化工学报 ›› 2020, Vol. 71 ›› Issue (6): 2795-2803.DOI: 10.11949/0438-1157.20200205
收稿日期:2020-02-28
修回日期:2020-04-07
出版日期:2020-06-05
发布日期:2020-06-05
通讯作者:
漆虹
作者简介:汪菊(1995—),女,硕士研究生,基金资助:
Ju WANG1( ),Shufeng NIU2,Ying FEI1,Hong QI1(
),Shufeng NIU2,Ying FEI1,Hong QI1( )
)
Received:2020-02-28
Revised:2020-04-07
Online:2020-06-05
Published:2020-06-05
Contact:
Hong QI
摘要:
以平均孔径为20 nm的Al2O3管式超滤膜为载体,经多巴胺改性后,利用压力驱动沉积法成功制备出能在水溶液中长期稳定的GO/Al2O3复合纳滤膜,并通过改变负载量实现了对GO层厚的调控。结果表明,随错流时间的延长,不同GO负载量下GO/Al2O3复合纳滤膜的纯水渗透系数均呈现先降低后稳定的趋势。且随着GO负载量的增加,稳态纯水渗透系数逐渐降低;当GO负载量增加到90 mg/m2后,GO/Al2O3复合纳滤膜对一二价盐的渗透系数与截留率均无显著变化。同时,由于盐测试过程中残余的盐离子在GO片层间产生了交联作用,从而导致随着在纯水中存放时间的延长,不同GO负载量的GO/Al2O3复合纳滤膜对一二价盐的截留率均呈上升趋势。GO负载量为140 mg/m2的GO/Al2O3复合纳滤膜在水中浸泡680 h后对1 mmol/L Na2SO4的截留率可达到91.0%。GO/Al2O3复合纳滤膜对四种一二价盐的截留率满足:R(Na2SO4) > R(MgSO4) > R(NaCl) > R(MgCl2)。
中图分类号:
汪菊, 牛淑锋, 费莹, 漆虹. GO/Al2O3复合纳滤膜的制备及其稳定性能研究[J]. 化工学报, 2020, 71(6): 2795-2803.
Ju WANG, Shufeng NIU, Ying FEI, Hong QI. Fabrication and stability of GO/Al2O3 composite nanofiltration membranes[J]. CIESC Journal, 2020, 71(6): 2795-2803.
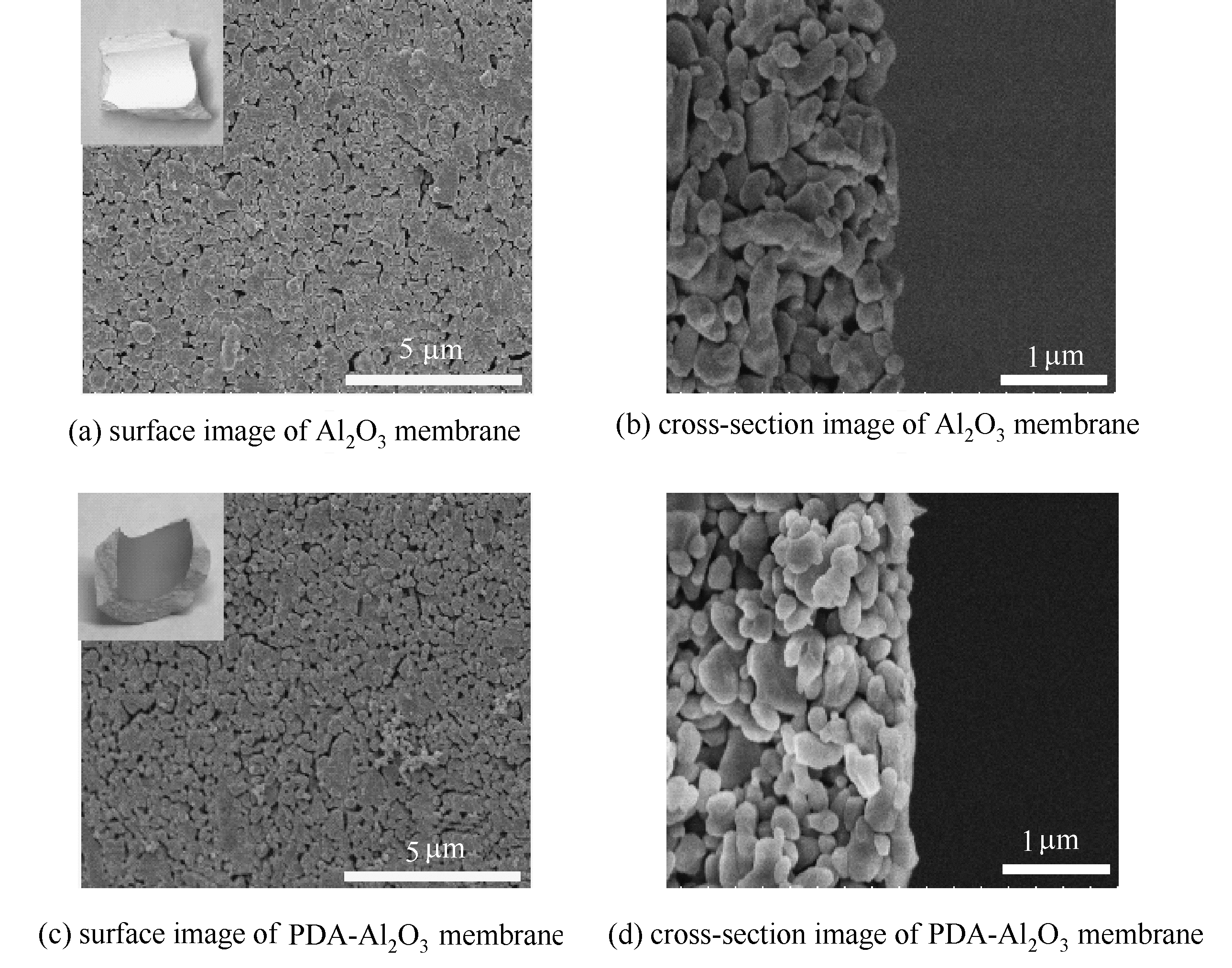
图3 Al2O3膜与PDA-Al2O3膜的SEM图(图(a)、(c)中内插图为相应膜表面的光学照片)
Fig.3 SEM images of Al2O3 membrane and PDA-Al2O3 membrane(The insets of (a) and (c) is the optical images of the corresponding membrane surface)

图5 不同GO负载量下GO/Al2O3复合纳滤膜的SEM照片[表面(a)~(d),断面(c)~(h)与水接触角(i)~(l)]
Fig.5 SEM images[surface(a)—(d),cross-section(c)—(h) and water contact angles(i)—(l)] of GO/Al2O3 composite nanofiltration membranes with different GO loading amounts
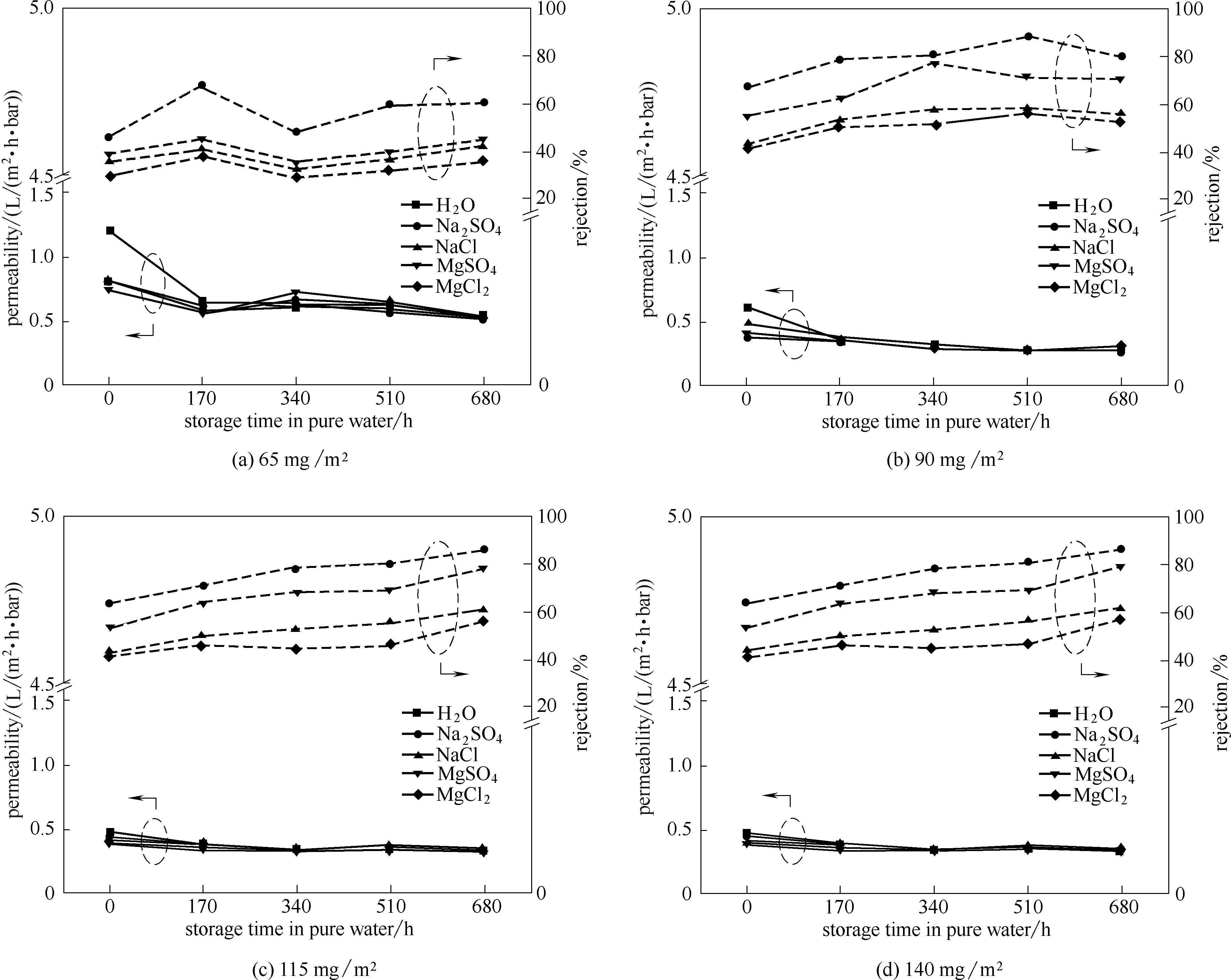
图8 GO/Al2O3复合纳滤膜对盐溶液的截留性能随浸泡时间延长的变化
Fig.8 Variation of rejection of GO/Al2O3 composite nanofiltration membranes (immersed in deionized water for different time) towards salt solutions
| 膜 | 分离体系 | 性能 | 稳定时间 |
|---|---|---|---|
| GO/Al2O3 (片式)[ | 水溶液中去除苯酚 (盲端过滤) | 对1 mmol/L苯酚水溶液的渗透率为1.153 L/(m2·h·bar),截留率为67.8% | 16 h |
| SG@GO/Al2O3 (管式)[ | 脱除水中的染料分子(EBT)(错流过滤) | 对0.2 mmol/L EBT的渗透率为33 L/(m2·h·bar),截留率为98% | 48 h |
| GO/PDA-Al2O3 (片式)[ | 海水渗透汽化 | 75℃下对含盐量3.5%(质量)的海水的渗透率为32.1 kg/(m2·h), 脱盐率为99.7% | 336 h |
| GO/Al2O3 (管式)(本文) | 单组分盐溶液截留 (错流过滤) | 对1 mmol/L Na2SO4的渗透率为0.2 L/(m2·h·bar),截留率为91.0% | 680 h |
表1 GO/Al2O3复合纳滤膜的性能及稳定性对比
Table 1 Comparison of properties including stability of GO/Al2O3 composite nanofiltration membranes
| 膜 | 分离体系 | 性能 | 稳定时间 |
|---|---|---|---|
| GO/Al2O3 (片式)[ | 水溶液中去除苯酚 (盲端过滤) | 对1 mmol/L苯酚水溶液的渗透率为1.153 L/(m2·h·bar),截留率为67.8% | 16 h |
| SG@GO/Al2O3 (管式)[ | 脱除水中的染料分子(EBT)(错流过滤) | 对0.2 mmol/L EBT的渗透率为33 L/(m2·h·bar),截留率为98% | 48 h |
| GO/PDA-Al2O3 (片式)[ | 海水渗透汽化 | 75℃下对含盐量3.5%(质量)的海水的渗透率为32.1 kg/(m2·h), 脱盐率为99.7% | 336 h |
| GO/Al2O3 (管式)(本文) | 单组分盐溶液截留 (错流过滤) | 对1 mmol/L Na2SO4的渗透率为0.2 L/(m2·h·bar),截留率为91.0% | 680 h |
| 1 | Shen H, Wang N, Ma K, et al. Tuning inter-layer spacing of graphene oxide laminates with solvent green to enhance its nanofiltration performance[J]. Journal of Membrane Science, 2017, 527: 43-50. |
| 2 | 高克, 许中煌, 洪昱斌, 等. 氧化石墨烯-陶瓷复合纳滤膜的层层自组装制备及其性能[J]. 化工学报, 2017, 68(5): 2177-2185. |
| Gao k, Xu Z H, Hong Y B, et al. Layer-by-layer self-assembly preparation and performance of GO-ceramics composite nanofiltration membrane[J]. CIESC Journal, 2017, 68(5): 2177-2185. | |
| 3 | 高以选, 叶凌碧. 膜分离技术基础[M]. 北京: 化学工业出版社, 2006: 193-195. |
| Gao Y X, Ye L B. Fundamentals of Membrane Separation Technology[M]. Beijing: Chemical Industry Press, 2006: 193-195. | |
| 4 | Chen L, Moon J H, Ma X, et al. High performance graphene oxide nanofiltration membrane prepared by electrospraying for wastewater purification[J]. Carbon, 2018, 130: 487-494. |
| 5 | 王晨颖, 汪菊, 漆虹. 多巴胺接枝的GO-Al2O3膜对Na+/Mg2+的分离性能[J]. 膜科学与技术, 2018, 38(5): 60-66+88. |
| Wang C Y, Wang J, Qi H. Separation of Na+/Mg2+ with dopamine grafted GO-Al2O3 membrane[J]. Membrane Science and Technology, 2018, 38(5): 60-66+88. | |
| 6 | 徐颜军, 徐泽海, 孟琴, 等. 新型还原氧化石墨烯/氮化碳复合纳滤膜制备及其性能[J]. 化工学报, 2019, 70(9): 3565-3572. |
| Xu Y J, Xu Z H, Meng Q, et al. Preparation and performance of novel rGO/uCN composite nanofiltration membrane[J]. CIESC Journal, 2019, 70(9): 3565-3572. | |
| 7 | Zhang Y, Zhang S, Chung T-S. Nanometric graphene oxide framework membranes with enhanced heavy metal removal via nanofiltration[J]. Environmental Science & Technology, 2015, 49(16): 10235-10242. |
| 8 | Joshi R K, Carbone P, Wang F C, et al. Precise and ultrafast molecular sieving through graphene oxide membranes[J]. Science, 2014, 343(6172): 752-754. |
| 9 | Liu G, Jin W, Xu N. Graphene-based membranes[J]. Chemical Society Reviews, 2015, 44(15): 5016-5030. |
| 10 | Han Y, Xu Z, Gao C. Ultrathin graphene nanofiltration membrane for water purification[J]. Advanced Functional Materials, 2013, 23(29): 3693-3700. |
| 11 | Huang K, Liu G, Lou Y, et al. A graphene oxide membrane with highly selective molecular separation of aqueous organic solution[J]. Angew. Chem. Int. Ed. Engl., 2014, 53(27): 6929-6932. |
| 12 | Lou Y, Liu G, Liu S, et al. A facile way to prepare ceramic-supported graphene oxide composite membrane via silane-graft modification[J]. Applied Surface Science, 2014, 307: 631-637. |
| 13 | Aba N F D, Chong J Y, Wang B, et al. Graphene oxide membranes on ceramic hollow fibers-microstructural stability and nanofiltration performance[J]. Journal of Membrane Science, 2015, 484: 87-94. |
| 14 | Li G, Shi L, Zeng G, et al. Efficient dehydration of the organic solvents through graphene oxid(GO)/ceramic composite membranes[J]. RSC Adv., 2014, 4(94): 52012-52015. |
| 15 | Zhang M, Mao Y, Liu G, et al. Molecular bridges stabilize graphene oxide membranes in water[J]. Angewandte Chemie, 2020, 59(4): 1689-1695. |
| 16 | Tsou C H, An Q F, Lo S C, et al. Effect of microstructure of graphene oxide fabricated through different self-assembly techniques on 1-butanol dehydration[J]. Journal of Membrane Science, 2015, 477: 93-100. |
| 17 | 王彩红, 孙婧, 季书馨, 等. 聚乙烯亚胺/多巴胺改性氧化硅固定碳酸酐酶[J]. 化工学报, 2019, 70(5): 233-239. |
| Wang C H, Sun J, Ji S X, et al. Immobilization of carbonic anhydrase on polyethylenimine/dopamine co-deposited SiO2[J]. CIESC Journal, 2019, 70(5): 233-239. | |
| 18 | Xu K, Feng B, Zhou C, et al. Synthesis of highly stable graphene oxide membranes on polydopamine functionalized supports for seawater desalination[J]. Chemical Engineering Science, 2016, 146: 159-165. |
| 19 | Hu X, Yu Y, Ren S, et al. Highly efficient removal of phenol from aqueous solutions using graphene oxide/Al2O3 composite membrane[J]. Journal of Porous Materials, 2017, 25(3): 719-726. |
| 20 |
Hu R, Zhao G, He Y, et al. The application feasibility of graphene oxide membranes for pressure-driven desalination in a dead-end flow system[J]. Desalination, 2020, 477. DOI: 10.1016/j.desal.2019.114271.
DOI URL |
| 21 | Tsuru T, Hironaka D, Yoshioka T, et al. Titania membranes for liquid phase separation: effect of surface charge on flux[J]. Separation & Purification Technology, 2001, 25(1/2/3): 307-314. |
| 22 | Gestel T V, Vandecasteele C, Buekenhoudt A, et al. Salt retention in nanofiltration with multilayer ceramic TiO2 membranes[J]. Journal of Membrane Science, 2002, 209(2): 379-389. |
| 23 | Voigt I, Richter H, Weyd M, et al. Treatment of oily and salty mining water by ceramic nanofiltration membranes[J]. Chemie Ingenieur Technik, 2019, 91(10): 1454-1459. |
| 24 | Zhe Y, Huang X, Wang J, et al. Novel polyethyleneimine/TMC-based nanofiltration membrane prepared on a polydopamine coated substrate[J]. Frontiers of Chemical Science and Engineering, 2018, 12(2): 273-282. |
| 25 | Pei S Z, Widjojo N, Chung T S, et al. Positively charged nanofiltration(NF) membranes via UV grafting on sulfonated polyphenylenesulfone(sPPSU) for effective removal of textile dyes from wastewater[J]. Journal of Membrane Science, 2012, 417: 52-60. |
| 26 | Ren X, Zhao C, Du S, et al. Fabrication of asymmetric poly(m-phenylene isophthalamide) nanofiltration membrane for chromium(VI) removal[J]. Journal of Environmental Sciences, 2010, (9): 35-41. |
| 27 | 孙位仕. 基于多巴胺改性的亲/疏水复合膜及其膜蒸馏性能[D]. 北京: 中国科学院大学(中国科学院过程工程研究所), 2018. |
| Sun W S. Hydrophilic/hydrophobic composite membranes based on the dopamine modification and its membrane distillation performance[D]. Beijing: University of Chinese Academy of Sciences (Institute of Process Engineering, Chinese Academy of Sciences), 2018. | |
| 28 | Wang Q, Zhao G, Li C, et al. Orderly stacked ultrathin graphene oxide membranes on a macroporous tubular ceramic substrate[J]. Journal of Membrane Science, 2019, 586: 177-184. |
| 29 | Meng N, Zhao W, Shamsaei E, et al. A low-pressure GO nanofiltration membrane crosslinked via ethylenediamine[J]. Journal of Membrane Science, 2018, 548: 363-371. |
| 30 | 马金霞. 中空纤维陶瓷-氧化石墨烯复合膜的制备及其去除水中布洛芬的性能研究[D]. 广州: 华南理工大学, 2017. |
| Ma J X. Fabrication and performance of hollow fiber ceramic-graphene-oxide composite membrane for removal of ibuprofen from water[D]. Guangzhou: South China University of Technology, 2017. | |
| 31 | 祝振鑫. 膜材料的亲水性、膜表面对水的湿润性和水接触角的关系[J]. 膜科学与技术, 2014, 34(2): 1-4. |
| Zhu Z X. Hydrophilicity,wettability and contact angle[J]. Membrane Science and Technology, 2014, 34(2): 1-4. | |
| 32 | Chong J Y, Wang B, Li K. Water transport through graphene oxide membranes: the roles of driving forces[J]. Chem. Commun(Camb), 2018, 54(20): 2554-2557. |
| 33 | Hu M, Mi B. Enabling graphene oxide nanosheets as water separation membranes[J]. Environ. Sci. Technol., 2013, 47(8): 3715-3723. |
| 34 | Zhang M, Sun J, Mao Y, et al. Effect of substrate on formation and nanofiltration performance of graphene oxide membranes[J]. Journal of Membrane Science, 2019, 574: 196-204. |
| 35 | Fornasiero F, Park H G, Holt J K, et al. Ion exclusion by sub-2-nm carbon nanotube pores[J]. Proc. Natl. Acad. Sci. USA, 2008, 105(45): 17250-17255. |
| 36 | Peeters J M M, Boom J P, Mulder M H V, et al. Retention measurements of nanofiltration membranes with electrolyte solutions[J]. Journal of Membrane Science, 1998, 145(2): 199-209. |
| 37 | Yeh C N, Raidongia K, Shao J, et al. On the origin of the stability of graphene oxide membranes in water[J]. Nature Chemistry, 2014, 7(2): 166-170. |
| 38 | Zhang M, Guan K, Shen J, et al. Nanoparticles@rGO membrane enabling highly-enhanced water permeability and structural stability with preserved selectivity[J]. AIChE Journal, 2017, 63(11): 5054-5063. |
| [1] | 江河, 袁俊飞, 王林, 邢谷雨. 均流腔结构对微细通道内相变流动特性影响的实验研究[J]. 化工学报, 2023, 74(S1): 235-244. |
| [2] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [3] | 郑玉圆, 葛志伟, 韩翔宇, 王亮, 陈海生. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192. |
| [4] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [5] | 蔡斌, 张效林, 罗倩, 党江涛, 左栗源, 刘欣梅. 导电薄膜材料的研究进展[J]. 化工学报, 2023, 74(6): 2308-2321. |
| [6] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [7] | 徐文超, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂E-1310对HCFC-141b水合物生成的影响[J]. 化工学报, 2023, 74(5): 2179-2185. |
| [8] | 吕阳光, 左培培, 杨正金, 徐铜文. 三嗪框架聚合物膜用于有机纳滤甲醇/正己烷分离[J]. 化工学报, 2023, 74(4): 1598-1606. |
| [9] | 王子健, 柯明, 李佳涵, 李舒婷, 孙巾茹, 童燕兵, 赵治平, 刘加英, 任璐. 短b轴ZSM-5分子筛制备方法及应用研究进展[J]. 化工学报, 2023, 74(4): 1457-1473. |
| [10] | 刘润竹, 储甜甜, 张孝阿, 王成忠, 张军营. α,ω-端羟基亚苯基氟硅聚合物的合成及性能[J]. 化工学报, 2023, 74(3): 1360-1369. |
| [11] | 陈向上, 马振杰, 任希华, 贾悦, 吕晓龙, 陈华艳. 三维网络萃取膜的制备及传质效率研究[J]. 化工学报, 2023, 74(3): 1126-1133. |
| [12] | 余后川, 任腾, 张宁, 姜晓滨, 代岩, 张晓鹏, 鲍军江, 贺高红. 二维氧化石墨烯膜离子选择性传递调控的研究进展[J]. 化工学报, 2023, 74(1): 303-312. |
| [13] | 杨宏欣, 李兴亚, 葛亮, 徐铜文. 含哌啶阳离子侧长链型一/二价阴离子选择性分离膜的制备[J]. 化工学报, 2022, 73(8): 3739-3748. |
| [14] | 朱江伟, 马鹏飞, 杜晓, 杨言言, 郝晓刚, 罗善霞. 基于可变价NiFe-LDH/rGO对磷酸根离子的特异性电控分离[J]. 化工学报, 2022, 73(7): 3057-3067. |
| [15] | 宋健斐, 孙立强, 解明, 魏耀东. 旋风分离器内气相旋转流不稳定性的实验研究[J]. 化工学报, 2022, 73(7): 2858-2864. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号