化工学报 ›› 2020, Vol. 71 ›› Issue (3): 1390-1397.DOI: 10.11949/0438-1157.20191448
收稿日期:2019-11-28
修回日期:2020-01-06
出版日期:2020-03-05
发布日期:2020-03-05
通讯作者:
殷娟娟
作者简介:李敬(1984—),男,博士,高级工程师,
Jing LI( ),Gang DU,Juanjuan YIN(
),Gang DU,Juanjuan YIN( )
)
Received:2019-11-28
Revised:2020-01-06
Online:2020-03-05
Published:2020-03-05
Contact:
Juanjuan YIN
摘要:
碳酸钴是一类典型的转换型负极锂电池材料,具有资源丰富、比容量高、安全可靠等优点,但是存在一些尚未解决的问题,例如导电性比较差,同时在锂离子的嵌入和脱出过程中体积变化严重。通过水热法制备了不同组分Zn掺杂ZnxCo1-xCO3 (x=0.12, 0.3, 0.5),通过调整Zn和Co原材料质量来控制Zn/Co的摩尔比,研究表明当Zn和Co的摩尔比为0.3∶0.7时,掺杂产物有良好的循环和倍率性能,锌离子的掺杂提高了锂离子电导率,对ZnxCo1-xCO3 (x=0.12, 0.3, 0.5)研究表明其在充放电过程中既有合金反应又有转换反应,提高了整个电极的导电性,进而表现出优异的电化学性能。
中图分类号:
李敬, 杜刚, 殷娟娟. ZnxCo1-xCO3碳酸盐负极材料的制备及其电化学性能研究[J]. 化工学报, 2020, 71(3): 1390-1397.
Jing LI, Gang DU, Juanjuan YIN. Preparation and electrochemical properties of ZnxCo1-xCO3 carbonateanode materials[J]. CIESC Journal, 2020, 71(3): 1390-1397.
| 理论掺杂样品 | 实际掺杂样品 |
|---|---|
| Zn0.33Co0.67CO3 | Zn0.12Co0.88CO3 |
| Zn0.5Co0.5CO3 | Zn0.3Co0.7CO3 |
| Zn0.67Co0.33CO3 | Zn0.5Co0.5CO3 |
表1 ZnxCo1-xCO3的理论掺杂量和实际掺杂量
Table 1 Theoretical doping and actual doping of ZnxCo1-xCO3
| 理论掺杂样品 | 实际掺杂样品 |
|---|---|
| Zn0.33Co0.67CO3 | Zn0.12Co0.88CO3 |
| Zn0.5Co0.5CO3 | Zn0.3Co0.7CO3 |
| Zn0.67Co0.33CO3 | Zn0.5Co0.5CO3 |
| 样品 | 振实密度/(g·cm-3) |
|---|---|
| CoCO3 | 1.96 |
| Zn0.12Co0.88CO3 | 2.11 |
| Zn0.3Co0.7CO3 | 2.2 |
| Zn0.5Co0.5CO3 | 2.25 |
表2 ZnxCo1-xCO3 (x=0,0.12,0.3,0.5)的振实密度
Table 2 High tap density of ZnxCo1-xCO3 (x=0,0.12,0.3,0.5)
| 样品 | 振实密度/(g·cm-3) |
|---|---|
| CoCO3 | 1.96 |
| Zn0.12Co0.88CO3 | 2.11 |
| Zn0.3Co0.7CO3 | 2.2 |
| Zn0.5Co0.5CO3 | 2.25 |
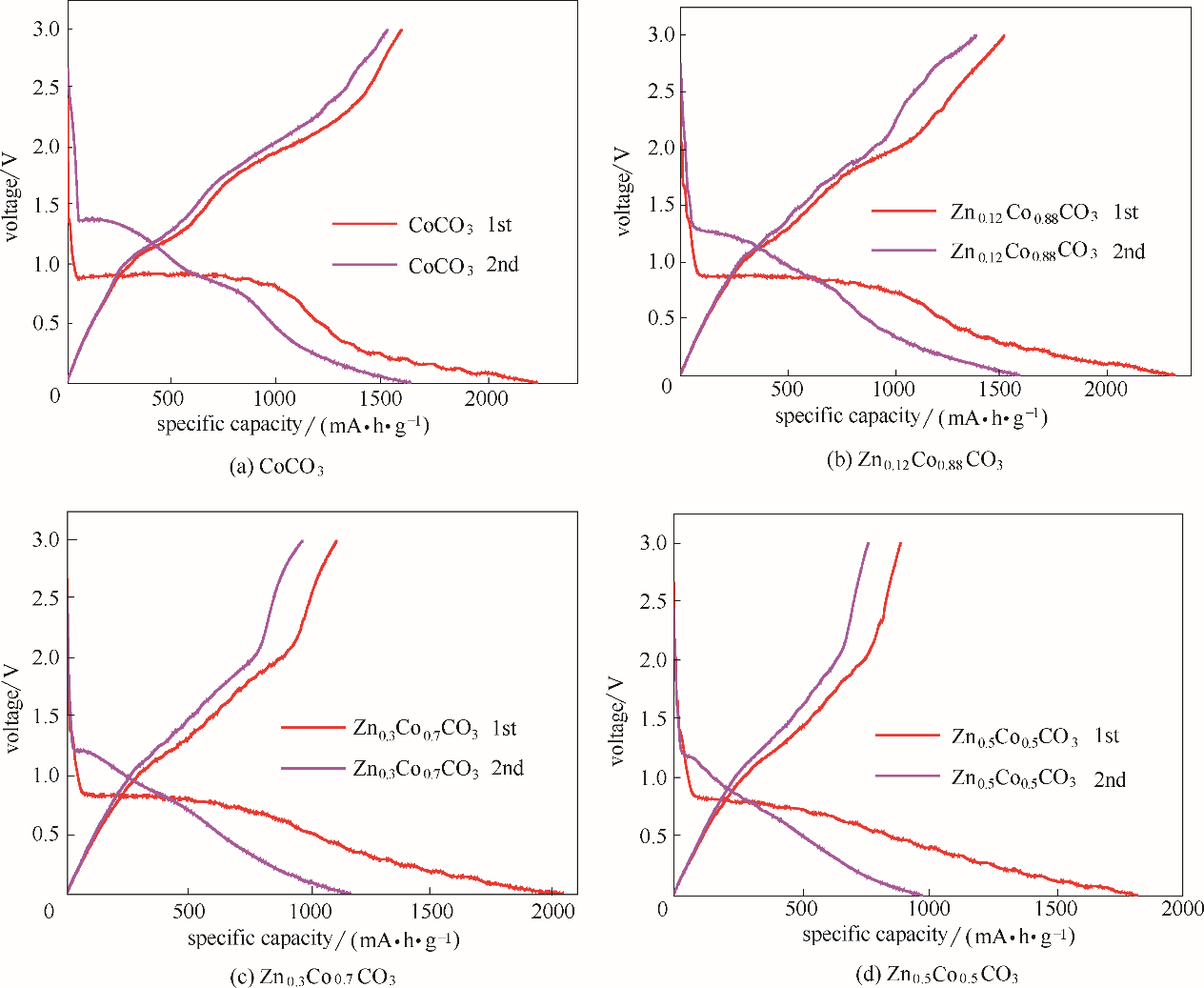
图6 ZnxCo1-xCO3(x=0,0.12,0.3,0.5)在0.1C的电流密度下前2圈的恒流充放电曲线
Fig.6 Constant current charge and discharge curve for first 2 turns at 0.1 C current density of ZnxCo1-xCO3(x=0,0.12,0.3,0.5)
| 样品 | 0.2 C | 0.5 C | 1 C | 2 C | 0.1 C |
|---|---|---|---|---|---|
| CoCO3 | 1146.7 | 787.7 | 479.4 | 227.8 | 921 |
| Zn0.12Co0.88CO3 | 767.8 | 580.9 | 546.1 | 353.4 | 769 |
| Zn0.3Co0.7CO3 | 1048.3 | 809.8 | 657.1 | 407.3 | 935.6 |
| Zn0.5Co0.5CO3 | 668.5 | 520.3 | 440.2 | 331.2 | 685.2 |
表3 图7(b)中ZnxCo1-xCO3 (x=0,0.12,0.3,0.5)在不同倍率下的容量
Table 3 Capacity of ZnxCo1-xCO3 (x=0,0.12,0.3,0.5) [Fig.7(b)] at different ratios/(mA·h·g-1)
| 样品 | 0.2 C | 0.5 C | 1 C | 2 C | 0.1 C |
|---|---|---|---|---|---|
| CoCO3 | 1146.7 | 787.7 | 479.4 | 227.8 | 921 |
| Zn0.12Co0.88CO3 | 767.8 | 580.9 | 546.1 | 353.4 | 769 |
| Zn0.3Co0.7CO3 | 1048.3 | 809.8 | 657.1 | 407.3 | 935.6 |
| Zn0.5Co0.5CO3 | 668.5 | 520.3 | 440.2 | 331.2 | 685.2 |
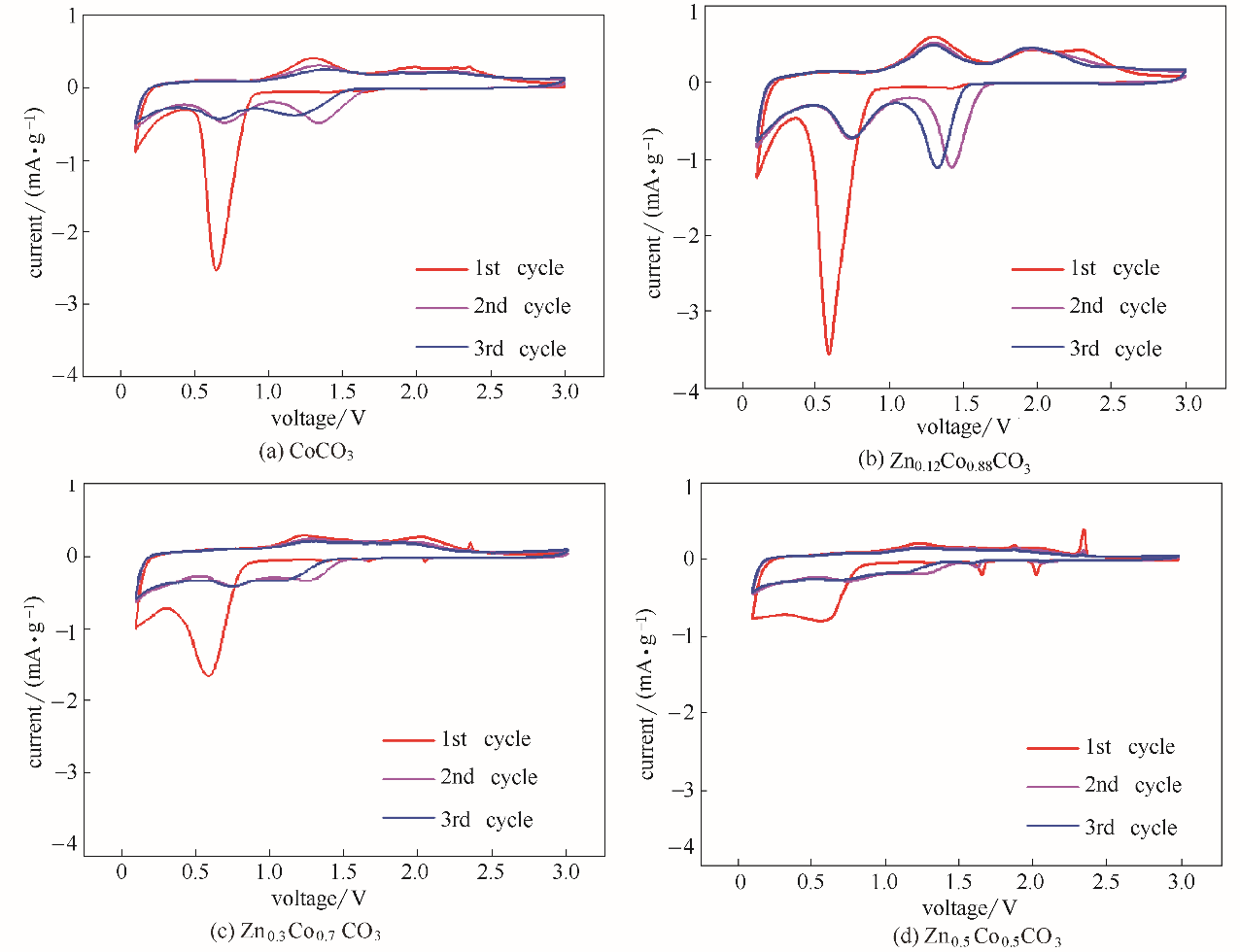
图8 ZnxCo1-xCO3 (x=0,0.12,0.3,0.5)在0.1 mV·s-1扫速下前3圈的CV曲线
Fig.8 CV curves of ZnxCo1-xCO3 (x=0,0.12,0.3,0.5) for the first 3 circles at scan rate of 0.1 mV·s-1
| 样品 | 循环次数 | Rs/Ω | RSEI/Ω | Rct/Ω |
|---|---|---|---|---|
| CoCO3 | 2 | 14.6 | 87.97 | 286.7 |
| Zn0.12Co0.88CO3 | 2 | 3.76 | 17.93 | 138.6 |
| Zn0.3Co0.7CO3 | 2 | 12.8 | 16.5 | 20.3 |
| Zn0.5Co0.5CO3 | 2 | 6.63 | 63.8 | 82.6 |
| CoCO3 | 102 | 4.124 | 150.2 | 223.7 |
| Zn0.12Co0.88CO3 | 102 | 14.7 | 281.4 | 406.1 |
| Zn0.3Co0.7CO3 | 102 | 7.65 | 103.6 | 137.9 |
| Zn0.5Co0.5CO3 | 102 | 1.35 | 137.8 | 162.4 |
表4 图9中的EIS曲线的拟合结果
Table 4 Fitting results of EIS curve in Fig.9
| 样品 | 循环次数 | Rs/Ω | RSEI/Ω | Rct/Ω |
|---|---|---|---|---|
| CoCO3 | 2 | 14.6 | 87.97 | 286.7 |
| Zn0.12Co0.88CO3 | 2 | 3.76 | 17.93 | 138.6 |
| Zn0.3Co0.7CO3 | 2 | 12.8 | 16.5 | 20.3 |
| Zn0.5Co0.5CO3 | 2 | 6.63 | 63.8 | 82.6 |
| CoCO3 | 102 | 4.124 | 150.2 | 223.7 |
| Zn0.12Co0.88CO3 | 102 | 14.7 | 281.4 | 406.1 |
| Zn0.3Co0.7CO3 | 102 | 7.65 | 103.6 | 137.9 |
| Zn0.5Co0.5CO3 | 102 | 1.35 | 137.8 | 162.4 |
| 1 | Yin J, Ding Z, Lei D, et al. Zn-substituted CoCO3 embedded in carbon nanotubes network as high performance anode for lithium-ion batteries[J]. Journal of Alloys and Compounds, 2017, 712: 605-612. |
| 2 | Wang L, Tang W, Jing Y, et al. Do transition metal carbonates have greater lithium storage capability than oxides? A case study of monodisperse CoCO3 and CoO microspindles[J]. ACS Applied Materials & Interfaces, 2014, 6(15): 12346-12352. |
| 3 | Ding Z, Yao B, Feng J, et al. Enhanced rate performance and cycling stability of a CoCO3–polypyrrole composite for lithium ion battery anodes[J]. Journal of Materials Chemistry A, 2013, 1(37): 11200-11209 |
| 4 | Mirhashemihaghighi S, León B, Pérez V C, et al. Lithium storage mechanisms and effect of partial cobalt substitution in manganese carbonate electrodes[J]. Inorganic Chemistry, 2012, 51(10): 5554-5560. |
| 5 | Giri A K, Pal P, Ananthakumar R, et al. 3D hierarchically assembled porous wrinkled-paper-like structure of ZnCo2O4 and Co-ZnO@C as anode materials for lithium-ion batteries [J].Crystal Growth & Design, 2014, 14(7): 3352-3359. |
| 6 | Liang K, Cheang T Y, Wen T, et al. Facile preparation of porous Mn2SnO4/Sn/C composite cubes as high performance anode material for lithium-ion batteries[J]. The Journal of Physical Chemistry C, 2016, 120(7): 3669-3676 |
| 7 | Qin Z, Hong B, Duan B, et al. Tributyl borate as a novel electrolyte additive to improve high voltage stability of lithium cobalt oxide in carbonate-based electrolyte[J]. Electrochimica Acta, 2018, 276: 412-416. |
| 8 | Tarascon J, Armand M. Issues and challenges facing rechargeable lithium batteries[J]. Nature, 2001,414: 359-367. |
| 9 | Ishikawa M, Tanaka H, Kawai T. Preparation of highly conductive Mn-doped Fe3O4 thin films with spin polarization at room temperature using a pulsed-laser deposition technique[J]. Applied Physics Letters, 2005, 86(22): 222504. |
| 10 | Courtel M F, Duncan H, Abu-Lebdeh Y, et al. High capacity anode materials for Li-ion batteries based on spinel metal oxides AMn2O4 (A= Co, Ni, and Zn) [J]. J. Mater. Chem., 2011, 21: 10206-10218. |
| 11 | Liu B, Zhang J, Wang X, et al. Hierarchical three-dimensional ZnCo2O4 nanowire arrays/carbon cloth anodes for a novel class of high-performance flexible lithium-ion batteries[J]. Nano Letters, 2012, 12(6): 3005-3011 |
| 12 | Wang H, Zhu Y, Yuan C, et al. Cobalt-phthalocyanine-derived ultrafine Co3O4 nanoparticles as high-performance anode materials for lithium ion batteries[J]. Applied Surface Science, 2017, 414: 398-404. |
| 13 | Jin R, Ma Y, Sun Y, et al. Manganese cobalt oxide (MnCo2O4) hollow spheres as high capacity anode materials for lithium‐ion batteries[J]. Energy Technology, 2017, 5(2): 293-299. |
| 14 | Sharma Y, Sharma N, Subba R G V, et al. Nanophase ZnCo2O4 as a high performance anode material for Li-ion batteries[J]. Advanced Functional Materials, 2007, 17(15): 2855-2861. |
| 15 | Lee C W, Seo S D, Kim D W, et al. Heteroepitaxial growth of ZnO nanosheet bands on ZnCo2O4 submicron rods toward high-performance Li ion battery electrodes[J]. Nano Research, 2013, 6(5): 348-355. |
| 16 | Sharma Y, Sharma N, Rao G V S, et al. Nano-phase (Cd1/3Zn1/3Co1/3) CO3: a high capacity anode material for Li-ion batteries//meeting abstracts [J].The Electrochemical Society, 2008 (4): 421-421. |
| 17 | Liu L, Mou L, Yu J, et al. Urchin-like CoO–C micro/nano hierarchical structures as high performance anode materials for Li-ion batteries[J]. RSC Advances, 2017, 7(5): 2637-2643. |
| 18 | Han X, Han X, Zhan W, et al. Preparation of 3D hierarchical porous Co3O4 nanostructures with enhanced performance in lithium-ion batteries[J]. RSC advances, 2018, 8(6): 3218-3224. |
| 19 | Huang G, Zhang F, Du X, et al. Metal organic frameworks route to in situ insertion of multiwalled carbon nanotubes in Co3O4 polyhedra as anode materials for lithium-ion batteries[J]. ACS Nano, 2015, 9(2): 1592-1599. |
| 20 | Zhang R, Zhang F, Feng J, et al. Green and facile synthesis of porous ZnCO3 as a novel anode material for advanced lithium-ion batteries[J]. Materials Letters, 2014, 118: 5-7. |
| 21 | Li T, Chen Z X, Cao Y L, et al. Transition-metal chlorides as conversion cathode materials for Li-ion batteries[J]. Electrochimica Acta, 2012, 68: 202-205. |
| 22 | Şahan H, Göktepe H, Yıldız S, et al. A novel and green synthesis of mixed phase CoO@ Co3O4@ C anode material for lithium ion batteries[J]. Ionics, 2019, 25(2): 447-455. |
| 23 | Zhao S, Wang Y, Liu R, et al. Full-molar-ratio synthesis and enhanced lithium storage properties of CoxFe1- xCO3 composites with an integrated lattice structure and an atomic-scale synergistic effect[J]. Journal of Materials Chemistry A, 2015, 3(33): 17181-17189. |
| 24 | Arkhangel'skaya Z P, Ivanova R P, Kas' yan T B, et al. Effect of electrolyte composition on the performance of electrodes in nickel-zinc batteries[J]. Russian Journal of Applied Chemistry, 2001, 74(9): 1479-1484. |
| 25 | 白晓波, 赵东江, 马松艳. 利用废旧锌锰电池制取盐和氧化锌的研究[J]. 应用化工, 2007, 36(8): 839-841. |
| Bai X B, Zhao D J, Ma S Y. Study on producing zinc salts and zinc oxide using the wasted Zn-Mn battery[J]. Applied Chemical Industry, 2007, 36(8): 839-841. | |
| 26 | Liu Q, Su X, Lei D, et al. Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping[J]. Nature Energy, 2018, 3(11): 936. |
| 27 | Yang Y, Huang G Y, Sun H, et al. Preparation and electrochemical properties of mesoporous NiCo2O4 double-hemisphere used as anode for lithium-ion battery[J]. Journal of Colloid and Interface Science, 2018, 529: 357-365. |
| 28 | Shi S, Zhang M, Deng T, et al. A facile strategy to construct binder-free flexible carbonate composite anode at low temperature with high performances for lithium-ion batteries[J]. Electrochimica Acta, 2017, 246: 1004-1015. |
| 29 | Zhang C, Xu D, Chen W, et al. Cockscomb-like Mn-doped MnxFe1- xCO3 as anode materials for a high-performance lithium-ion battery[J]. Journal of Applied Electrochemistry, 2017, 47(2): 157-166. |
| 30 | Zhang F, Zhang R, Liang G, et al. Carboxylated carbon nanotube anchored MnCO3 nanocomposites as anode materials for advanced lithium-ion batteries[J]. Materials Letters, 2013, 111:165-168. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [3] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [4] | 康飞, 吕伟光, 巨锋, 孙峙. 废锂离子电池放电路径与评价研究[J]. 化工学报, 2023, 74(9): 3903-3911. |
| [5] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [6] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [7] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [8] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [9] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [10] | 何晓崐, 刘锐, 薛园, 左然. MOCVD生长AlN单晶薄膜的气相和表面化学反应综述[J]. 化工学报, 2023, 74(7): 2800-2813. |
| [11] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [12] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [13] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [14] | 王志龙, 杨烨, 赵真真, 田涛, 赵桐, 崔亚辉. 搅拌时间和混合顺序对锂离子电池正极浆料分散特性的影响[J]. 化工学报, 2023, 74(7): 3127-3138. |
| [15] | 李彬, 徐正虎, 姜爽, 张天永. 双氧水催化氧化法清洁高效合成促进剂CBS[J]. 化工学报, 2023, 74(7): 2919-2925. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号