化工学报 ›› 2021, Vol. 72 ›› Issue (9): 4759-4767.DOI: 10.11949/0438-1157.20210026
李媛1,2( ),张飞飞1,2,王丽1,2,杨江峰1,2(
),张飞飞1,2,王丽1,2,杨江峰1,2( ),李立博1,2,李晋平1,2
),李立博1,2,李晋平1,2
收稿日期:2021-01-08
修回日期:2021-03-01
出版日期:2021-09-05
发布日期:2021-09-05
通讯作者:
杨江峰
作者简介:李媛(1994—),女,硕士研究生,基金资助:
Yuan LI1,2( ),Feifei ZHANG1,2,Li WANG1,2,Jiangfeng YANG1,2(
),Feifei ZHANG1,2,Li WANG1,2,Jiangfeng YANG1,2( ),Libo LI1,2,Jinping LI1,2
),Libo LI1,2,Jinping LI1,2
Received:2021-01-08
Revised:2021-03-01
Online:2021-09-05
Published:2021-09-05
Contact:
Jiangfeng YANG
摘要:
氧化亚氮(N2O)是仅次于CO2和CH4的第三大温室气体,对其捕集具有资源回收和减排温室气体的双重价值。本文通过添加氢氟酸和盐酸合成了末端具有不同阴离子的MIL-101Cr材料:MIL-101(Cr)-F和MIL-101(Cr)-Cl,通过XRD、BET、SEM等对样品进行了表征,测试并分析了两种样品对N2O和N2的吸附性能,进行了选择性和吸附热的计算以及混合气体的穿透模拟。研究结果表明,MIL-101(Cr)-Cl拥有目前最高的N2O吸附容量(6.43 mmol/g,298 K)和N2O/N2选择性(267),混合气体(N2O/N2=0.1%/99.9%)穿透模拟结果显示MIL-101(Cr)-Cl具有更加优异的微量N2O捕获能力。
中图分类号:
李媛, 张飞飞, 王丽, 杨江峰, 李立博, 李晋平. MIL-101Cr-F/Cl用于N2O的捕集研究[J]. 化工学报, 2021, 72(9): 4759-4767.
Yuan LI, Feifei ZHANG, Li WANG, Jiangfeng YANG, Libo LI, Jinping LI. Study on the capture of N2O by MIL-101Cr-F/Cl[J]. CIESC Journal, 2021, 72(9): 4759-4767.
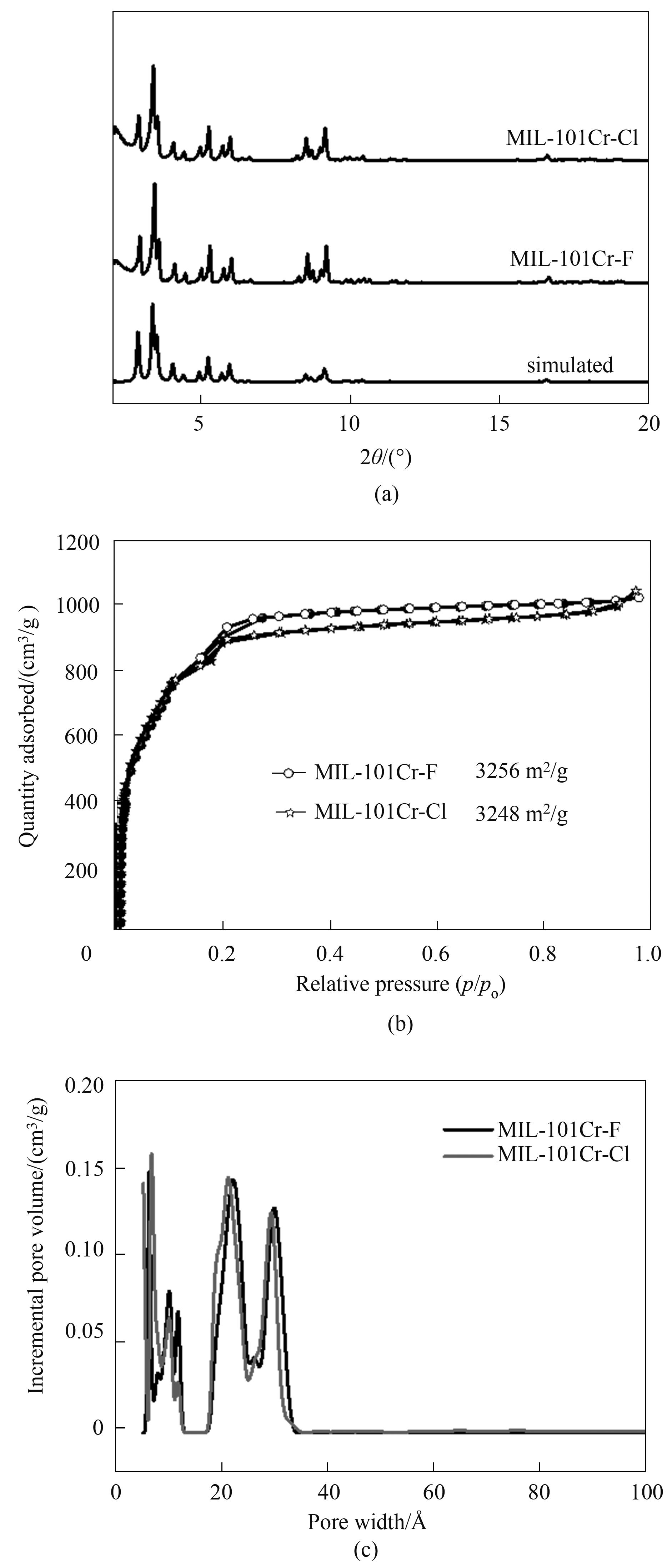
图2 MIL-101(Cr)-F/Cl的XRD谱图(a);77 K下的N2吸脱附等温线(b)和孔径分布 (c)
Fig.2 XRD pattern (a); N2 adsorption-desorption isotherms(b) and pore size distribution (c) at 77 K of MIL-101(Cr)-F/Cl
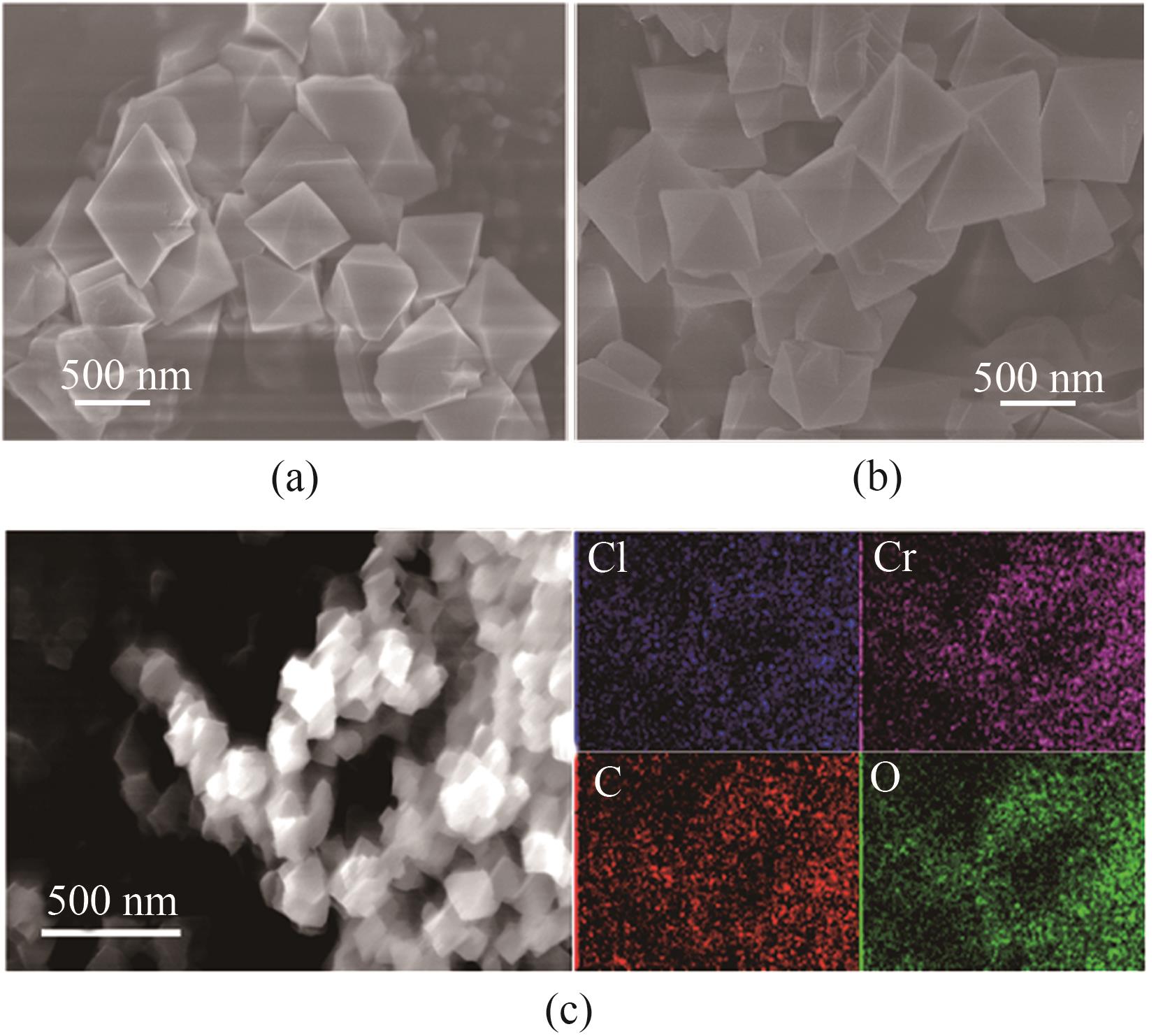
图3 MIL-101(Cr)-F/Cl的SEM图[(a)、(b)]和MIL-101(Cr)-Cl的EDX元素分布(c)
Fig.3 SEM images [(a),(b)] of MIL-101(Cr)-F/Cl and EDX mapping images (c) of MIL-101(Cr)-Cl
| 吸附剂 | N2O吸附量/(mmol/g) | 温度/K | 文献 |
|---|---|---|---|
| Silicalite-1 | 1.75 | 298 | [ |
| Zeolite-5A | 4.10 | 298 | [ |
| MOF-5 | 0.90 | 298 | [ |
| ZIF-7 | 2.50 | 298 | [ |
| Ni-MOF | 2.81 | 298 | [ |
| MIL-100Cr | 5.78 | 298 | [ |
| ED-MIL-100Cr | 2.14 | 298 | [ |
| MIL-101Cr-F | 3.26 | 298 | 本文 |
| MIL-101Cr-Cl | 6.43 | 298 | 本文 |
表1 298 K、1 bar下N2O在多孔材料上的吸附容量
Table 1 N2O adsorption capacity on several porous materials at 298 K, 1 bar
| 吸附剂 | N2O吸附量/(mmol/g) | 温度/K | 文献 |
|---|---|---|---|
| Silicalite-1 | 1.75 | 298 | [ |
| Zeolite-5A | 4.10 | 298 | [ |
| MOF-5 | 0.90 | 298 | [ |
| ZIF-7 | 2.50 | 298 | [ |
| Ni-MOF | 2.81 | 298 | [ |
| MIL-100Cr | 5.78 | 298 | [ |
| ED-MIL-100Cr | 2.14 | 298 | [ |
| MIL-101Cr-F | 3.26 | 298 | 本文 |
| MIL-101Cr-Cl | 6.43 | 298 | 本文 |
| 参数 | 数值 | |
|---|---|---|
| 孔隙率 | 0.53 | |
| 堆积密度/(kg/m3) | 233 | |
| 吸附剂半径/m | 1×10-5 | |
| 床层空隙率 | 0.2 | |
| 床层高度/m | 0.16 | |
| 床层半径/m | 4×10-3 | |
| 传质系数(N2O) | 0.02 | |
| 传质系数(N2) | 0.03 |
表2 吸附剂及吸附床参数设置
Table 2 Parameter setting of adsorbent and adsorption bed
| 参数 | 数值 | |
|---|---|---|
| 孔隙率 | 0.53 | |
| 堆积密度/(kg/m3) | 233 | |
| 吸附剂半径/m | 1×10-5 | |
| 床层空隙率 | 0.2 | |
| 床层高度/m | 0.16 | |
| 床层半径/m | 4×10-3 | |
| 传质系数(N2O) | 0.02 | |
| 传质系数(N2) | 0.03 |
| 1 | Lashof D A, Ahuja D R. Relative contributions of greenhouse gas emissions to global warming[J]. Nature, 1990, 344(6266): 529-531. |
| 2 | Wuebbles D J. Nitrous oxide: no laughing matter[J]. Science, 2009, 326(5949): 56-57. |
| 3 | Ravishankara A R, Daniel J S, Portmann R W. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century[J]. Science, 2009, 326(5949): 123-125. |
| 4 | Xiao D J, Bloch E D, Mason J A, et al. Oxidation of ethane to ethanol by N2 O in a metal-organic framework with coordinatively unsaturated iron(Ⅱ) sites[J]. Nature Chemistry, 2014, 6(7): 590-595. |
| 5 | Kay S. Synthetic chemistry with nitrous oxide[J]. Chemical Society Reviews, 2015, 44(17): 6375-6386. |
| 6 | Tian H, Xu R, Canadell J G, et al. A comprehensive quantification of global nitrous oxide sources and sinks[J]. Nature, 2020, 586(7828): 248-256. |
| 7 | Konsolakis M. Recent advances on nitrous oxide (N2O) decomposition over non-noble-metal oxide catalysts: catalytic performance, mechanistic considerations, and surface chemistry aspects[J]. ACS Catalysis, 2015, 5(11): 6397-6421. |
| 8 | Hamilton S M, Hopkins W S, Harding D J, et al. Infrared induced reactivity on the surface of isolated size-selected clusters: dissociation of N2O on rhodium clusters[J]. Journal of the American Chemical Society, 2010, 132(5): 1448-1449. |
| 9 | Jabłońska M, Palkovits R. It is no laughing matter: nitrous oxide formation in diesel engines and advances in its abatement over rhodium-based catalysts[J]. Catalysis Science & Technology, 2016, 6(21): 7671-7687. |
| 10 | Jurado A, Borges A V, Brouyère S. Dynamics and emissions of N2O in groundwater: a review[J]. Science of the Total Environment, 2017, 584/585: 207-218. |
| 11 | Wang L, Zhang F F, Wang C, et al. Ethylenediamine-functionalized metal organic frameworks MIL-100(Cr) for efficient CO2/N2O separation[J]. Separation and Purification Technology, 2020, 235: 116219. |
| 12 | Chen D L, Wang N W, Wang F F, et al. Utilizing the gate-opening mechanism in ZIF-7 for adsorption discrimination between N2O and CO2[J]. The Journal of Physical Chemistry C, 2014, 118(31): 17831-17837. |
| 13 | 赵铎, 陈聚良, 史红军, 等. 一种己二酸尾气中氮氧化物的脱除装置: 206587600U[P]. 2017-10-27. |
| Zhao D, Chen J L, Shi H J, et al. A device and method for removing nitrogen oxides in adipic acid tail gases: 206587600U[P]. 2017-10-27. | |
| 14 | Tsai M L, Hadt R G, Vanelderen P, et al. [Cu2O]2+ active site formation in Cu-ZSM-5: geometric and electronic structure requirements for N2O activation[J]. Journal of the American Chemical Society, 2014, 136(9): 3522-3529. |
| 15 | Zhang F M, Chen X, Zhuang J, et al. Direct oxidation of benzene to phenol by N2O over meso-Fe-ZSM-5 catalysts obtained via alkaline post-treatment[J]. Catalysis Science & Technology, 2011, 1(7): 1250-1255. |
| 16 | Zeng R, Feller M, Diskin-Posner Y, et al. CO oxidation by N2O homogeneously catalyzed by ruthenium hydride pincer complexes indicating a new mechanism[J]. Journal of the American Chemical Society, 2018, 140(23): 7061-7064. |
| 17 | Tolman W. Binding and activation of N2O at transition-metal centers: recent mechanistic insights[J]. Angewandte Chemie International Edition, 2010, 49(6): 1018-1024. |
| 18 | Park K S, Ni Z, Côté A P, et al. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(27): 10186-10191. |
| 19 | Hartmann N J, Wu G, Hayton T W. Synthesis and reactivity of a nickel(Ⅱ) thioperoxide complex: demonstration of sulfide-mediated N2O reduction[J]. Chemical Science, 2018, 9(31): 6580-6588. |
| 20 | Perez-Ramirez J, Santiago M. Metal-substituted hexaaluminates for high-temperature N2O abatement[J]. Chemical Communications, 2007, 38(6): 619-621. |
| 21 | 周丽, 丁剑木. 空分装置中有害气体的危害与控制[J]. 冶金动力, 2020, 39(5): 30-33. |
| Zhou L, Ding J M. The harms and control of harmful gases in air separation unit[J]. Metallurgical Power, 2020, 39(5): 30-33. | |
| 22 | Harvey M J, Sperlich P, Clough T J, et al. Global research alliance N2O chamber methodology guidelines: recommendations for air sample collection, storage, and analysis[J]. Journal of Environmental Quality, 2020, 49(5): 1110-1125. |
| 23 | Groen J C, Pérez-Ramírez J, Zhu W D. Adsorption of nitrous oxide on silicalite-1[J]. Journal of Chemical & Engineering Data, 2002, 47(3): 587-589. |
| 24 | Saha D, Bao Z B, Jia F, et al. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A[J]. Environmental Science & Technology, 2010, 44(5): 1820-1826. |
| 25 | Li L B, Lin R B, Krishna R, et al. Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites[J]. Science, 2018, 362(6413): 443-446. |
| 26 | Lin R B, Xiang S C, Xing H B, et al. Exploration of porous metal-organic frameworks for gas separation and purification[J]. Coordination Chemistry Reviews, 2019, 378: 87-103. |
| 27 | Li J R, Kuppler R J, Zhou H C. Selective gas adsorption and separation in metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1477. |
| 28 | 杨江峰, 欧阳坤, 张倬铭, 等. 脱氧剂Fe-MOF-74的制备及氧气吸附失活规律[J]. 化工学报, 2015, 66(8): 3262-3267. |
| Yang J F, Ouyang K, Zhang Z M, et al. Preparation and oxygen adsorption of deoxidation agent Fe-MOF-74 and its deactivation[J]. CIESC Journal, 2015, 66(8): 3262-3267. | |
| 29 | Jiang J, Furukawa H, Zhang Y B, et al. High methane storage working capacity in metal-organic frameworks with acrylate links[J]. Journal of the American Chemical Society, 2016, 138(32): 10244-10251. |
| 30 | 张倬铭, 杨江峰, 陈杨, 等. 一维直孔道MOFs对CH4/N2和CO2/CH4的分离[J]. 化工学报, 2015, 66(9): 3549-3555. |
| Zhang Z M, Yang J F, Chen Y, et al. Separation of CH4/N2 and CO2/CH4 mixtures in one dimension channel MOFs[J]. CIESC Journal, 2015, 66(9): 3549-3555. | |
| 31 | Yang J F, Wang Y, Li L B, et al. Protection of open-metal V(Ⅲ) sites and their associated CO2/CH4/N2/O2/H2O adsorption properties in mesoporous V-MOFs[J]. Journal of Colloid and Interface Science, 2015, 456: 197-205. |
| 32 | Kloutse F A, Gauthier W, Hourri A, et al. Study of competitive adsorption of the N2O-CO2-CH4-N2 quaternary mixture on CuBTC[J]. Separation and Purification Technology, 2020, 235: 116211. |
| 33 | Saha D, Deng S G. Adsorption equilibrium, kinetics, and enthalpy of N2O on zeolite 4A and 13X[J]. Journal of Chemical & Engineering Data, 2010, 55(9): 3312-3317. |
| 34 | Zhang X P, Chen W J, Shi W, et al. Highly selective sorption of CO2 and N2O and strong gas-framework interactions in a nickel(ⅱ) organic material[J]. Journal of Materials Chemistry A, 2016, 4(41): 16198-16204. |
| 35 | Yang J F, Du B J, Liu J Q, et al. MIL-100Cr with open Cr sites for a record N2O capture[J]. Chemical Communications, 2018, 54(100): 14061-14064. |
| 36 | Hamon L, Serre C, Devic T, et al. Comparative study of hydrogen sulfide adsorption in the MIL-53(Al, Cr, Fe), MIL-47(V), MIL-100(Cr), and MIL-101(Cr) metal-organic frameworks at room temperature[J]. Journal of the American Chemical Society, 2009, 131(25): 8775-8777. |
| 37 | Férey G, Mellot-Draznieks C, Serre C, et al. A chromium terephthalate-based solid with unusually large pore volumes and surface area[J]. Science, 2005, 309(5743): 2040-2042. |
| 38 | Malouche A, Blanita G, Dan L P, et al. Hydrogen absorption in 1 nm Pd clusters confined in MIL-101(Cr)[J]. Journal of Materials Chemistry A, 2017, 5(44): 23043-23052. |
| 39 | Myers A L, Prausnitz J M. Thermodynamics of mixed-gas adsorption[J]. AIChE Journal, 1965, 11(1): 121-127. |
| 40 | Kumar K V, Gadipelli S, Wood B, et al. Characterization of the adsorption site energies and heterogeneous surfaces of porous materials[J]. Journal of Materials Chemistry A, 2019, 7(17): 10104-10137. |
| 41 | Yang J F, Bai H H, Shang H, et al. Experimental and simulation study on efficient CH4/N2 separation by pressure swing adsorption on silicalite-1 pellets[J]. Chemical Engineering Journal, 2020, 388: 124222. |
| 42 | Xu M, Deng S G. Efficient screening of novel adsorbents for coalbed methane recovery[J]. Journal of Colloid and Interface Science, 2020, 565: 131-141. |
| 43 | Berdonosova E A, Kovalenko K A, Polyakova E V, et al. Influence of anion composition on gas sorption features of Cr-MIL-101 metal-organic framework[J]. The Journal of Physical Chemistry C, 2015, 119(23): 13098-13104. |
| 44 | Zhao M, Yuan K, Wang Y, et al. Metal-organic frameworks as selectivity regulators for hydrogenation reactions[J]. Nature, 2016, 539(7627): 76-80. |
| 45 | Yoon J W, Chang H, Lee S J, et al. Selective nitrogen capture by porous hybrid materials containing accessible transition metal ion sites[J]. Nature Materials, 2017, 16(5): 526-531. |
| 46 | 尚华, 白洪灏, 刘佳奇, 等. CH4-N2在自支撑颗粒型Silicalite-1上的吸附分离及PSA模拟[J]. 化工学报, 2020, 71(5): 2088-2098. |
| Shang H, Bai H H, Liu J Q, et al. PSA simulation and adsorption separation of CH4-N2 by self-supporting pellets Silicalite-1[J]. CIESC Journal, 2020, 71(5): 2088-2098. |
| [1] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [2] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [3] | 彭晓婉, 郭笑楠, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8浆液法分离CH4/N2的双吸收-吸附塔工艺流程建模与模拟[J]. 化工学报, 2023, 74(2): 784-795. |
| [4] | 孟金琳, 汪宇, 张群锋, 叶光华, 周兴贵. 介孔材料低温氮气吸脱附的孔道网络模型[J]. 化工学报, 2023, 74(2): 893-903. |
| [5] | 韩昌亮, 辛镜青, 于广滨, 刘俊秀, 许麒澳, 姚安卡, 尹鹏. 微通道内超临界氮气三维热流场实验与数值模拟[J]. 化工学报, 2022, 73(2): 653-662. |
| [6] | 付凤艳, 邢广恩. 碱性燃料电池用阴离子交换膜的研究进展[J]. 化工学报, 2021, 72(S1): 42-52. |
| [7] | 戴琼斌, 刘宏斌, 夏启斌, 周欣, 李忠. 一种新的颗粒炭材料的制备及其高效分离甲烷氮气性能[J]. 化工学报, 2021, 72(8): 4196-4203. |
| [8] | 刘璇, 马溢昌, 张秋根, 刘庆林. 富勒烯交联季铵化聚苯醚阴离子交换膜的制备[J]. 化工学报, 2021, 72(7): 3849-3855. |
| [9] | 韩笑,陈雨亭,苏宝根,鲍宗必,张治国,杨亦文,任其龙,杨启炜. 己烷异构体吸附分离材料研究进展[J]. 化工学报, 2021, 72(7): 3445-3465. |
| [10] | 巩有奎, 李美玲, 孙洪伟. 不同NO3-浓度An/A/O-SBR系统PAOs-GAOs竞争及N2O释放特性[J]. 化工学报, 2021, 72(3): 1675-1683. |
| [11] | 刘元伟, 董晨初, 廖俊斌, 王超, 陈权, 沈江南. 不同侧链BPPO阴离子交换膜的制备及其抗污染性能[J]. 化工学报, 2021, 72(3): 1732-1741. |
| [12] | 李建惠, 兰天昊, 陈杨, 杨江峰, 李立博, 李晋平. MOF复合材料在气体吸附分离中的研究进展[J]. 化工学报, 2021, 72(1): 167-179. |
| [13] | 陈润道, 郑芳, 郭立东, 杨启炜, 张治国, 杨亦文, 任其龙, 鲍宗必. 稀有气体Xe/Kr吸附分离研究进展[J]. 化工学报, 2021, 72(1): 14-26. |
| [14] | 郑沐云, 万宇驰, 吕瑞涛. 电催化氮气还原合成氨催化材料研究进展[J]. 化工学报, 2020, 71(6): 2481-2491. |
| [15] | 尚华, 白洪灏, 刘佳奇, 杨江峰, 李晋平. CH4-N2在自支撑颗粒型Silicalite-1上的吸附分离及PSA模拟[J]. 化工学报, 2020, 71(5): 2088-2098. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号