化工学报 ›› 2021, Vol. 72 ›› Issue (4): 2076-2085.DOI: 10.11949/0438-1157.20200895
朱慧红1( ),茆志伟2,杨涛1,冯翔2(
),茆志伟2,杨涛1,冯翔2( ),金浩1,彭冲1,3(
),金浩1,彭冲1,3( ),杨朝合2,王继锋1,方向晨1
),杨朝合2,王继锋1,方向晨1
收稿日期:2020-07-06
修回日期:2020-09-25
出版日期:2021-04-05
发布日期:2021-04-05
通讯作者:
冯翔,彭冲
作者简介:朱慧红(1978—),女,硕士, 高级工程师,基金资助:
ZHU Huihong1( ),MAO Zhiwei2,YANG Tao1,FENG Xiang2(
),MAO Zhiwei2,YANG Tao1,FENG Xiang2( ),JIN Hao1,PENG Chong1,3(
),JIN Hao1,PENG Chong1,3( ),YANG Chaohe2,WANG Jifeng1,FANG Xiangchen1
),YANG Chaohe2,WANG Jifeng1,FANG Xiangchen1
Received:2020-07-06
Revised:2020-09-25
Online:2021-04-05
Published:2021-04-05
Contact:
FENG Xiang,PENG Chong
摘要:
随着原油供应趋于劣质化和严格的环保法规出台,沸腾床渣油加氢技术引起了广泛关注。采用挤压成型法和STRONG沸腾床的特殊成型法分别制备了圆柱形和球形Ni-Mo/Al2O3催化剂,系统地研究了催化剂的颗粒形貌对活性相和渣油加氢性能的影响。采用XRD、N2物理吸脱附、H2-TPR、HRTEM、XPS和电子微探针分析等手段对催化剂进行了表征。结果表明,球形催化剂具有活性更强的Type Ⅱ类型活性位点、更优异的孔道结构性质和更好的流化性能,这使得其具有更高的渣油加氢活性。球形催化剂中的金属和载体之间相互作用较弱,这有利于形成更高硫化程度和堆垛层数的Ni-Mo-S Ⅱ型活性相,这种活性相在渣油加氢中具有更高的活性。此外,球形催化剂具有比圆柱形催化剂更大的孔径和孔体积,这有利于大分子杂质在孔道中的扩散和活性位点上的吸附,并且使得金属沉积物均匀分布在球形催化剂中,而不是集中分布在孔口。而且球形催化剂尺寸更小,可能更易于流化,这增强了催化剂的传质性能。
中图分类号:
朱慧红, 茆志伟, 杨涛, 冯翔, 金浩, 彭冲, 杨朝合, 王继锋, 方向晨. 催化剂形貌对沸腾床渣油加氢Ni-Mo/Al2O3 催化剂活性位的影响机制[J]. 化工学报, 2021, 72(4): 2076-2085.
ZHU Huihong, MAO Zhiwei, YANG Tao, FENG Xiang, JIN Hao, PENG Chong, YANG Chaohe, WANG Jifeng, FANG Xiangchen. Influence mechanism of catalyst morphology on the active sites of Ni-Mo/ Al2O3 catalyst for ebullated bed residue hydrogenation[J]. CIESC Journal, 2021, 72(4): 2076-2085.
| 催化剂 | 长度/mm | 直径/mm | 体积当量直径/mm | 比表面积/(m2·g-1) | 平均孔径/nm | 孔体积/(ml·g-1) |
|---|---|---|---|---|---|---|
| 球形 | — | 0.4~0.5 | 0.4~0.5 | 163 | 15.34 | 0.625 |
| 圆柱形 | 3~5 | 0.8 | 1.42~1.69 | 169 | 13.61 | 0.575 |
表1 球形和圆柱形Ni-Mo/Al2O3催化剂的结构性质
Table 1 Structural and textural properties of spherical and cylindrical Ni-Mo/Al2O3 catalysts
| 催化剂 | 长度/mm | 直径/mm | 体积当量直径/mm | 比表面积/(m2·g-1) | 平均孔径/nm | 孔体积/(ml·g-1) |
|---|---|---|---|---|---|---|
| 球形 | — | 0.4~0.5 | 0.4~0.5 | 163 | 15.34 | 0.625 |
| 圆柱形 | 3~5 | 0.8 | 1.42~1.69 | 169 | 13.61 | 0.575 |
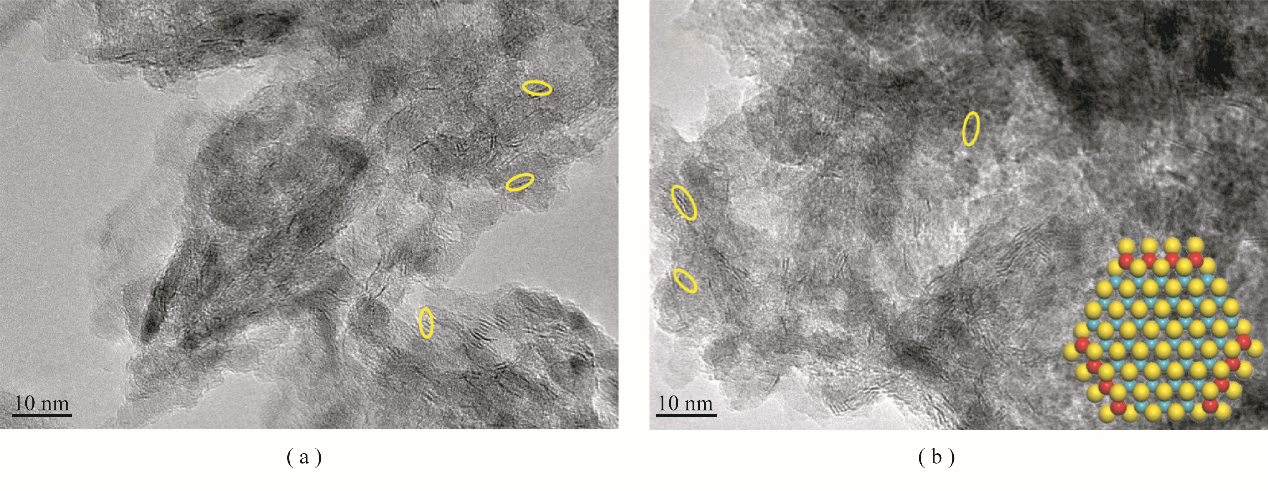
图3 圆柱形(a)和球形(b) Ni-Mo/Al2O3催化剂的HRTEM图以及Ni-Mo-S纳米簇模型结构(S:黄色;Mo:蓝色;Ni:红色)
Fig.3 HRTEM images of the cylindrical (a) and spherical (b) Ni-Mo/Al2O3 catalysts and Ni-Mo-S nanocluster model (S: yellow; Mo: blue; Ni: red)
| 催化剂 | 球形 | 圆柱形 |
|---|---|---|
| 最大颗粒长度/nm | 6.5 | 4.41 |
| 平均颗粒长度/nm | 3.67 | 2.94 |
| 平均堆垛层数 | 2.46 | 2.07 |
| 1~2层百分比/% | 55.2 | 69.8 |
| 3~4层百分比/% | 41.8 | 28.3 |
| ≥ 5层百分比/% | 3 | 1.9 |
| 分散度 | 29.8 | 36.9 |
表2 球形和圆柱形Ni-Mo/Al2O3催化剂的HRTEM分析结果
Table 2 HRTEM analysis data of spherical and cylindrical Ni-Mo/Al2O3催化剂
| 催化剂 | 球形 | 圆柱形 |
|---|---|---|
| 最大颗粒长度/nm | 6.5 | 4.41 |
| 平均颗粒长度/nm | 3.67 | 2.94 |
| 平均堆垛层数 | 2.46 | 2.07 |
| 1~2层百分比/% | 55.2 | 69.8 |
| 3~4层百分比/% | 41.8 | 28.3 |
| ≥ 5层百分比/% | 3 | 1.9 |
| 分散度 | 29.8 | 36.9 |

图6 失活的球形[(a),(c)]和圆柱形[(b),(d)]催化剂颗粒横截面上Ni、V、Fe和C的电子微探针分析和分布曲线
Fig.6 Electron probe microanalysis and distribution curves of Ni, V, Fe and C on the cross-section of spent cylindrical [(a), (b)] and spherical [(c), (d)] catalyst pellets
| 参数 | 数值 |
|---|---|
| H含量/% | 10.58 |
| C含量/% | 85.41 |
| S含量/% | 3.64 |
| Ni/V含量/(μg·g-1) | 62.94/160 |
| CCR含量/% | 20.3 |
| 密度(20℃)/(g·cm-3) | 1.0102 |
| 四组分分析 | |
| 饱和分/% | 19.63 |
| 芳香分/% | 49.53 |
| 胶质/% | 27.64 |
| 沥青质/% | 3.2 |
| >500℃收率/% | 96.1 |
表3 减压渣油的性质
Table 3 Properties of the vacuum residue
| 参数 | 数值 |
|---|---|
| H含量/% | 10.58 |
| C含量/% | 85.41 |
| S含量/% | 3.64 |
| Ni/V含量/(μg·g-1) | 62.94/160 |
| CCR含量/% | 20.3 |
| 密度(20℃)/(g·cm-3) | 1.0102 |
| 四组分分析 | |
| 饱和分/% | 19.63 |
| 芳香分/% | 49.53 |
| 胶质/% | 27.64 |
| 沥青质/% | 3.2 |
| >500℃收率/% | 96.1 |
| 28 | Sun S H, Wang G, Fang X C, et al. Development of catalyst for STRONG ebullated-bed residue hydrotreating process[J]. Petroleum Refinery Engineering, 2011, 41(12): 26-30. |
| 29 | Peng C, Guo R, Feng X, et al. Tailoring the structure of Co-Mo/mesoporous γ-Al2O3 catalysts by adding multi-hydroxyl compound: a 3000 kt/a industrial-scale diesel ultra-deep hydrodesulfurization study[J]. Chemical Engineering Journal, 2019, 377: 119706. |
| 30 | Li G C, Lu X L, Tang Z, et al. Preparation of NiMo/γ-Al2O3 catalysts with large pore size for vacuum residue hydrotreatment[J]. Materials Research Bulletin, 2013, 48(11): 4526-4530. |
| 31 | Lai W K, Pang L Q, Zheng J B, et al. Efficient one pot synthesis of mesoporous NiMo-Al2O3 catalysts for dibenzothiophene hydrodesulfurization[J]. Fuel Processing Technology, 2013, 110: 8-16. |
| 32 | Zaikovskii V I, Plyasova L M, Burmistrov V A, et al. Sulphide catalysts on silica as a support(Ⅱ):High resolution electron microscopy data[J]. Applied Catalysis, 1984, 11(1): 15-27. |
| 33 | Li Y P, Zhang T T, Liu D P, et al. Study of the promotion effect of citric acid on the active NiMoS phase in NiMo/Al2O3 catalysts[J]. Industrial & Engineering Chemistry Research, 2019, 58(37): 17195-17206. |
| 34 | Hensen E J M, Kooyman P J, van der Meer Y, et al. The relation between morphology and hydrotreating activity for supported MoS2 particles[J]. Journal of Catalysis, 2001, 199(2): 224-235. |
| 35 | Topsøe H. The role of Co-Mo-S type structures in hydrotreating catalysts[J]. Applied Catalysis A: General, 2007, 322: 3-8. |
| 36 | Kobayashi K, Nagai M. Active sites of sulfided NiMo/Al2O3 catalysts for 4, 6-dimethyldibenzothiophene hydrodesulfurization-effects of Ni and Mo components, sulfidation, citric acid and phosphate addition[J]. Catalysis Today, 2017, 292: 74-83. |
| 37 | Li H F, Li M F, Nie H. Tailoring the surface characteristic of alumina for preparation of highly active NiMo/Al2O3 hydrodesulfurization catalyst[J]. Microporous and Mesoporous Materials, 2014, 188: 30-36. |
| 38 | López Cordero R, Gil Llambias F J, López Agudo A. Temperature-programmed reduction and zeta potential studies of the structure of Mo/O3Al2O3 and Mo/O3SiO2 catalysts effect of the impregnation pH and molybdenum loading[J]. Applied Catalysis, 1991, 74(1): 125-136. |
| 39 | Scheffer B, Arnoldy P, Moulijn J A. Sulfidability and hydrodesulfurization activity of Mo catalysts supported on alumina, silica, and carbon[J]. Journal of Catalysis, 1988, 112(2): 516-527. |
| 1 | BP. BP Statistical Review of World Energy 2019[EB/OL]. [2020-05-06]. . |
| 2 | Umana B, Zhang N, Smith R. Development of vacuum residue hydrodesulphurization-hydrocracking models and their integration with refinery hydrogen networks[J]. Industrial & Engineering Chemistry Research, 2016, 55(8): 2391-2406. |
| 3 | Kim S H, Kim K D, Lee Y K. Effects of dispersed MoS2 catalysts and reaction conditions on slurry phase hydrocracking of vacuum residue[J]. Journal of Catalysis, 2017, 347: 127-137. |
| 4 | Marafi A, Albazzaz H, Rana M S. Hydroprocessing of heavy residual oil: opportunities and challenges[J]. Catalysis Today, 2019, 329: 125-134. |
| 5 | Kim K D, Lee Y K. Active phase of dispersed MoS2 catalysts for slurry phase hydrocracking of vacuum residue[J]. Journal of Catalysis, 2019, 369: 111-121. |
| 6 | Danial-Fortain P, Gauthier T, Merdrignac I, et al. Reactivity study of Athabasca vacuum residue in hydroconversion conditions[J]. Catalysis Today, 2010, 150(3/4): 255-263. |
| 7 | Castañeda L C, Muñoz J A D, Ancheyta J. Current situation of emerging technologies for upgrading of heavy oils[J]. Catalysis Today, 2014, 220/221/222: 248-273. |
| 8 | Jeong H R, Lee Y K. Comparison of unsupported WS2 and MoS2 catalysts for slurry phase hydrocracking of vacuum residue[J]. Applied Catalysis A: General, 2019, 572: 90-96. |
| 9 | Marques J, Guillaume D, Merdrignac I, et al. Effect of catalysts acidity on residues hydrotreatment[J]. Applied Catalysis B: Environmental, 2011, 101(3/4): 727-737. |
| 10 | Kohli K, Prajapati R, Maity S K, et al. Accelerated pre-coking of NiMo/γ-Al2O3 catalyst: effect on the hydroprocessing activity of vacuum residue[J]. Fuel, 2019, 235: 437-447. |
| 11 | Liu B, Zhao K D, Chai Y M, et al. Slurry phase hydrocracking of vacuum residue in the presence of presulfided oil-soluble MoS2 catalyst[J]. Fuel, 2019, 246: 133-140. |
| 12 | Speight J G. The Desulfurization of Heavy Oils and Residua[M]. CRC Press, 1999. |
| 13 | Kim S H, Kim K D, Lee D, et al. Structure and activity of dispersed Co, Ni, or Mo sulfides for slurry phase hydrocracking of vacuum residue[J]. Journal of Catalysis, 2018, 364: 131-140. |
| 14 | Lee H S, Nguyen-Huy C, Pham T T, et al. ZrO2-impregnated red mud as a novel catalyst for steam catalytic cracking of vacuum residue[J]. Fuel, 2016, 165: 462-467. |
| 15 | Díaz-Boffelli G, Ancheyta J, Muñoz J A D, et al. Experimental study and economic analysis of heavy oil partial upgrading by solvent deasphalting-hydrotreating[J]. Energy & Fuels, 2018, 32(1): 55-59. |
| 16 | Che Y J, Hao J H, Zhang J H, et al. Vacuum residue thermal cracking: product yield determination and characterization using thermogravimetry-Fourier transform infrared spectrometry and a fluidized bed reactor[J]. Energy & Fuels, 2018, 32(2): 1348-1357. |
| 17 | Kaminski T, Husein M M. Thermal cracking of atmospheric residue versus vacuum residue[J]. Fuel Processing Technology, 2018, 181: 331-339. |
| 18 | Liu J K, Fang X C, Yang T. Novel ebullated bed residue hydrocracking process[J]. Energy & Fuels, 2017, 31(6): 6568-6579. |
| 19 | Galiasso Tailleur R, Caprioli L. Catalyst pore plugging effects on hydrocracking reactions in an Ebullated bed reactor operation[J]. Catalysis Today, 2005, 109(1/2/3/4): 185-194. |
| 20 | Morel F, Kressmann S, Harlé V, et al. Processes and catalysts for hydrocracking of heavy oil and residues[J]. Studies in Surface Science and Catalysis, 1997, 106: 1-16. |
| 21 | Sahu R, Song B J, Jeon Y P, et al. Upgrading of vacuum residue in batch type reactor using Ni-Mo supported on goethite catalyst[J]. Journal of Industrial and Engineering Chemistry, 2016, 35: 115-122. |
| 22 | Lauritsen J V, Kibsgaard J, Olesen G H, et al. Location and coordination of promoter atoms in Co- and Ni-promoted MoS2-based hydrotreating catalysts[J]. Journal of Catalysis, 2007, 249(2): 220-233. |
| 23 | Eijsbouts S, van den Oetelaar L C A, van Puijenbroek R R. MoS2 morphology and promoter segregation in commercial Type 2 Ni-Mo/Al2O3 and Co-Mo/Al2O3 hydroprocessing catalysts[J]. Journal of Catalysis, 2005, 229(2): 352-364. |
| 24 | Al-Dalama K, Stanislaus A. A comparative study of the influence of chelating agents on the hydrodesulfurization (HDS) activity of alumina and silica-alumina-supported CoMo catalysts[J]. Energy & Fuels, 2006, 20(5): 1777-1783. |
| 25 | Pashigreva A V, Klimov O V, Bukhtiyarova G A, et al. The superior activity of the CoMo hydrotreating catalysts, prepared using citric acid: what's the reason?[J]. Studies in Surface Science and Catalysis, 2010, 175: 109-116. |
| 26 | Escobar J, Barrera M C, Gutiérrez A W, et al. Benzothiophene hydrodesulfurization over NiMo/alumina catalysts modified by citric acid. Effect of addition stage of organic modifier[J]. Fuel Processing Technology, 2017, 156: 33-42. |
| 27 | Ramírez J, Castillo-Villalón P, Gutiérrez-Alejandre A, et al. Interaction of different molecules with the hydrogenation and desulfurization sites of NiMoS supported particles with different morphology[J]. Catalysis Today, 2020, 353: 99-111. |
| 28 | 孙素华, 王刚, 方向晨, 等. STRONG沸腾床渣油加氢催化剂研究及工业放大[J]. 炼油技术与工程, 2011, 41(12): 26-30. |
| 40 | Fan Y, Xiao H, Shi G, et al. Citric acid-assisted hydrothermal method for preparing NiW/USY-Al2O3 ultradeep hydrodesulfurization catalysts[J]. Journal of Catalysis, 2011, 279(1): 27-35. |
| 41 | Lai W K, Song W J, Pang L Q, et al. The effect of starch addition on combustion synthesis of NiMo-Al2O3 catalysts for hydrodesulfurization[J]. Journal of Catalysis, 2013, 303: 80-91. |
| 42 | Gray M R. Upgrading Petroleum Residues and Heavy Oils[M]. CRC Press,1994 |
| 43 | Zhu H H, Mao Z W, Liu B, et al. Regulating catalyst morphology to boost the stability of Ni-Mo/Al2O3 catalyst for ebullated-bed residue hydrotreating[J]. Green Energy & Environment, 2020,In Press. |
| 44 | Mitra-Kirtley S, Mullins O C, Ralston C Y, et al. Sulfur characterization in asphaltene, resin, and oil fractions of two crude oils[J]. ACS Division of Fuel Chemistry, Preprints, 1999, 44(4): 763-767. |
| 45 | Ancheyta J, Rana M S, Furimsky E. Hydroprocessing of heavy petroleum feeds: tutorial[J]. Catalysis Today, 2005, 109(1/2/3/4): 3-15. |
| 46 | Vradman L, Landau M V. Structure-function relations in supported Ni-W sulfide hydrogenation catalysts[J]. Catalysis Letters, 2001, 77(1/2/3): 47-54. |
| 47 | Rana M, Ancheyta J, Ramírez J. Characteristics of heavy oil hydroprocessing catalysts[M]//Hydroprocessing of Heavy Oils and Residua. CRC Press, 2007: 121-190. |
| [1] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [2] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 曹跃, 余冲, 李智, 杨明磊. 工业数据驱动的加氢裂化装置多工况切换过渡状态检测[J]. 化工学报, 2023, 74(9): 3841-3854. |
| [5] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [6] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [7] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [8] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [9] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [10] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [11] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [12] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [13] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [14] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [15] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号