化工学报 ›› 2025, Vol. 76 ›› Issue (5): 1960-1972.DOI: 10.11949/0438-1157.20241308
• 综述与专论 • 上一篇
程刘惠美( ), 闫军营, 刘慧情, 王治澎, 王报英, 徐铜文, 汪耀明(
), 闫军营, 刘慧情, 王治澎, 王报英, 徐铜文, 汪耀明( )
)
收稿日期:2024-11-15
修回日期:2025-03-21
出版日期:2025-05-25
发布日期:2025-06-13
通讯作者:
汪耀明
作者简介:程刘惠美(2001—),女,硕士研究生,clhm0212@mail.ustc.edu.cn
基金资助:
Liuhuimei CHENG( ), Junying YAN, Huiqing LIU, Zhipeng WANG, Baoying WANG, Tongwen XU, Yaoming WANG(
), Junying YAN, Huiqing LIU, Zhipeng WANG, Baoying WANG, Tongwen XU, Yaoming WANG( )
)
Received:2024-11-15
Revised:2025-03-21
Online:2025-05-25
Published:2025-06-13
Contact:
Yaoming WANG
摘要:
双极膜作为一种高性能复合膜,在盐制酸碱、能量存储与转化、酸碱液流电池等领域得到了广泛应用。在反向偏压下,水体系中双极膜中间层的水分子可解离成H+和OH-,与阴阳离子交换膜配合构成双极膜电渗析广泛应用于高盐废水脱盐同时酸碱化。除水体系外,双极膜电渗析也可应用于水-醇体系,生产水溶性较差的有机酸。同时,双极膜也可实现醇的解离,使其解离为氢离子和烷氧根离子,应用于金属醇盐生产、有机物绿色合成等。目前醇水体系研究较少,因此,本文聚焦于醇水体系双极膜电渗析研究进展,深度比较水、醇解离的机理和电化学性质。同时也指出双极膜应用于醇体系的瓶颈问题,包括解离电阻高、双极膜溶胀造成同离子泄漏严重等,最后分析为实现双极膜水-醇混合体系以及醇体系更广泛的应用,仍需积极探索其混合体系的解离机理,制备适合醇体系的双极膜等。
中图分类号:
程刘惠美, 闫军营, 刘慧情, 王治澎, 王报英, 徐铜文, 汪耀明. 双极膜电渗析在醇水体系的应用研究进展[J]. 化工学报, 2025, 76(5): 1960-1972.
Liuhuimei CHENG, Junying YAN, Huiqing LIU, Zhipeng WANG, Baoying WANG, Tongwen XU, Yaoming WANG. Progress of bipolar membrane electrodialysis for non-aqueous systems[J]. CIESC Journal, 2025, 76(5): 1960-1972.
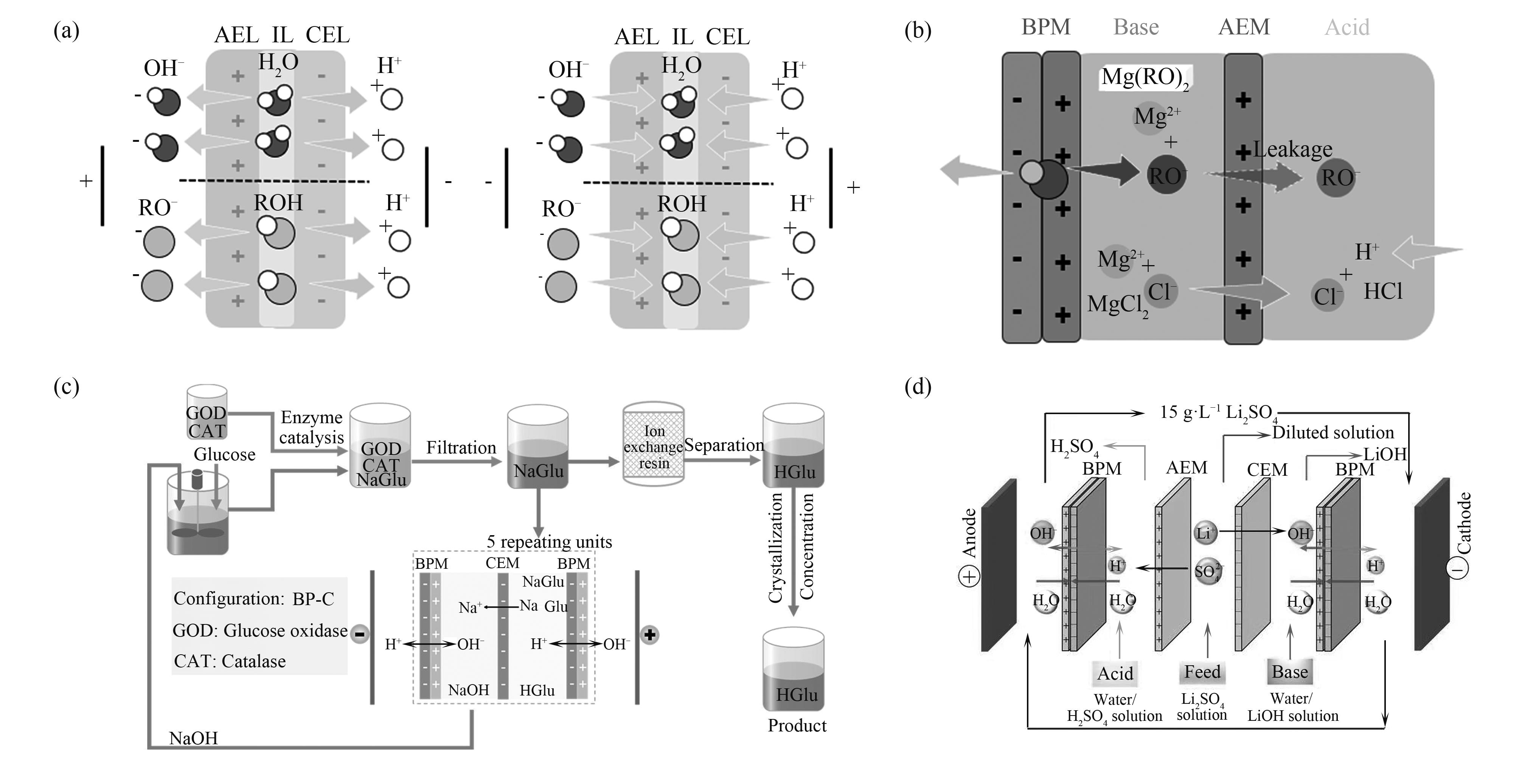
图1 (a)双极膜界面层水/醇缔合/解离示意图;(b)醇盐生产;(c)葡萄糖酸生产[12];(d)氢氧化锂生产示意图[8]
Fig. 1 (a) Schematic diagram of water/alcohol conjugation/dissociation at the interfacial layer of the bipolar membrane; (b) Schematic diagram of alkoxides production; (c) Gluconic acid production; (d) Lithium hydroxide production[8]
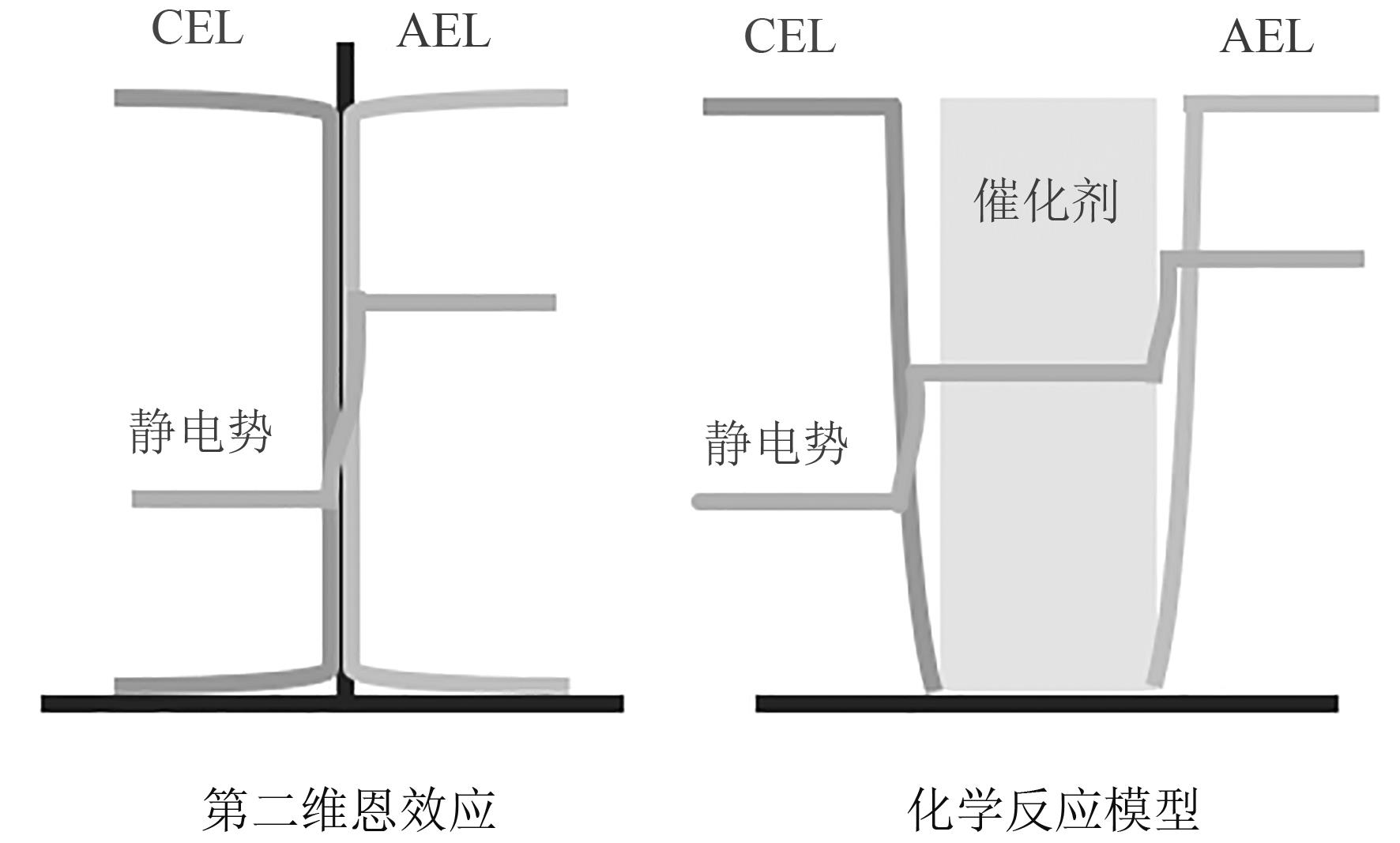
图2 双极膜水解离模型(图中显示了 CEL和 AEL上离子的电荷密度,以及BPM IL 上的静电势)
Fig. 2 Water dissociation model of the bipolar membranes (the plots show the charge density of the ions on the CEL and AEL, and the electrostatic potential on the BPM IL)
| 溶剂 | 化学式 | 介电常数 | pKSH | 黏度 |
|---|---|---|---|---|
| 水 | H2O | 78.4 | 14.00 | 1.00 |
| 甲醇 | CH3OH | 32.7 | 16.71 | 0.611 |
| 乙醇 | CH3CH2OH | 24.6 | 18.90 | 1.19 |
| 丙醇 | CH3CH2CH2OH | 20.5 | 19.43 | 2.20 |
表1 溶剂性质汇总[47]
Table 1 Summary of the main properties of different solvents[47]
| 溶剂 | 化学式 | 介电常数 | pKSH | 黏度 |
|---|---|---|---|---|
| 水 | H2O | 78.4 | 14.00 | 1.00 |
| 甲醇 | CH3OH | 32.7 | 16.71 | 0.611 |
| 乙醇 | CH3CH2OH | 24.6 | 18.90 | 1.19 |
| 丙醇 | CH3CH2CH2OH | 20.5 | 19.43 | 2.20 |
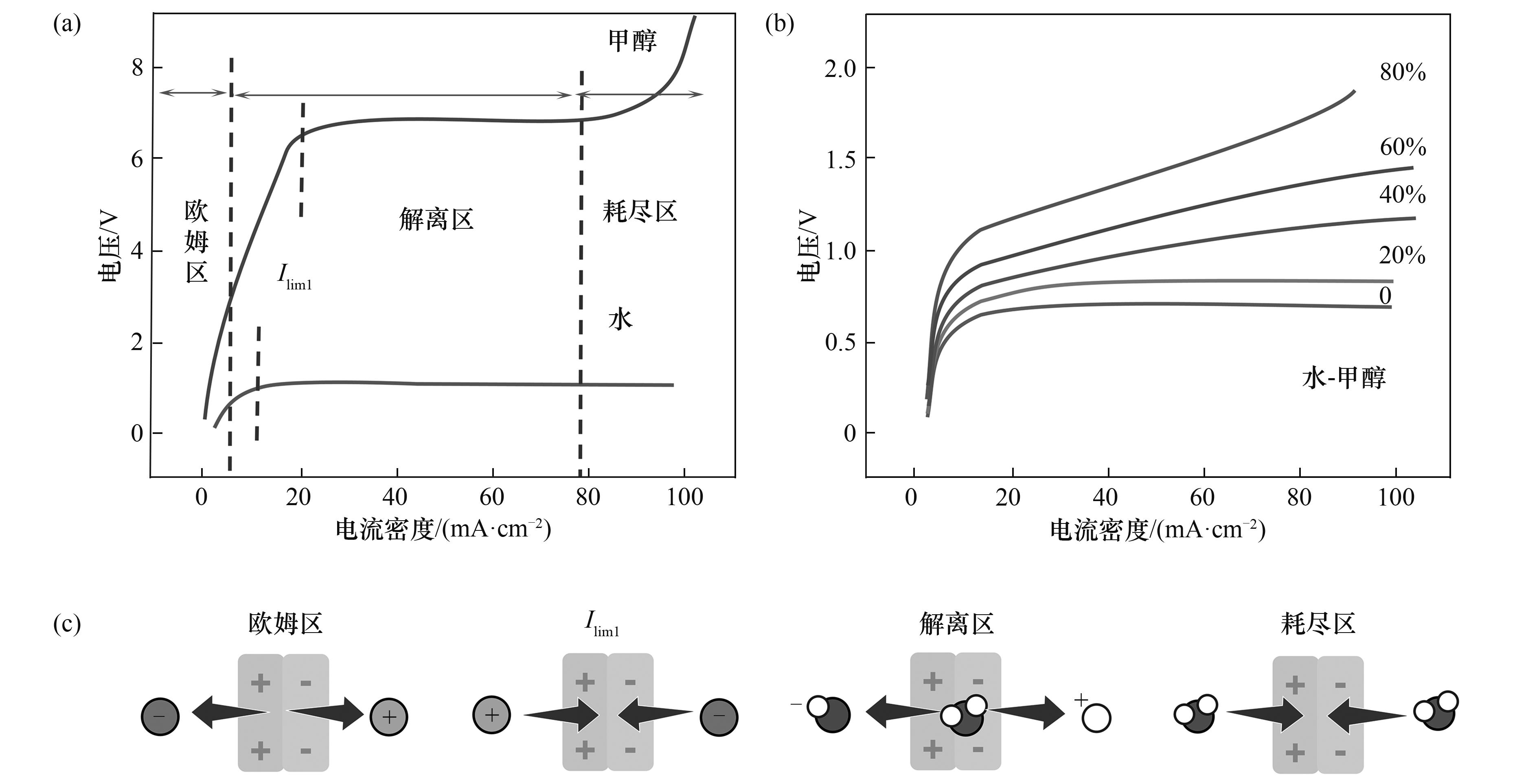
图4 (a)水/甲醇[49]和(b)水-甲醇体系的I-V曲线示意图;(c)电流-电压曲线划分示意图[49]
Fig. 4 (a) I-V plot for water/methanol[49]; (b) I-V curve schematic for water-methanol system; (c) Schematic of current-voltage curve division[49]
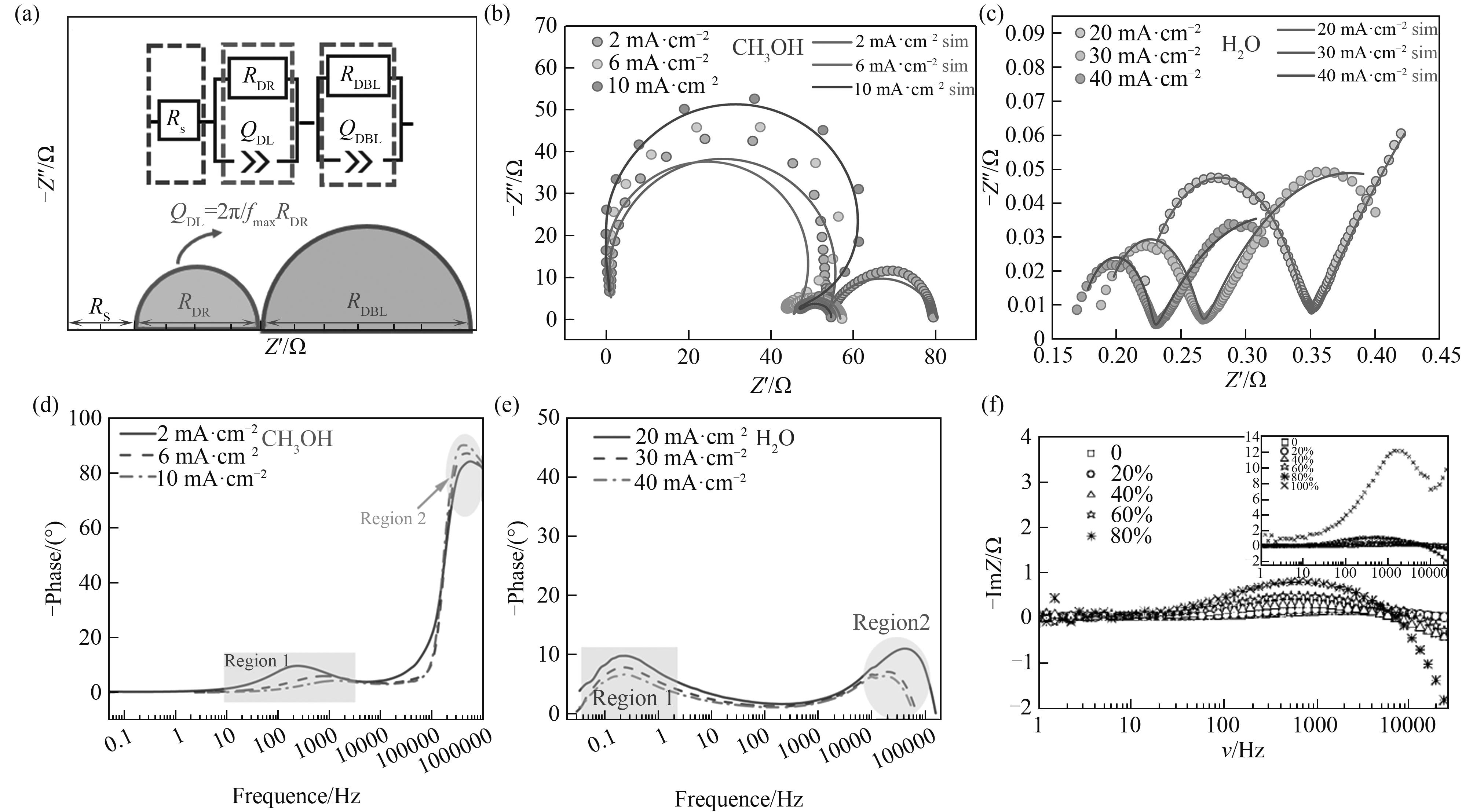
图5 (a)双极膜典型Nyquist谱图及等效电路(EEC);(b)、(c)水/甲醇阻抗谱图[32];(d)、(e)水/甲醇Bode谱图[32];(f)水-醇阻抗变化图[54]
Fig. 5 (a) Typical Nyquist spectroscopy and equivalent circuits (EECs) of bipolar membranes; (b),(c) Water/methanol impedance profiles[32]; (d),(e) Water/methanol Bode profiles[32]; (f) Water-alcohol impedance spectroscopy[54]
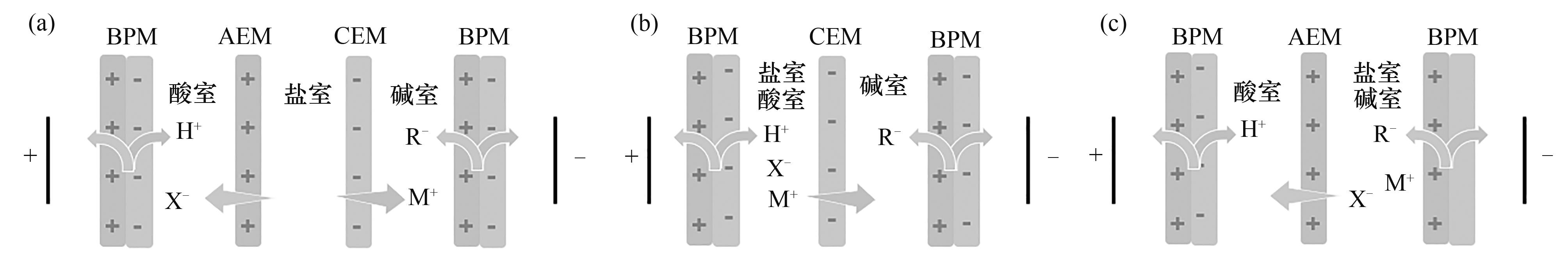
图6 BMED典型膜堆构型图:(a)三隔室BP-A-C;(b)两隔室BP-C;(c)两隔室BP-A
Fig. 6 Typical membrane stack configuration of BMED: (a) three-compartment BP-A-C; (b) two-compartment BP-C; (c) two-compartment BP-A
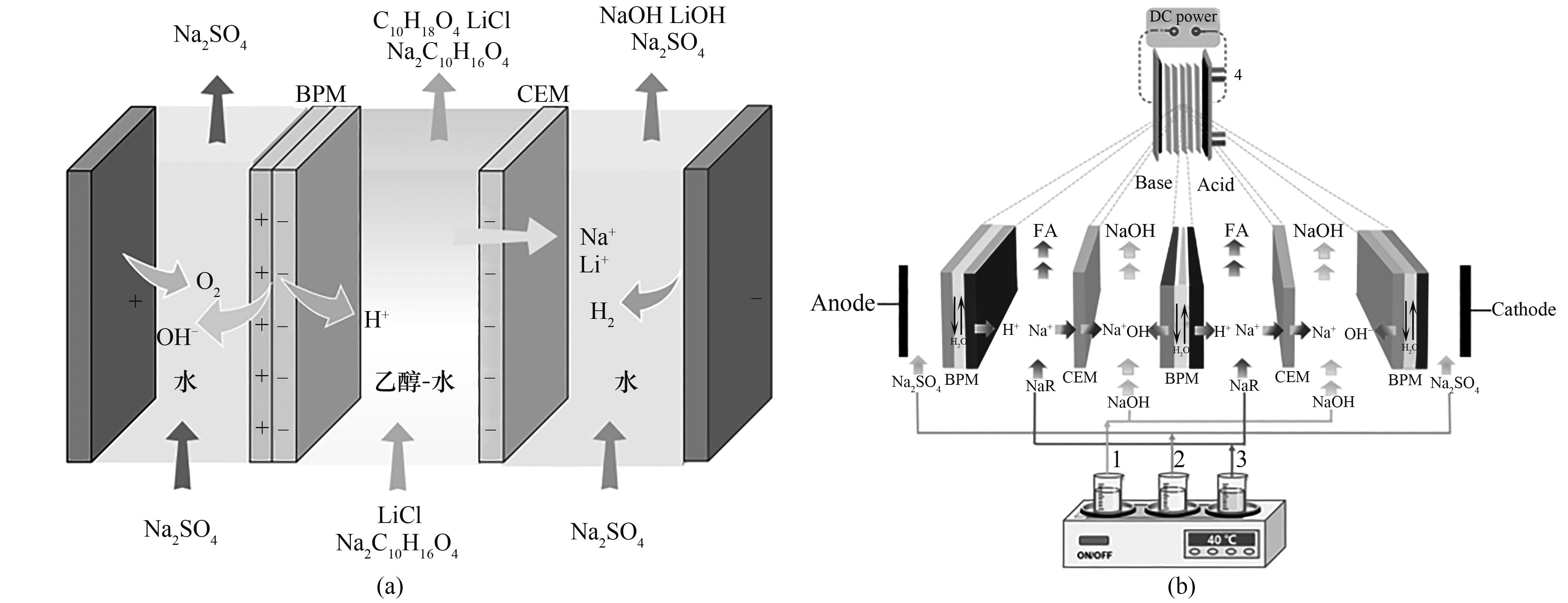
图7 水-醇混合体系生产癸二酸(a)和阿魏酸(b)[30]的膜堆构型
Fig.7 Membrane stack configurations of water-alcohol mixed solutions for the production of arachidonic acid (a) and ferulic acid (b)[30]
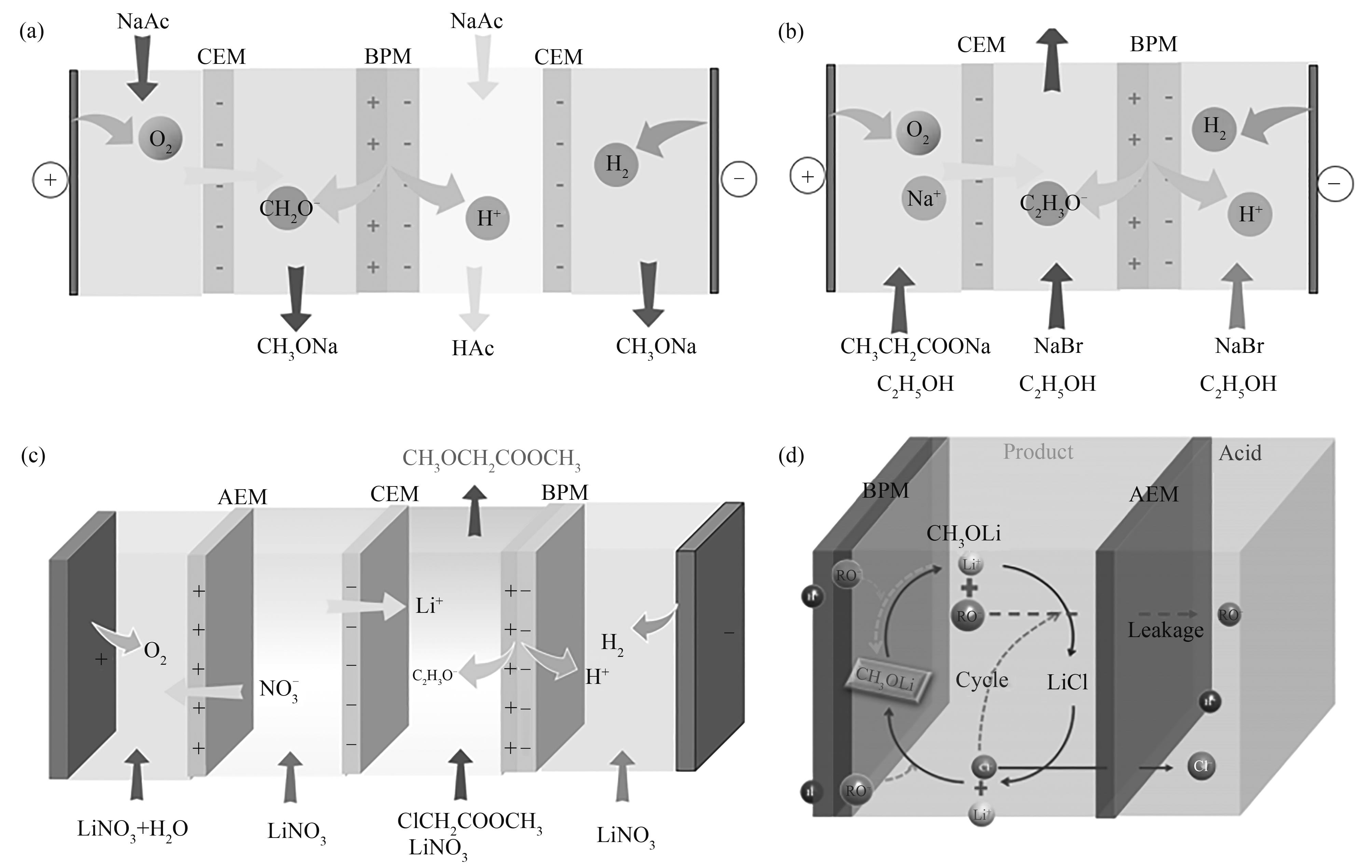
图8 醇体系中甲醇解离(a)、乙醇解离(b)、绿色化学合成甲氧基乙酸甲酯(c)和甲醇锂生产(d)[32]的膜堆构型
Fig.8 Membrane stack configurations for methanol dissociation (a), ethanol dissociation (b), green chemistry synthesis of methyl methoxyacetate (c) and lithium methanol production in alcohol system (d)[32]
| 1 | Frilette V J. Preparation and characterization of bipolar ion exchange membranes[J]. The Journal of Physical Chemistry, 1956, 60(4): 435-439. |
| 2 | Mauro A. Space charge regions in fixed charge membranes and the associated property of capacitance[J]. Biophysical Journal, 1962, 2(2 Pt 1): 179-198. |
| 3 | Bassignana I C, Reiss H. Ion transport and water dissociation in bipolar ion exchange membranes[J]. Journal of Membrane Science, 1983, 15(1): 27-41. |
| 4 | Peng S K, Xu X, Lu S F, et al. A self-humidifying acidic-alkaline bipolar membrane fuel cell[J]. Journal of Power Sources, 2015, 299: 273-279. |
| 5 | Dai J C, Dong Y C, Gao P, et al. A sandwiched bipolar membrane for all vanadium redox flow battery with high coulombic efficiency[J]. Polymer, 2018, 140: 233-239. |
| 6 | Chen R Y. Redox flow batteries: mitigating cross-contamination via bipolar redox-active materials and bipolar membranes[J]. Current Opinion in Electrochemistry, 2023, 37: 101188. |
| 7 | Metlay A S, Chyi B, Yoon Y, et al. Three-chamber design for aqueous acid-base redox flow batteries[J]. ACS Energy Letters, 2022, 7(3): 908-913. |
| 8 | Chen X, Ruan X Y, Kentish S E, et al. Production of lithium hydroxide by electrodialysis with bipolar membranes[J]. Separation and Purification Technology, 2021, 274: 119026. |
| 9 | Noguchi M, Nakamura Y, Shoji T, et al. Simultaneous removal and recovery of boron from waste water by multi-step bipolar membrane electrodialysis[J]. Journal of Water Process Engineering, 2018, 23: 299-305. |
| 10 | Fu R, Wang H Y, Yan J Y, et al. Ion injection bipolar membrane electrodialysis realizes over 8 mol/L NaOH conversion from a brine stream[J]. AIChE Journal, 2024, 70(4): e18345. |
| 11 | Yan J Y, Yan H Y, Wang H Y, et al. Bipolar membrane electrodialysis for clean production of L-10-camphorsulfonic acid: from laboratory to industrialization[J]. AIChE Journal, 2022, 68(2): e17490. |
| 12 | Wang H Y, Yan J Y, Fu R, et al. Bipolar membrane electrodialysis for cleaner production of gluconic acid: valorization of the regenerated base for the upstream enzyme catalysis[J]. Industrial & Engineering Chemistry Research, 2022, 61(22): 7634-7644. |
| 13 | Jiang C X, Chen B L, Xu Z A, et al. Ion-“distillation” for isolating lithium from lake brine[J]. AIChE Journal, 2022, 68(6): e17710. |
| 14 | Bi J T, Chen T Y, Xie Y, et al. Bipolar membrane electrodialysis integrated with in situ CO2 absorption for simulated seawater concentrate utilization, carbon storage and production of sodium carbonate[J]. Journal of Environmental Sciences, 2024, 142: 21-32. |
| 15 | Ye W Y, Huang J, Lin J Y, et al. Environmental evaluation of bipolar membrane electrodialysis for NaOH production from wastewater: conditioning NaOH as a CO2 absorbent[J]. Separation and Purification Technology, 2015, 144: 206-214. |
| 16 | Nagasawa H, Yamasaki A, Iizuka A, et al. A new recovery process of carbon dioxide from alkaline carbonate solution via electrodialysis[J]. AIChE Journal, 2009, 55(12): 3286-3293. |
| 17 | Siritanaratkul B, Forster M, Greenwell F, et al. Zero-gap bipolar membrane electrolyzer for carbon dioxide reduction using acid-tolerant molecular electrocatalysts[J]. Journal of the American Chemical Society, 2022, 144(17): 7551-7556. |
| 18 | Li Y J, Wang R Y, Shi S Y, et al. Bipolar membrane electrodialysis for ammonia recovery from synthetic urine: experiments, modeling, and performance analysis[J]. Environmental Science and Technology, 2021, 55(21): 14886-14896. |
| 19 | Ben Ali M A, Rakib M, Laborie S, et al. Coupling of bipolar membrane electrodialysis and ammonia stripping for direct treatment of wastewaters containing ammonium nitrate[J]. Journal of Membrane Science, 2004, 244(1/2): 89-96. |
| 20 | Xu Z A, Wan L, Liao Y W, et al. Continuous ammonia electrosynthesis using physically interlocked bipolar membrane at 1000 mA·cm-2 [J]. Nature Communications, 2023, 14(1): 1619. |
| 21 | Oener S Z, Twight L P, Lindquist G A, et al. Thin cation-exchange layers enable high-current-density bipolar membrane electrolyzers via improved water transport[J]. ACS Energy Letters, 2021, 6(1): 1-8. |
| 22 | Marin D H, Perryman J T, Hubert M A, et al. Hydrogen production with seawater-resilient bipolar membrane electrolyzers[J]. Joule, 2023, 7(4): 765-781. |
| 23 | Lee L, Kim D. Poly(arylene ether ketone)-based bipolar membranes for acid-alkaline water electrolysis applications[J]. Journal of Materials Chemistry A, 2021, 9(9): 5485-5496. |
| 24 | Park E J, Arges C G, Xu H, et al. Membrane strategies for water electrolysis[J]. ACS Energy Letters, 2022, 7(10): 3447-3457. |
| 25 | Blommaert M A, Subramanian S, Yang K L, et al. High indirect energy consumption in AEM-based CO2 electrolyzers demonstrates the potential of bipolar membranes[J]. ACS Applied Materials & Interfaces, 2022, 14(1): 557-563. |
| 26 | Chen Y Y, Wrubel J A, Ellis Klein W, et al. High-performance bipolar membrane development for improved water dissociation[J]. ACS Applied Polymer Materials, 2020, 2(11): 4559-4569. |
| 27 | Li Y C, Yan Z F, Hitt J, et al. Bipolar membranes inhibit product crossover in CO2 electrolysis cells[J]. Advanced Sustainable Systems, 2018, 2(4): 1700187. |
| 28 | Xie K, Miao R K, Ozden A, et al. Bipolar membrane electrolyzers enable high single-pass CO2 electroreduction to multicarbon products[J]. Nature Communications, 2022, 13(1): 3609. |
| 29 | Zhang F, Huang C H, Xu T W. Production of sebacic acid using two-phase bipolar membrane electrodialysis[J]. Industrial & Engineering Chemistry Research, 2009, 48(16): 7482-7488. |
| 30 | Ma X X, Liu W L, Li C R, et al. Bipolar membrane electrodialysis for efficient production of ferulic acid in alcohol/water mixed solvent[J]. Separation and Purification Technology, 2024, 341: 126876. |
| 31 | Liu X H, Li Q H, Jiang C X, et al. Bipolar membrane electrodialysis in aqua–ethanol medium: production of salicylic acid[J]. Journal of Membrane Science, 2015, 482: 76-82. |
| 32 | Yan J Y, Li R R, Wang H Y, et al. Alcohol splitting with bipolar membranes for the production of metal alkoxides: alcohol splitting behaviour and ion transport kinetics[J]. Chemical Engineering Science, 2024, 286: 119657. |
| 33 | Sridhar S, Feldmann C. Electrodialysis in a non-aqueous medium: a clean process for the production of acetoacetic ester[J]. Journal of Membrane Science, 1997, 124(2): 175-179. |
| 34 | Li Q H, Huang C H, Xu T W. Bipolar membrane electrodialysis in an organic medium: production of methyl methoxyacetate[J]. Journal of Membrane Science, 2009, 339(1/2): 28-32. |
| 35 | Loh Y Y, Nagao K, Hoover A J, et al. Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds[J]. Science, 2017, 358(6367): 1182-1187. |
| 36 | Grossman G. Water dissociation effects in ion transport through composite membrane[J]. The Journal of Physical Chemistry, 1976, 80(14): 1616-1625. |
| 37 | Simons R. Electric field effects on proton transfer between ionizable groups and water in ion exchange membranes[J]. Electrochimica Acta, 1984, 29(2): 151-158. |
| 38 | Onsager L. Deviations from Ohm’s law in weak electrolytes[J]. The Journal of Chemical Physics, 1934, 2(9): 599-615. |
| 39 | Ramírez P, Manzanares J A, Mafé S. Water dissociation effects in ion transport through anion exchange membranes with thin cation exchange surface films[J]. Berichte der Bunsengesellschaft Für Physikalische Chemie, 1991, 95(4): 499-503. |
| 40 | Ge Z, Shehzad M A, Yang X, et al. High-performance bipolar membrane for electrochemical water electrolysis[J]. Journal of Membrane Science, 2022, 656: 120660. |
| 41 | Krol J J, Wessling M, Strathmann H. Concentration polarization with monopolar ion exchange membranes: current-voltage curves and water dissociation[J]. Journal of Membrane Science, 1999, 162(1/2): 145-154. |
| 42 | Timashev S F, Kirganova E V. Mechanism of the electrolytic decomposition of water-molecules in bipolar ion-exchange membranes[J]. Soviet Electrochemistry, 1981, 17(3): 366-369. |
| 43 | Zabolotskii V I, Gnusin N, Shel'deshov N V. The current-voltage characteristic of the transition region in MB-1 bipolar membranes[J]. Soviet Electrochemistry, 1984, 20(10): 1238-1243. |
| 44 | Strathmann H, Krol J J, Rapp H J, et al. Limiting current density and water dissociation in bipolar membranes[J]. Journal of Membrane Science, 1997, 125(1): 123-142. |
| 45 | Pärnamäe R, Mareev S, Nikonenko V, et al. Bipolar membranes: a review on principles, latest developments, and applications[J]. Journal of Membrane Science, 2021, 617: 118538. |
| 46 | Mafé S, Ramı́rez P, Alcaraz A. Electric field-assisted proton transfer and water dissociation at the junction of a fixed-charge bipolar membrane[J]. Chemical Physics Letters, 1998, 294(4/5): 406-412. |
| 47 | Onishi N, Minagawa M, Tanioka A, et al. Current-voltage characteristics and solvent dissociation of bipolar membranes in organic solvents[J]. Membranes, 2022, 12(12): 1236. |
| 48 | Bruggeman D A G. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen (I): Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen[J]. Annalen der Physik, 1935, 416(8): 665-679. |
| 49 | Abdu S, Sricharoen K, Wong J E, et al. Catalytic polyelectrolyte multilayers at the bipolar membrane interface[J]. ACS Applied Materials & Interfaces, 2013, 5(21): 10445-10455. |
| 50 | Blommaert M A, Vermaas D A, Izelaar B, et al. Electrochemical impedance spectroscopy as a performance indicator of water dissociation in bipolar membranes[J]. Journal of Materials Chemistry A, 2019, 7(32): 19060-19069. |
| 51 | Balster J, Srinkantharajah S, Sumbharaju R, et al. Tailoring the interface layer of the bipolar membrane[J]. Journal of Membrane Science, 2010, 365(1/2): 389-398. |
| 52 | Giesbrecht P K, Freund M S. Recent advances in bipolar membrane design and applications[J]. Chemistry of Materials, 2020, 32(19): 8060-8090. |
| 53 | Yan Z F, Zhu L, Li Y C, et al. The balance of electric field and interfacial catalysis in promoting water dissociation in bipolar membranes[J]. Energy & Environmental Science, 2018, 11(8): 2235-2245. |
| 54 | 刘小菏, 李秋花, 葛亮, 等. 水-乙醇体系对双极膜中间界面层的影响[J]. 化工学报, 2016, 67(1):309-314. |
| Liu X H, Li Q H, Ge L, et al. Influence of agua-ethanol medium on properties of intermediate layer of a bipolar membrane [J]. CIESC Journal, 2016, 67(1):309-314. | |
| 55 | Yan K X, Hang X F, Liu J S, et al. Preparation of hypophosphorous acid by bipolar membrane electrodialysis: process optimization and phosphorous acid minimization[J]. Industrial & Engineering Chemistry Research, 2019, 58(47): 21855-21863. |
| 56 | Wang Y M, Zhang X, Xu T W. Integration of conventional electrodialysis and electrodialysis with bipolar membranes for production of organic acids[J]. Journal of Membrane Science, 2010, 365(1/2): 294-301. |
| 57 | Jaime Ferrer J S, Laborie S, Durand G, et al. Formic acid regeneration by electromembrane processes[J]. Journal of Membrane Science, 2006, 280(1/2): 509-516. |
| 58 | Szczygiełda M, Antczak J, Prochaska K. Separation and concentration of succinic acid from post-fermentation broth by bipolar membrane electrodialysis (EDBM)[J]. Separation and Purification Technology, 2017, 181: 53-59. |
| 59 | Yan J Y, Yu W S, Wang Z H, et al. Review on high-performance polymeric bipolar membrane design and novel electrochemical applications[J]. Aggregate, 2024, 5(4): e527. |
| 60 | Alvarez F, Alvarez R, Coca J, et al. Salicylic acid production by electrodialysis with bipolar membranes [J]. Journal of Membrane Science, 1997, 123(1): 61-69. |
| 61 | Kameche M, Xu F N, Innocent C, et al. Electrodialysis in water-ethanol solutions: application to the acidification of organic salts[J]. Desalination, 2003, 154(1): 9-15. |
| 62 | Luo G S, Shan X Y, Qi X, et al. Two-phase electro-electrodialysis for recovery and concentration of citric acid[J]. Separation and Purification Technology, 2004, 38(3): 265-271. |
| 63 | Yi S S, Lu Y C, Luo G S. Separation and concentration of lactic acid by electro-electrodialysis[J]. Separation and Purification Technology, 2008, 60(3): 308-314. |
| 64 | Rottiers T, der B Bruggen van, Pinoy L. Production of salicylic acid in a three compartment bipolar membrane electrodialysis configuration[J]. Journal of Industrial and Engineering Chemistry, 2017, 54: 190-199. |
| 65 | Chou T J, Tanioka A. Current–voltage curves of composite bipolar membrane in alcohol–water solutions[J]. The Journal of Physical Chemistry B, 1998, 102(40): 7866-7870. |
| 66 | Sridhar S. Electrodialysis in a non-aqueous medium: production of sodium methoxide[J]. Journal of Membrane Science, 1996, 113(1): 73-79. |
| 67 | Li Q H, Huang C H, Xu T W. Ethanol splitting in bipolar membranes: evidence from NMR analysis[J]. Journal of Membrane Science, 2008, 325(1): 20-22. |
| 68 | Li Q H, Huang C H, Xu T W. Alcohol splitting for the production of methyl methoxyacetate: integration of ion-exchange with bipolar membrane electrodialysis[J]. Journal of Membrane Science, 2011, 367(1/2): 314-318. |
| 69 | Tomilov A P. Electrochemical syntheses in absolute alcohols (review)[J]. Russian Journal of Electrochemistry, 2000, 36(2): 100-116. |
| 70 | Zheng Y Z, Wang N N, Luo J J, et al. Hydrogen-bonding interactions between [BMIM][BF4] and acetonitrile[J]. Physical Chemistry Chemical Physics, 2013, 15(41): 18055. |
| 71 | Hou H Y, Huang Y R, Bai B F, et al. Volumetric properties and molecular interactions of binary mixtures imidazolium acetates-ethanol at 293.15 K[J]. Chemical Journal of Chinese Universities, 2014, 35(1): 121-126. |
| 72 | Oener S Z, Foster M J, Boettcher S W. Accelerating water dissociation in bipolar membranes and for electrocatalysis[J]. Science, 2020, 369(6507): 1099-1103. |
| 73 | Vermaas D A, Wiegman S, Nagaki T, et al. Ion transport mechanisms in bipolar membranes for (photo)electrochemical water splitting[J]. Sustainable Energy & Fuels, 2018, 2(9): 2006-2015. |
| 74 | Greben V P, Pivovarov N Y, Kovarskii N Y, et al. Influence of ionite nature on physicochemical properties of bipolar ion-exchange membranes[J]. Zhurnal FizicheskoiI Khimii, 1978, 52(10): 2641-2645. |
| 75 | Chen L, Xu Q C, Oener S Z, et al. Design principles for water dissociation catalysts in high-performance bipolar membranes[J]. Nature Communications, 2022, 13(1): 3846. |
| 76 | Bui J C, Lees E W, Marin D H, et al. Multi-scale physics of bipolar membranes in electrochemical processes[J]. Nature Chemical Engineering, 2024, 1: 45-60. |
| 77 | Xu Z A, Liao Y W, Pang M B, et al. A chemically interlocked bipolar membrane achieving stable water dissociation for high output ammonia electrosynthesis[J]. Energy & Environmental Science, 2023, 16(9): 3815-3824. |
| 78 | Sasmal S, Chen L, Sarma P V, et al. Materials descriptors for advanced water dissociation catalysts in bipolar membranes[J]. Nature Materials, 2024, 23(10): 1421-1427. |
| 79 | Zhu B T, Dong B, Wang F, et al. Unraveling a bifunctional mechanism for methanol-to-formate electro-oxidation on nickel-based hydroxides[J]. Nature Communications, 2023, 14(1): 1686. |
| 80 | Chen W, Xie C, Wang Y Y, et al. Activity origins and design principles of nickel-based catalysts for nucleophile electrooxidation[J]. Chem, 2020, 6(11): 2974-2993. |
| 81 | Zuo P P, Ye C C, Jiao Z R, et al. Near-frictionless ion transport within triazine framework membranes[J]. Nature, 2023, 617(7960): 299-305. |
| 82 | Xu J, Jiang R, Qiu Z, et al. Conjugated microporous polymer for membrane separation: a review [J]. Separation and Purification Technology, 2025, 362: 131795. |
| 83 | Zuo P P, Xu T W. Constructing hydrophilic polymer membranes with microporosity for aqueous redox flow batteries[J]. ChemSusChem, 2025: e202402562. |
| 84 | Peng K, Zhang C, Fang J K, et al. Constructing microporous ion exchange membranes via simple hypercrosslinking for pH-neutral aqueous organic redox flow batteries[J]. Angewandte Chemie International Edition, 2024, 63(37): e202407372. |
| [1] | 邱知, 谭明. 聚离子液体膜的制备及其在低钠高钾健康酱油中的应用[J]. 化工学报, 2024, 75(S1): 244-250. |
| [2] | 谢慧慧, 姜佳鑫, 王鑫, 李正, 郭鑫, 吕欣然, 王凌云, 刘杨. 深共晶溶剂聚合物包覆膜传输分离铂、钯的研究[J]. 化工学报, 2024, 75(S1): 235-243. |
| [3] | 胡军勇, 胡亚丽, 谭学诣, 黄佳欣, 张乐炜, 曾俊立, 刘晓奕, 陶源. 基于LiCl-NH4Cl水溶液多级逆电渗析性能的实验研究[J]. 化工学报, 2024, 75(7): 2670-2679. |
| [4] | 王灵洁, 高海龙, 靳继鹏, 王志浩, 李见波. 海水中的污染物对逆电渗析电堆性能的影响[J]. 化工学报, 2024, 75(2): 695-705. |
| [5] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [6] | 朱兴驰, 郭志远, 纪志永, 汪婧, 张盼盼, 刘杰, 赵颖颖, 袁俊生. 选择性电渗析镁锂分离过程模拟优化[J]. 化工学报, 2023, 74(6): 2477-2485. |
| [7] | 闫军营, 王皝莹, 李瑞瑞, 符蓉, 蒋晨啸, 汪耀明, 徐铜文. 选择性电渗析:机遇与挑战[J]. 化工学报, 2023, 74(1): 224-236. |
| [8] | 杨宏欣, 李兴亚, 葛亮, 徐铜文. 含哌啶阳离子侧长链型一/二价阴离子选择性分离膜的制备[J]. 化工学报, 2022, 73(8): 3739-3748. |
| [9] | 杨珊珊, 姚宇洋, 董云迪, 徐志鹏, 高尚上, 阮慧敏, 沈江南. 基于二苯并-18-冠-6基体改性的K+选择性离子交换膜的制备及性能研究[J]. 化工学报, 2022, 73(4): 1781-1793. |
| [10] | 孙博, 王建伟, 张小松. 基于电渗析的溶液再生传质模型及性能分析[J]. 化工学报, 2021, 72(S1): 218-226. |
| [11] | 刘元伟, 董晨初, 廖俊斌, 王超, 陈权, 沈江南. 不同侧链BPPO阴离子交换膜的制备及其抗污染性能[J]. 化工学报, 2021, 72(3): 1732-1741. |
| [12] | 唐元晖, 孙文文, 李太雨, 毛鹏, 金义凡, 汪林, 林亚凯, 王晓琳. 双极膜电渗析法麦草畏生产废水的资源化利用研究[J]. 化工学报, 2021, 72(12): 6328-6339. |
| [13] | 祝海涛, 杨波, 吴雅琴, 高从堦. 电渗析脱盐过程离子传递现象的数值模拟[J]. 化工学报, 2020, 71(8): 3518-3526. |
| [14] | 徐士鸣, 刘志强, 吴曦, 张又文, 胡军勇, 吴德兵, 冷强, 金东旭, 王平. 溶液浓差能驱动的逆电渗析反应器制氢实验研究[J]. 化工学报, 2020, 71(5): 2283-2291. |
| [15] | 王超, 潘能修, 鲁丹, 廖俊斌, 沈江南, 高从堦. 电渗析用季铵化聚氯乙烯均相阴离子交换膜的制备[J]. 化工学报, 2019, 70(4): 1620-1627. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号