化工学报 ›› 2021, Vol. 72 ›› Issue (10): 5319-5329.DOI: 10.11949/0438-1157.20210378
收稿日期:2021-03-15
修回日期:2021-05-18
出版日期:2021-10-05
发布日期:2021-10-05
通讯作者:
修志龙
作者简介:周惠(1994—),女,硕士研究生,
Hui ZHOU1( ),Zhifeng TIAN2,Xiaowei TANG2,Zhilong XIU1(
),Zhifeng TIAN2,Xiaowei TANG2,Zhilong XIU1( )
)
Received:2021-03-15
Revised:2021-05-18
Online:2021-10-05
Published:2021-10-05
Contact:
Zhilong XIU
摘要:
巴氏芽孢八叠球菌(Sporosarcina pasteurii)产生的脲酶可水解尿素,生成的CO2与氯化钙反应获得碳酸钙晶体,不同形式的脲酶溶液对碳酸钙晶型有显著影响。用发酵液上清液中的脲酶催化,可以获得由纳米级球霰石自组装成的介孔空心微米纯球霰石;用菌体中的脲酶催化可获得100%的方解石。红外光谱分析表明,细胞破碎后的粗脲酶溶液获得的椭圆形碳酸钙受到富含羟基物质的影响。进一步探究了脲酶活性和反应物浓度对球霰石的影响,并发现获得的纯球霰石在7 d内非常稳定,有望作为药物载体使用。
中图分类号:
周惠,田志锋,唐小微,修志龙. 脲酶驱动不同晶型碳酸钙微纳米颗粒的制备[J]. 化工学报, 2021, 72(10): 5319-5329.
Hui ZHOU,Zhifeng TIAN,Xiaowei TANG,Zhilong XIU. Urease-driven preparation of calcium carbonate micro-nanoparticles with different polymorphs[J]. CIESC Journal, 2021, 72(10): 5319-5329.
| 脲酶溶液 | 脲酶活性/(U/L) | 蛋白质含量/(g/L) | 比活性/(U/g) |
|---|---|---|---|
| fermentation broth | 3.12 ± 0.02 | 0.77 ± 0.03 | 4.06 ± 0.12 |
| supernatant | 1.24 ± 0.01 | 0.29 ± 0.04 | 4.28 ± 0.12 |
| bacterial cell | 3.75 ± 0.16 | 0.62 ± 0.02 | 6.05 ± 0.01 |
| crude enzyme solution | 5.75 ± 0.08 | 0.87 ± 0.03 | 6.63 ± 0.21 |
表1 不同溶液的脲酶活性测定结果
Table 1 The results of urease activity in different solutions
| 脲酶溶液 | 脲酶活性/(U/L) | 蛋白质含量/(g/L) | 比活性/(U/g) |
|---|---|---|---|
| fermentation broth | 3.12 ± 0.02 | 0.77 ± 0.03 | 4.06 ± 0.12 |
| supernatant | 1.24 ± 0.01 | 0.29 ± 0.04 | 4.28 ± 0.12 |
| bacterial cell | 3.75 ± 0.16 | 0.62 ± 0.02 | 6.05 ± 0.01 |
| crude enzyme solution | 5.75 ± 0.08 | 0.87 ± 0.03 | 6.63 ± 0.21 |
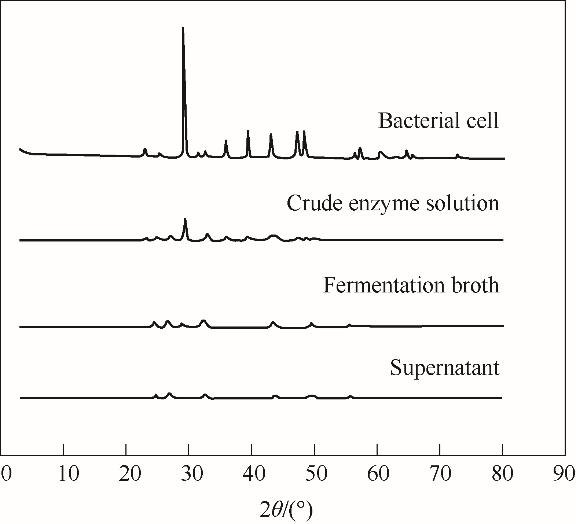
图3 不同脲酶溶液催化获得CaCO3沉淀的XRD图像(Bacterial cells / Crude enzyme solution / Fermentation broth / Supernatant 分别代表菌体/粗酶液/发酵液/上清液
Fig.3 XRD images of CaCO3 precipitation obtained by urease in different solutions
| 脲酶溶液 | 球霰石 | 方解石 |
|---|---|---|
| 发酵液 | 88.10% | 11.90% |
| 上清液 | 100.00% | 0 |
| 菌体 | 0 | 100.00% |
| 粗酶液 | 53.26% | 46.74% |
表2 不同脲酶溶液中球霰石和方解石的占比
Table 2 Proportion of vaterite and calcite by urease in different solutions
| 脲酶溶液 | 球霰石 | 方解石 |
|---|---|---|
| 发酵液 | 88.10% | 11.90% |
| 上清液 | 100.00% | 0 |
| 菌体 | 0 | 100.00% |
| 粗酶液 | 53.26% | 46.74% |
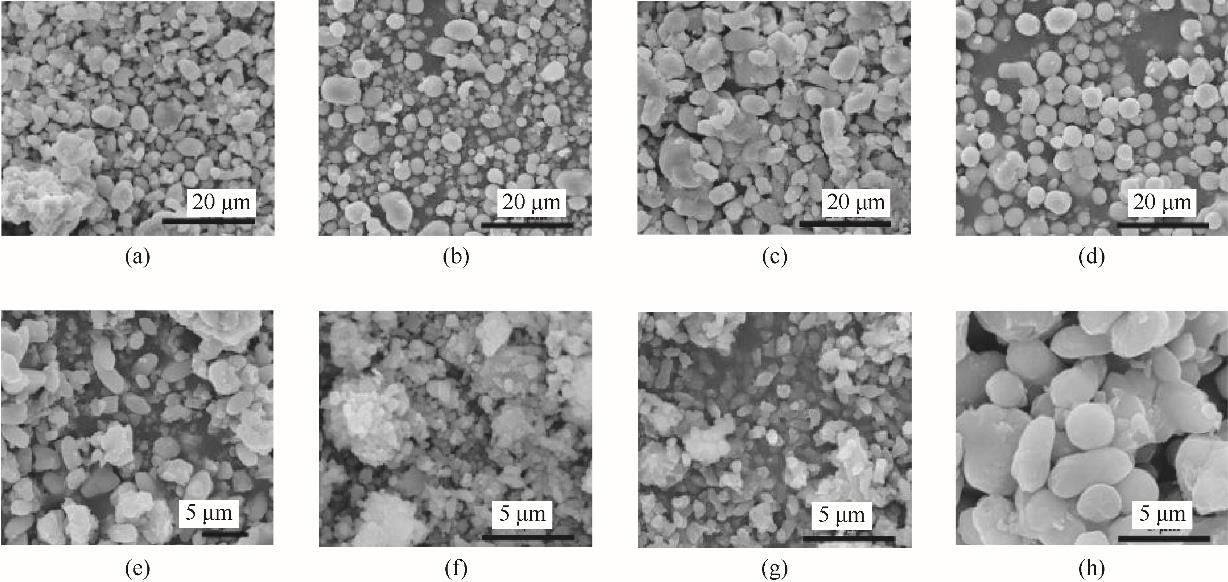
图6 脲酶活性对球霰石的影响[(a)~(d)的反应时间<1 min; (e)~(h)的反应时间是30 min]urease activity/(mmol/(L·min)): (a),(e) 2; (b),(f) 4; (c),(g) 8; (d),(h)16
Fig.6 Effect of urease activity on vaterite
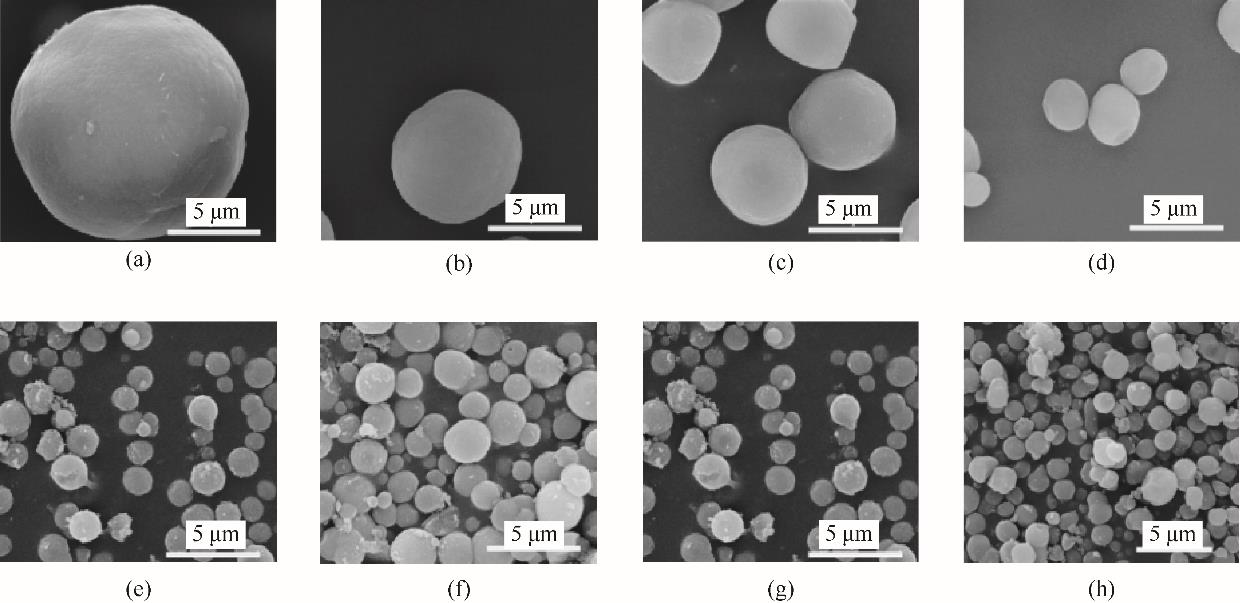
图7 反应物浓度对球霰石的影响[(a)~(d)的反应时间<1 min; (e)~(h)的反应时间是30 min]reactant concentration: (a),(e) 0.05 mol/L; (b),(f) 0.1 mol/L; (c),(g) 0.3 mol/L; (d),(h) 0.5 mol/L
Fig.7 Effect of reactant concentration on vaterite
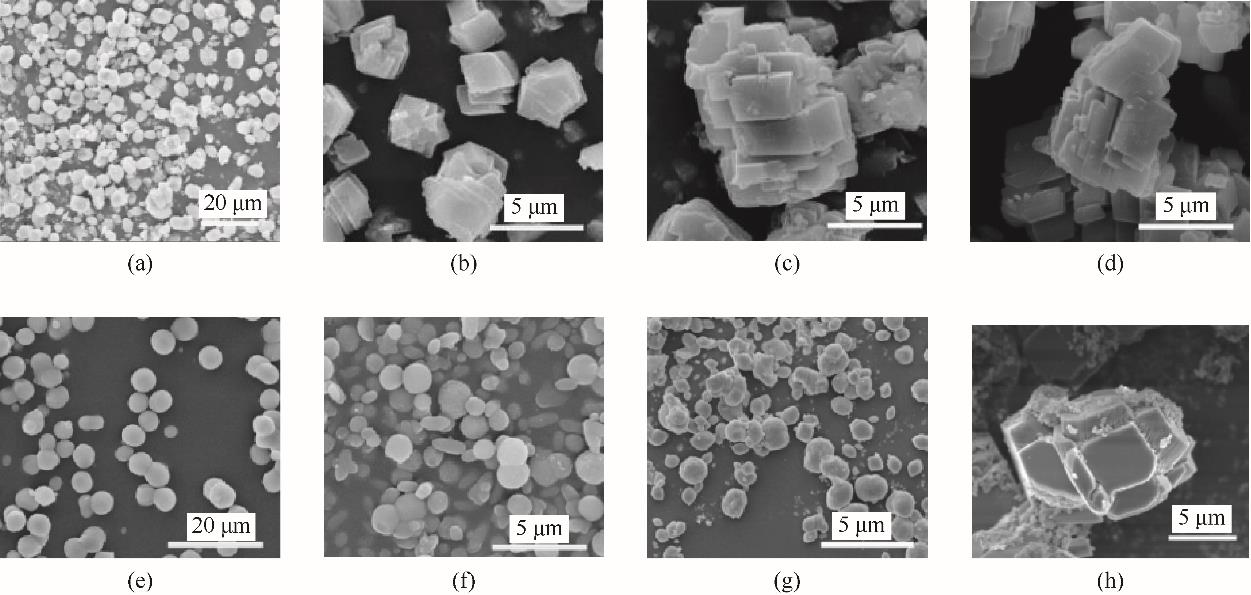
图9 方解石和球霰石在不同时间的SEM图(a)~(d)分别是对照组在<1 min、30 min、7 d、14 d时的晶体形态;(e)~(h)分别是纯球霰石组在<1 min、30 min、7 d、14 d时的晶体形态
Fig.9 SEM of calcite and vaterite at different time(a)—(d) were the crystal morphology of the control group at <1 min, 30 min, 7 d and 14 d, respectively; (e)—(h) were the crystal morphology of the pure vaterite group at <1 min, 30 min, 7 d and 14 d, respectively
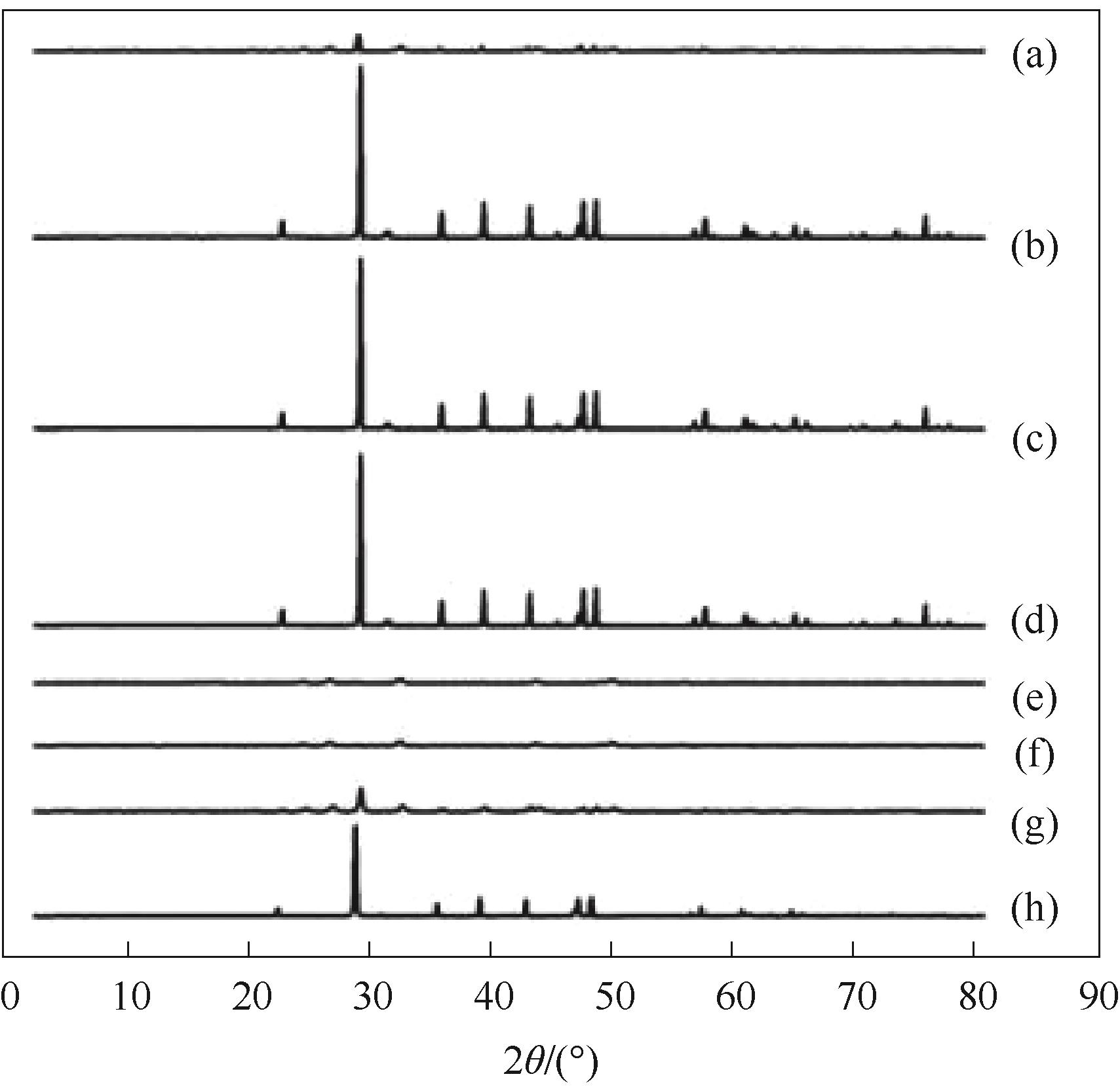
图10 对照组和纯球霰石组在不同时间的XRD谱图(a)对照组,<1 min;(b)对照组,30 min;(c)对照组,7 d;(d)对照组,14 d;(e)球霰石组,<1 min;(f)球霰石组,30 min;(g)球霰石组,7 d;(h)球霰石组,14 d
Fig.10 XRD of control group and pure vaterite group at different time(a) control group, < 1 min; (b) control group, 30 min; (c) control group, 7 d; (d) control group, 14 d; (e) vaterite group, < 1 min; (f) vaterite group, 30 min; (g) vaterite group, 7 d; (h) vaterite group, 14 d
| 晶体类型 | <1 min | 30 min | 7 d | 14 d | |
|---|---|---|---|---|---|
| 对照组 | 方解石 | 96.81% | 100% | 100% | 100% |
| 球霰石 | 3.19% | 0 | 0 | 0 | |
| 纯球霰石组 | 方解石 | 0 | 0 | 29.21% | 100% |
| 球霰石 | 100% | 100% | 70.78% | 0 | |
表3 对照组和纯球霰石组在不同时间球霰石和方解石的占比
Table 3 Proportion of vaterite and calcite in control group and pure vaterite group at different time
| 晶体类型 | <1 min | 30 min | 7 d | 14 d | |
|---|---|---|---|---|---|
| 对照组 | 方解石 | 96.81% | 100% | 100% | 100% |
| 球霰石 | 3.19% | 0 | 0 | 0 | |
| 纯球霰石组 | 方解石 | 0 | 0 | 29.21% | 100% |
| 球霰石 | 100% | 100% | 70.78% | 0 | |
| 1 | Patil V J, Patil U D, Kulkarni R D, et al. Synthesis of nano CaCO3/acrylic co-polymer latex composites for interior decorative paints[J]. Polymer Composites, 2018, 39(4): 1350-1360. |
| 2 | Al-Attar F, Al-Samhan M. Nano CaCO3 incorporation with polypropylene to reduce film water vapor permeability for packaging application[J]. Asian Journal of Scientific Research, 2020, 13(4): 275-283. |
| 3 | Wang H M, Chen Y Z, Zhang Z J. Enhanced ink-absorption performance of inkjet printing paper-based patterns with core-shell-structure CaCO3@SiO2 pigments[J]. Nordic Pulp & Paper Research Journal, 2019, 34(4): 525-533. |
| 4 | Morsy F A, El-Sheikh S M, Barhoum A. Nano-silica and SiO2/CaCO3 nanocomposite prepared from semi-burned rice straw ash as modified papermaking fillers[J]. Arabian Journal of Chemistry, 2019, 12(7): 1186-1196. |
| 5 | 王训遒, 蒋登高. 纳米碳酸钙复合丙烯酸涂料的流变性[J]. 化工学报, 2007, 58(1): 238-247. |
| Wang X Q, Jiang D G. Rheological property of nanometer calcium carbonate composite acrylic coatings[J]. Journal of Chemical Industry and Engineering (China), 2007, 58(1): 238-247. | |
| 6 | Boyjoo Y, Pareek V K, Liu J. Synthesis of micro and nano-sized calcium carbonate particles and their applications[J]. Journal of Materials Chemistry A, 2014, 2(35): 14270-14288. |
| 7 | Gong L F, Qian S H, Wang W, et al. Influence of nano-additives (nano-PTFE and nano-CaCO3) on tribological properties of food-grade aluminum-based grease[J]. Tribology International, 2021, 160: 107014. |
| 8 | Maleki Dizaj S, Sharifi S, Ahmadian E, et al. An update on calcium carbonate nanoparticles as cancer drug/gene delivery system[J]. Expert Opinion on Drug Delivery, 2019, 16(4): 331-345. |
| 9 | Trofimov A, Ivanova A, Zyuzin M, et al. Porous inorganic carriers based on silica, calcium carbonate and calcium phosphate for controlled/modulated drug delivery: fresh outlook and future perspectives[J]. Pharmaceutics, 2018, 10(4): 167. |
| 10 | Wang S, Ni D Z, Yue H, et al. Exploration of antigen induced CaCO3 nanoparticles for therapeutic vaccine[J]. Small, 2018, 14(14): 1704272. |
| 11 | Batool A, Valiyaveettil S. Coprecipitation—an efficient method for removal of polymer nanoparticles from water[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(35): 13481-13487. |
| 12 | Zhang X D, Shi D Y, Li X, et al. Nanoscale dispersing of zero-valent iron on CaCO3 and their significant synergistic effect in high performance removal of lead[J]. Chemosphere, 2019, 224: 390-397. |
| 13 | Fathy M, Zayed M A, Moustafa Y M. Synthesis and applications of CaCO3/HPC core-shell composite subject to heavy metals adsorption processes[J]. Heliyon, 2019, 5(8): e02215. |
| 14 | Akiyama M, Kawasaki S. Biogeochemical simulation of microbially induced calcite precipitation with Pararhodobacter sp. strain SO1[J]. Acta Geotechnica, 2019, 14(3): 685-696. |
| 15 | Wen K J, Li Y, Amini F, et al. Impact of bacteria and urease concentration on precipitation kinetics and crystal morphology of calcium carbonate[J]. Acta Geotechnica, 2020, 15(1): 17-27. |
| 16 | Zheng T W. Bacteria-induced facile biotic calcium carbonate precipitation[J]. Journal of Crystal Growth, 2021, 563: 126096. |
| 17 | Trushina D B, Bukreeva T V, Antipina M N. Size-controlled synthesis of vaterite calcium carbonate by the mixing method: aiming for nanosized particles[J]. Crystal Growth & Design, 2016, 16(3): 1311-1319. |
| 18 | Oral Ç M, Ercan B. Influence of pH on morphology, size and polymorph of room temperature synthesized calcium carbonate particles[J]. Powder Technology, 2018, 339: 781-788. |
| 19 | Wei W, Ma G H, Hu G, et al. Preparation of hierarchical hollow CaCO3 particles and the application as anticancer drug carrier[J]. Journal of the American Chemical Society, 2008, 130(47): 15808-15810. |
| 20 | Polat S. Experimental investigations on the effects of asparagine and serine on the polymorphism of calcium carbonate[J]. Advanced Powder Technology, 2020, 31(10): 4282-4291. |
| 21 | 张一江, 柳鑫华, 陈智慧, 等. L-半胱氨酸改性聚环氧琥珀酸的合成及其阻垢缓蚀性能[J]. 化工学报, 2016, 67(10): 4344-4355. |
| Zhang Y J, Liu X H, Chen Z H, et al. Synthesis of L-cysteine modified polyepoxysuccinic acid and evaluation of its inhibition on scale deposition and corrosion[J]. CIESC Journal, 2016, 67(10): 4344-4355. | |
| 22 | Liu X, Li K X, Wu C Q, et al. Egg white-assisted preparation of inorganic functional materials: a sustainable, eco-friendly, low-cost and multifunctional method[J]. Ceramics International, 2019, 45(18): 23869-23889. |
| 23 | Yang D, Yan Y, Yang X, et al. A basic protein, N25, from a mollusk modifies calcium carbonate morphology and shell biomineralization[J]. Journal of Biological Chemistry, 2019, 294(21): 8371-8383. |
| 24 | Wei Y, Xu H, Xu S M, et al. Synthesis and characterization of calcium carbonate on three kinds of microbial cells templates[J]. Journal of Crystal Growth, 2020, 547: 125755. |
| 25 | Singh A, Singh S K. The impact of various drug ferrying additives on phase transitions behavior of calcite, vaterite and aragonite[J]. Phase Transitions, 2019, 92(11): 990-1008. |
| 26 | Bastrzyk A, Fiedot-Toboła M, Polowczyk I, et al. Effect of a lipopeptide biosurfactant on the precipitation of calcium carbonate[J]. Colloids and Surfaces B: Biointerfaces, 2019, 174: 145-152. |
| 27 | Chaparro-Acuña S P, Becerra-Jiménez M L, Martínez-Zambrano J J, et al. Soil bacteria that precipitate calcium carbonate: mechanism and applications of the process[J]. Acta Agronómica, 2018, 67(2): 277-288. |
| 28 | Seifan M, Berenjian A. Application of microbially induced calcium carbonate precipitation in designing bio self-healing concrete[J]. World Journal of Microbiology and Biotechnology, 2018, 34(11): 1-15. |
| 29 | Ortega-Villamagua E, Gudiño-Gomezjurado M, Palma-Cando A. Microbiologically induced carbonate precipitation in the restoration and conservation of cultural heritage materials[J]. Molecules, 2020, 25(23): 5499. |
| 30 | Ghosh T, Bhaduri S, Montemagno C, et al. Sporosarcina pasteurii can form nanoscale calcium carbonate crystals on cell surface[J]. PLoS One, 2019, 14(1): e0210339. |
| 31 | Zhang W C, Ju Y, Zong Y W, et al. In situ real-time study on dynamics of microbially induced calcium carbonate precipitation at a single-cell level[J]. Environmental Science & Technology, 2018, 52(16): 9266-9276. |
| 32 | Zehner J, Røyne A, Wentzel A, et al. Microbial-induced calcium carbonate precipitation: an experimental toolbox for in situ and real-time investigation of micro-scale pH evolution[J]. RSC Advances, 2020, 10(35): 20485-20493. |
| 33 | Zehner J, Røyne A, Sikorski P. Calcite seed-assisted microbial induced carbonate precipitation(MICP)[J]. PLoS One, 2021, 16(2): e0240763. |
| 34 | Gorospe C M, Han S H, Kim S G, et al. Effects of different calcium salts on calcium carbonate crystal formation by Sporosarcina pasteurii KCTC 3558[J]. Biotechnology and Bioprocess Engineering, 2013, 18(5): 903-908. |
| 35 | Whiffin V S. Microbial CaCO3 precipitation for the production of biocement[D]. Western Australia: Murdoch University, 2004. |
| 36 | Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding[J]. Analytical Biochemistry, 1976, 72(1/2): 248-254. |
| 37 | Seepma S Y M H, Ruiz-Hernandez S E, Nehrke G, et al. Controlling CaCO3 particle size with {Ca2+}: {CO32-} ratios in aqueous environments[J]. Crystal Growth & Design, 2021, 21(3): 1576-1590. |
| 38 | Tobler D J, Cuthbert M O, Greswell R B, et al. Comparison of rates of ureolysis between Sporosarcina pasteurii and an indigenous groundwater community under conditions required to precipitate large volumes of calcite[J]. Geochimica et Cosmochimica Acta, 2011, 75(11): 3290-3301. |
| 39 | Aizenberg J, Black A J, Whitesides G M. Oriented growth of calcite controlled by self-assembled monolayers of functionalized alkanethiols supported on gold and silver[J]. Journal of the American Chemical Society, 1999, 121(18): 4500-4509. |
| 40 | Deng H, Wang S, Wang X, et al. Two competitive nucleation mechanisms of calcium carbonate biomineralization in response to surface functionality in low calcium ion concentration solution[J]. Regenerative Biomaterials, 2015, 2(3): 187-195. |
| 41 | Fang P A, Conway J F, Margolis H C, et al. Hierarchical self-assembly of amelogenin and the regulation of biomineralization at the nanoscale[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(34): 14097-14102. |
| 42 | Meldrum F C, Cölfen H. Controlling mineral morphologies and structures in biological and synthetic systems[J]. Chemical Reviews, 2008, 108(11): 4332-4432. |
| 43 | Massi M, Ogden M I, Jones F. Investigating vaterite phase stabilisation by a tetrazole molecule during calcium carbonate crystallisation[J]. Journal of Crystal Growth, 2012, 351(1): 107-114. |
| 44 | 李云钊, 宋兴福, 孙玉柱, 等. 反应-萃取-结晶过程制备碳酸钙的晶型转变与结晶机理[J]. 化工学报, 2015, 66(10): 4007-4015. |
| Li Y Z, Song X F, Sun Y Z, et al. Polymorph transformation and formation mechanism of calcium carbonate during reactive extraction-crystallization process[J]. CIESC Journal, 2015, 66(10): 4007-4015. | |
| 45 | Sergeeva A, Sergeev R, Lengert E, et al. Composite magnetite and protein containing CaCO3 crystals. external manipulation and vaterite→calcite recrystallization-mediated release performance[J]. ACS Applied Materials & Interfaces, 2015, 7(38): 21315-21325. |
| 46 | Fang Z, He W, Tu T, et al. An efficient and green pathway for continuous Friedel-Crafts acylation over α-Fe2O3 and CaCO3 nanoparticles prepared in the microreactors[J]. Chemical Engineering Journal, 2018, 331: 443-449. |
| [1] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [2] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [3] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [4] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [5] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [6] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [7] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [8] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [9] | 段重达, 姚小伟, 朱家华, 孙静, 胡南, 李广悦. 环境因素对克雷白氏杆菌诱导碳酸钙沉淀的影响[J]. 化工学报, 2023, 74(8): 3543-3553. |
| [10] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [11] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [12] | 仪显亨, 周骛, 蔡小舒, 蔡天意. 光纤后向动态光散射测量纳米颗粒的浓度适用范围研究[J]. 化工学报, 2023, 74(8): 3320-3328. |
| [13] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [14] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [15] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号