温等离子体系统1—气瓶;2—质量流量控制器;3—高压电极;4—等离子体高压发生器;5—示波器;6—油浴;7—等离子体反应器(填充催化剂);8—接地极;9—积硫槽;10—冷阱;11—气相色谱分析仪;12—碱液处理
Fig.1 Schematic diagram of the non-thermal plasma experimental set-up
| Si/Al比 | 比表面积/(m2/g) | 介电常数ε |
|---|---|---|
| 25 | 305 | 3.15 |
| 38 | 318 | 3.27 |
| 50 | 324 | 3.37 |
| 80 | 306 | 3.42 |
| 200 | 307 | 3.49 |
表1 不同Si/Al比ZSM-5催化剂的比表面积及介电常数
Table 1 Specific surface area and dielectric constant of ZSM-5 catalysts with various Si/Al molar ratios
| Si/Al比 | 比表面积/(m2/g) | 介电常数ε |
|---|---|---|
| 25 | 305 | 3.15 |
| 38 | 318 | 3.27 |
| 50 | 324 | 3.37 |
| 80 | 306 | 3.42 |
| 200 | 307 | 3.49 |
| Si/Al比 | H2S转化率/% | CO2转化率/% |
|---|---|---|
| 25 | 5.0 | 0.9 |
| 38 | 2.9 | 0.8 |
| 50 | 3.5 | 1.3 |
| 80 | 5.3 | 1.4 |
| 200 | 5.9 | 1.2 |
表2 无等离子体下不同Si/Al比ZSM-5催化剂上热转化H2S-CO2反应结果
Table 2 The thermal conversion of H2S-CO2 in the presence of packing various ZSM-5 catalysts without non-thermal plasma
| Si/Al比 | H2S转化率/% | CO2转化率/% |
|---|---|---|
| 25 | 5.0 | 0.9 |
| 38 | 2.9 | 0.8 |
| 50 | 3.5 | 1.3 |
| 80 | 5.3 | 1.4 |
| 200 | 5.9 | 1.2 |
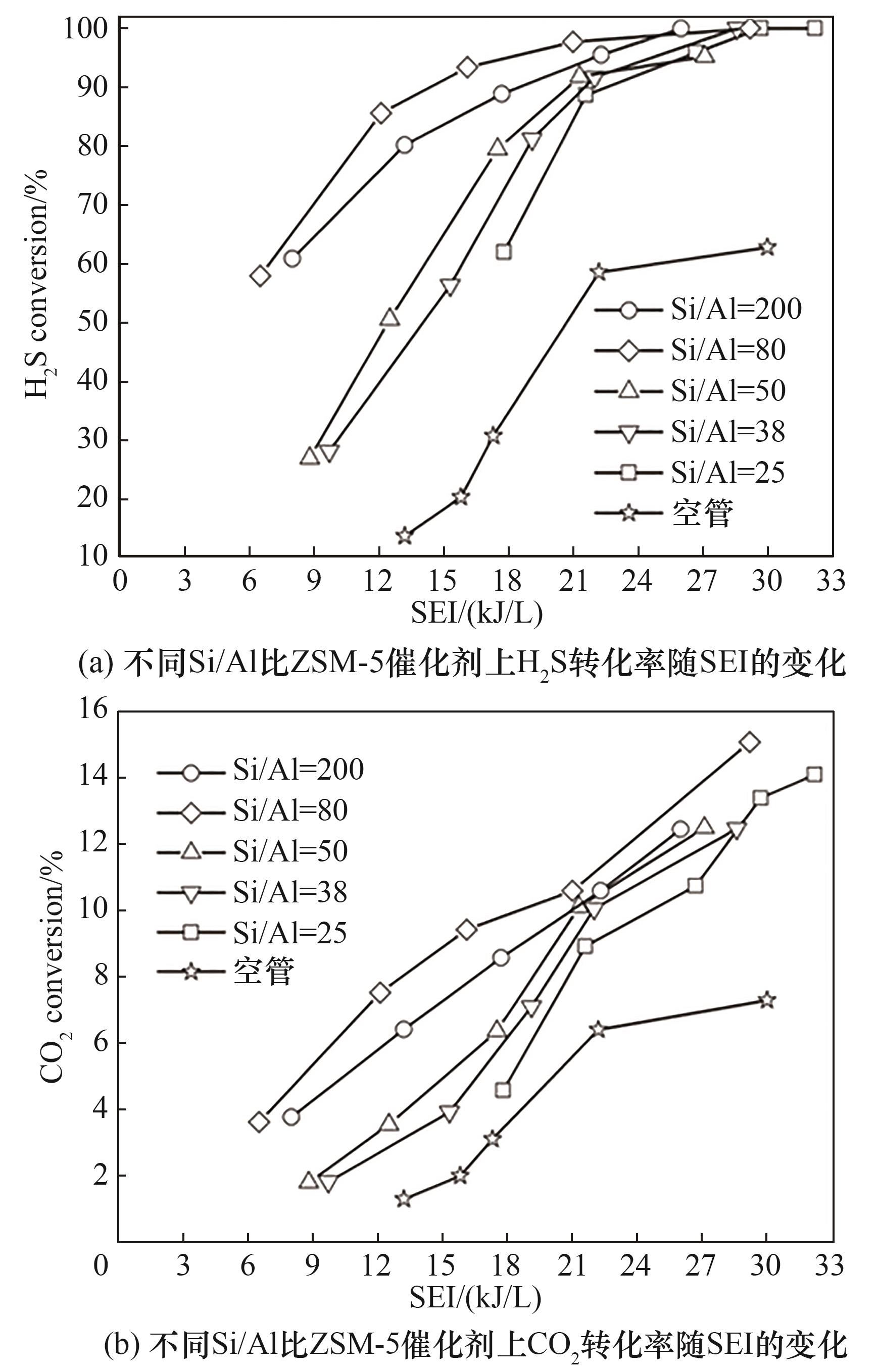
图4 不同Si/Al比ZSM-5催化剂上H2S-CO2转化性能随SEI的变化(反应条件:原料气H2S/CO2为1∶4; N2浓度20%; 反应气流量200 ml/min; 填充体积15 ml)
Fig.4 H2S-CO2 conversion as a function of SEI in the presence of packing various ZSM-5 catalysts in non-thermal plasma(feed: H2S/CO2 molar ratio = 1∶4; 20%(vol) N2 in H2S-CO2 gas; feed flow rate 200 ml/min; material bed volume 15 ml)
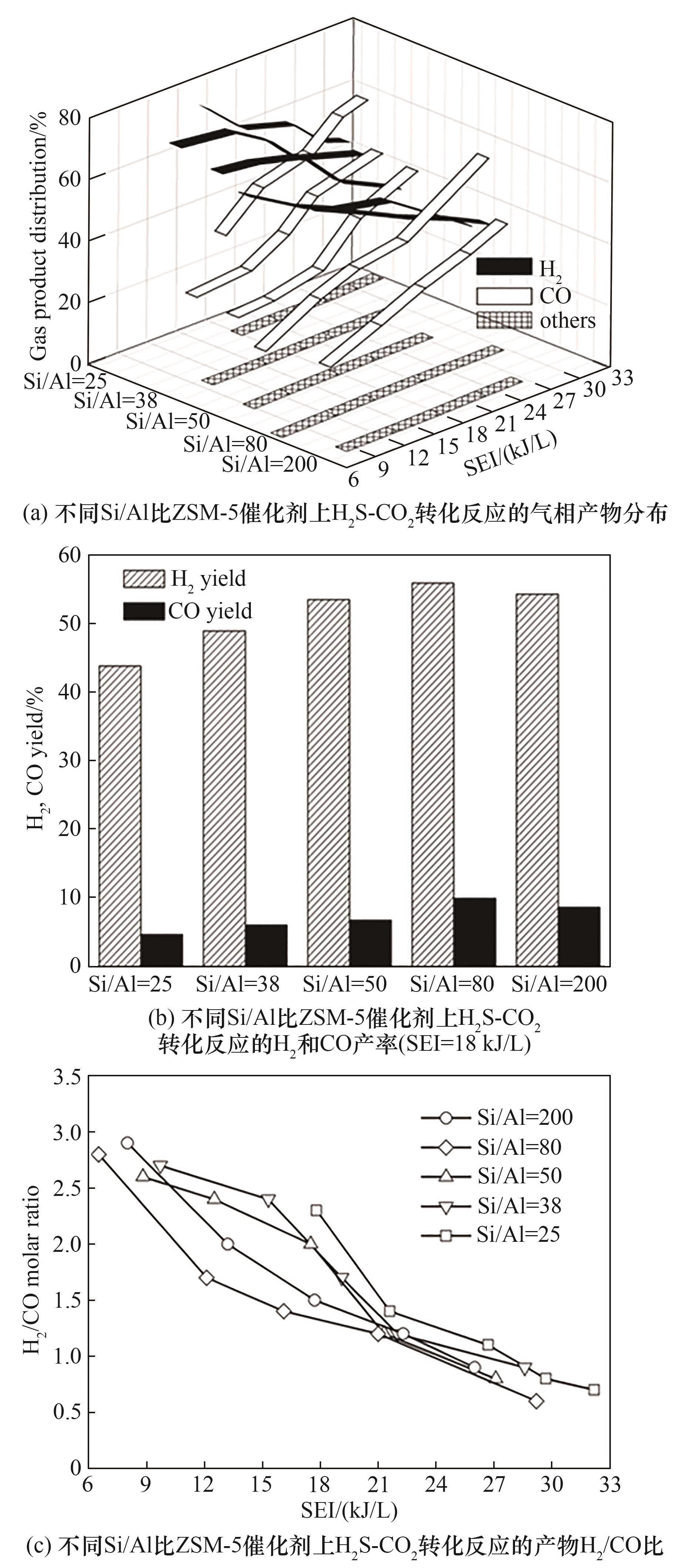
图5 不同Si/Al比ZSM-5催化剂上H2S-CO2转化反应气相产物分布、H2及CO产率、H2/CO比(反应条件:原料气H2S/CO2为1∶4; N2浓度20%; 反应气流量200 ml/min; 填充体积15 ml)
Fig.5 Gaseous product distributions, H2 and CO yields and H2/CO molar ratios in H2S-CO2 conversion with packing various ZSM-5 catalysts in non-thermal plasma(feed: H2S/CO2 molar ratio = 1∶4; 20%(vol) N2 in H2S-CO2 gas; feed flow rate 200 ml/min; material bed volume 15 ml)
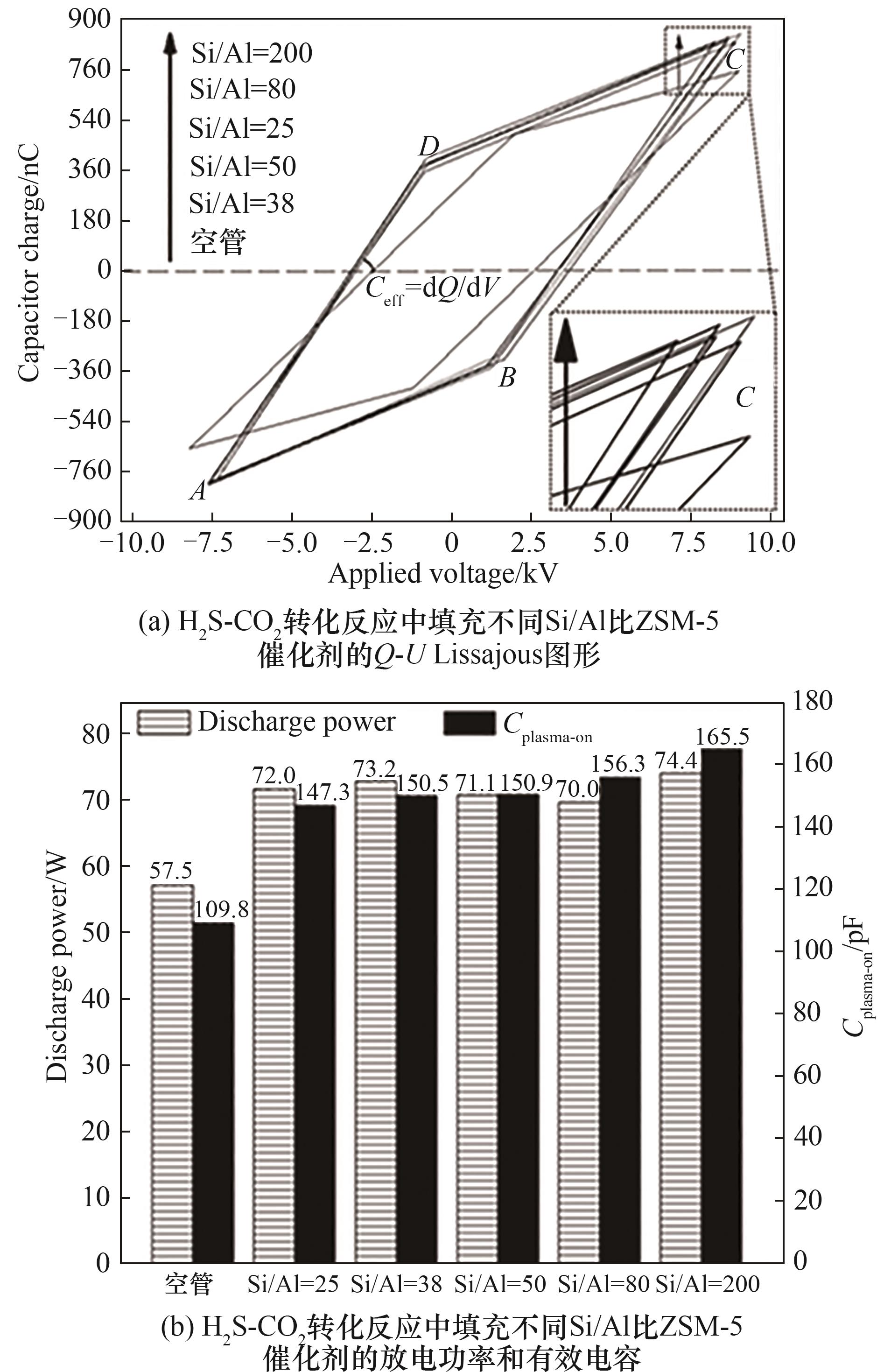
图6 H2S-CO2转化反应中填充不同Si/Al比ZSM-5催化剂的Q-U Lissajous图形、放电功率和有效电容(反应条件:原料气H2S/CO2=1∶4; N2浓度20%; 反应气流量200 ml/min; 填充体积15 ml; 输入功率95 W)
Fig.6 Q-U Lissajous figures, discharge power and effective capacitance in H2S-CO2 conversion with packing various ZSM-5 catalysts in non-thermal plasma(feed: H2S/CO2 molar ratio = 1∶4; 20%(vol) N2 in H2S-CO2 gas; feed flow rate 200 ml/min; material bed volume 15 ml; input power 95 W)
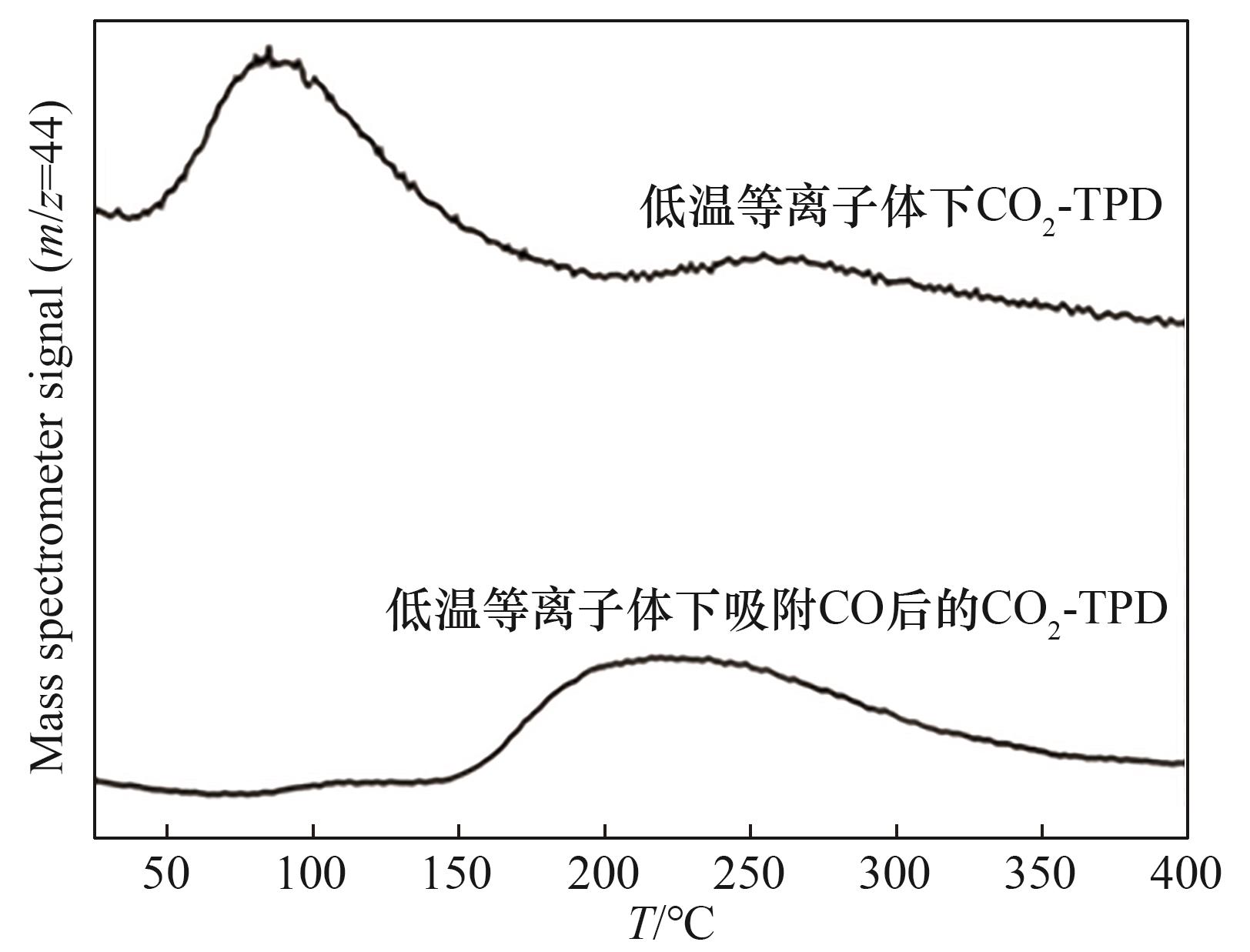
图13 低温等离子体下吸附CO后ZSM-5催化剂(Si/Al比为80)的CO2-TPD谱图
Fig.13 CO2-TPD profiles of ZSM-5 catalyst with Si/Al molar ratio of 80 after CO adsorption in non-thermal plasma
| 1 | Hendrickson R G, Chang A, Hamilton R J. Co-worker fatalities from hydrogen sulfide[J]. American Journal of Industrial Medicine, 2004, 45(4): 346-350. |
| 2 | 刘昌俊, 郭秋婷, 叶静云, 等. 二氧化碳转化催化剂研究进展及相关问题思考[J]. 化工学报, 2016, 67(1): 6-13. |
| Liu C J, Guo Q T, Ye J Y, et al. Perspective on catalyst investigation for CO2 conversion and related issues[J]. CIESC Journal, 2016, 67(1): 6-13. | |
| 3 | Bai S T, de Smet G, Liao Y, et al. Homogeneous and heterogeneous catalysts for hydrogenation of CO2 to methanol under mild conditions[J]. Chemical Society Reviews, 2021, 50(7): 4259-4298. |
| 4 | Liu J L, Park H W, Chung W J, et al. High-efficient conversion of CO2 in AC-pulsed tornado gliding arc plasma[J]. Plasma Chemistry and Plasma Processing, 2016, 36(2): 437-449. |
| 5 | Kim S C, Lim M S, Chun Y N. Reduction characteristics of carbon dioxide using a plasmatron[J]. Plasma Chemistry and Plasma Processing, 2014, 34(1): 125-143. |
| 6 | Zhang K, Mukhriza T, Liu X T, et al. A study on CO2 and CH4 conversion to synthesis gas and higher hydrocarbons by the combination of catalysts and dielectric-barrier discharges[J]. Applied Catalysis A: General, 2015, 502: 138-149. |
| 7 | Schieweck B G, Jürling-Will P, Klankermayer J. Structurally versatile ligand system for the ruthenium catalyzed one-pot hydrogenation of CO2 to methanol[J]. ACS Catalysis, 2020, 10(6): 3890-3894. |
| 8 | 吴秀章. 煤制低碳烯烃工艺与工程[M]. 北京: 化学工业出版社, 2014: 111. |
| Wu X Z. Coal-to-olefins Technology and Engineering [M]. Beijing: Chemical Industry Press, 2014: 111. | |
| 9 | Fridman A. Plasma Chemistry[M]. New York:Cambridge University Press, 2008: 13. |
| 10 | Saleem F, Zhang K, Harvey A P. Decomposition of benzene as a tar analogue in CO2 and H2 carrier gases, using a non-thermal plasma[J]. Chemical Engineering Journal, 2019, 360: 714-720. |
| 11 | Ellmer K, Lichtenberger D. Plasma diagnostics by energy resolved quadrupole mass spectrometry of a reactive magnetron sputtering discharge from an Fe target in Ar-H2S atmospheres[J]. Surface and Coatings Technology, 1995, 74/75: 586-593. |
| 12 | Lan L Y, Wang A J, Wang Y. CO2 hydrogenation to lower hydrocarbons over ZSM-5-supported catalysts in a dielectric-barrier discharge plasma reactor[J]. Catalysis Communications, 2019, 130: 105761. |
| 13 | Zhao L, Wang Y, Jin L, et al. Decomposition of hydrogen sulfide in non-thermal plasma aided by supported CdS and ZnS semiconductors[J]. Green Chemistry, 2013, 15(6): 1509-1513. |
| 14 | 周柒, 丁红蕾, 郭得通, 等. CO2催化氢化制清洁能源的研究进展及趋势[J]. 化工学报, 2020, 71(8): 3428-3443. |
| Zhou Q, Ding H L, Guo D T, et al. Recent advances in catalytic methods of CO2 hydrogenation to clean energy[J]. CIESC Journal, 2020, 71(8): 3428-3443. | |
| 15 | Zhao L, Liu X Z, Mu X L, et al. Highly selective conversion of H2S-CO2 to syngas by combination of non-thermal plasma and MoS2/Al2O3[J]. Journal of CO2 Utilization, 2020, 37: 45-54. |
| 16 | 房克功, 赵璐, 李文斌, 等. 转化二氧化碳和硫化氢混合气制取合成气的方法及装置: 107244652B[P]. 2019-12-06. |
| Fang K G, Zhao L, Li W B, et al. Method and device for preparing synthetic gas by converting carbon dioxide and hydrogen sulfide mixture: 107244652B[P]. 2019-12-06. | |
| 17 | Cundy C S, Cox P A. The hydrothermal synthesis of zeolites: precursors, intermediates and reaction mechanism[J]. Microporous and Mesoporous Materials, 2005, 82(1/2): 1-78. |
| 18 | Derouane E G, Determmerie S, Gabelica Z, et al. Synthesis and characterization of ZSM-5 type zeolites Ⅰ. Physico-chemical properties of precursors and intermediates[J]. Applied Catalysis, 1981, 1(3/4): 201-224. |
| 19 | Kim H H, Lee Y H, Ogata A, et al. Plasma-driven catalyst processing packed with photocatalyst for gas-phase benzene decomposition[J]. Catalysis Communications, 2003, 4(7): 347-351. |
| 20 | Zhao L, Wang Y, Li X, et al. Hydrogen production via decomposition of hydrogen sulfide by synergy of non-thermal plasma and semiconductor catalysis[J]. International Journal of Hydrogen Energy, 2013, 38(34): 14415-14423. |
| 21 | Pour A N, Zare M, Kamali Shahri S M, et al. Catalytic behaviors of bifunctional Fe-HZSM-5 catalyst in Fischer-Tropsch synthesis[J]. Journal of Natural Gas Science and Engineering, 2009, 1(6): 183-189. |
| 22 | 滕加伟, 赵国良, 谢在库, 等. ZSM-5分子筛晶粒尺寸对C4烯烃催化裂解制丙烯的影响[J]. 催化学报, 2004, 25(8): 602-606. |
| Teng J W, Zhao G L, Xie Z K, et al. Effect of ZSM-5 zeolite crystal size on propylene production from catalytic cracking of C4 olefins[J]. Chinese Journal of Catalysis, 2004, 25(8): 602-606. | |
| 23 | Chen H L, Lee H M, Chen S H, et al. Review of plasma catalysis on hydrocarbon reforming for hydrogen production—interaction, integration, and prospects[J]. Applied Catalysis B: Environmental, 2008, 85(1/2): 1-9. |
| 24 | Zhang X, Liu Y, Zhang M T, et al. Synergy between β-Mo2C nanorods and non-thermal plasma for selective CO2 reduction to CO[J]. Chem, 2020, 6(12): 3312-3328. |
| 25 | Diao Y N, Zhang X, Liu Y, et al. Plasma-assisted dry reforming of methane over Mo2C-Ni/Al2O3 catalysts: effects of β-Mo2C promoter[J]. Applied Catalysis B: Environmental, 2022, 301: 120779. |
| 26 | Liu X Z, Zhao L, Li Y, et al. Ni-Mo sulfide semiconductor catalyst with high catalytic activity for one-step conversion of CO2 and H2S to syngas in non-thermal plasma[J]. Catalysts, 2019, 9(6): 525. |
| 27 | Zeng Y X, Tu X. Plasma-catalytic hydrogenation of CO2 for the cogeneration of CO and CH4 in a dielectric barrier discharge reactor: effect of argon addition[J]. Journal of Physics D: Applied Physics, 2017, 50(18): 184004. |
| 28 | Liang W J, Fang H P, Li J, et al. Performance of non-thermal DBD plasma reactor during the removal of hydrogen sulfide[J]. Journal of Electrostatics, 2011, 69(3): 206-213. |
| 29 | Zhang X H, Lin L, Zhang T, et al. Catalytic dehydration of lactic acid to acrylic acid over modified ZSM-5 catalysts[J]. Chemical Engineering Journal, 2016, 284: 934-941. |
| 30 | 邹吉军. 等离子体处理制备高效催化剂的基础研究[D]. 天津: 天津大学, 2005. |
| Zou J J. On the preparation of highly efficient catalysts using cold plasma treatment[D]. Tianjin: Tianjin University, 2005. | |
| 31 | Snoeckx R, Bogaerts A. Plasma technology—a novel solution for CO2 conversion? [J]. Chemical Society Reviews, 2017, 46(19): 5805-5863. |
| 32 | Zhang L P, Karakas G, Ozkan U S. NiMoS/γ-Al2O3 catalysts: the nature and the aging behavior of active sites in HDN reactions[J]. Journal of Catalysis, 1998, 178(2): 457-465. |
| 33 | Zhao L, Wang Y, Wang A J, et al. Cr-doped ZnS semiconductor catalyst with high catalytic activity for hydrogen production from hydrogen sulfide in non-thermal plasma[J]. Catalysis Today, 2019, 337: 83-89. |
| 34 | Zhao G B, John S, Zhang J J, et al. Production of hydrogen and sulfur from hydrogen sulfide in a nonthermal-plasma pulsed corona discharge reactor[J]. Chemical Engineering Science, 2007, 62(8): 2216-2227. |
| 35 | Lacroix M, Dumonteil C, Breysse M, et al. Hydrogen activation on alumina supported MoS2 based catalysts: role of the promoter[J]. Journal of Catalysis, 1999, 185(1): 219-222. |
| 36 | Wang A Q, Ma L, Cong Y, et al. Unique properties of Ir/ZSM-5 catalyst for NO reduction with CO in the presence of excess oxygen[J]. Applied Catalysis B: Environmental, 2003, 40(4): 319-329. |
| 37 | Lupinetti A J, Fau S, Frenking G, et al. Theoretical analysis of the bonding between CO and positively charged atoms[J]. The Journal of Physical Chemistry A, 1997, 101(49): 9551-9559. |
| [1] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [2] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [3] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [4] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [5] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [6] | 韩晨, 司徒友珉, 朱斌, 许建良, 郭晓镭, 刘海峰. 协同处理废液的多喷嘴粉煤气化炉内反应流动研究[J]. 化工学报, 2023, 74(8): 3266-3278. |
| [7] | 张曼铮, 肖猛, 闫沛伟, 苗政, 徐进良, 纪献兵. 危废焚烧处理耦合有机朗肯循环系统工质筛选与热力学优化[J]. 化工学报, 2023, 74(8): 3502-3512. |
| [8] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [9] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [10] | 陈朝光, 贾玉香, 汪锰. 以低浓度废酸驱动中和渗析脱盐的模拟与验证[J]. 化工学报, 2023, 74(6): 2486-2494. |
| [11] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [12] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [13] | 李晨曦, 刘永峰, 张璐, 刘海峰, 宋金瓯, 何旭. O2/CO2氛围下正庚烷的燃烧机理研究[J]. 化工学报, 2023, 74(5): 2157-2169. |
| [14] | 葛泽峰, 吴雨青, 曾名迅, 查振婷, 马宇娜, 侯增辉, 张会岩. 灰化学成分对生物质气化特性的影响规律[J]. 化工学报, 2023, 74(5): 2136-2146. |
| [15] | 白天昊, 王晓雯, 杨梦滋, 段新伟, 米杰, 武蒙蒙. 类水滑石衍生锌基氧化物高温煤气脱硫过程中COS释放行为及其抑制研究[J]. 化工学报, 2023, 74(4): 1772-1780. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号