化工学报 ›› 2021, Vol. 72 ›› Issue (9): 4698-4707.DOI: 10.11949/0438-1157.20210060
收稿日期:2021-01-11
修回日期:2021-05-25
出版日期:2021-09-05
发布日期:2021-09-05
通讯作者:
樊星
作者简介:李泽严(1995—),男,硕士研究生,基金资助:Received:2021-01-11
Revised:2021-05-25
Online:2021-09-05
Published:2021-09-05
Contact:
Xing FAN
摘要:
尿素-选择性催化还原技术低温下运行时尿素分解不彻底,易形成缩二脲、三聚氰酸和三聚氰胺等副产物。本研究将TiO2催化剂与介质阻挡放电等离子体相结合,在程序升温条件下考察了载气中有无O2时引入等离子体前后TiO2催化尿素分解副产物水解的性能。结果表明:TiO2表面缩二脲、三聚氰酸和三聚氰胺分别在43~261℃、217~300℃和199~300℃水解生成NH3和CO2,载气中有无O2对催化水解过程几乎无影响。引入等离子体后缩二脲、三聚氰酸和三聚氰胺水解所需温度显著降低,载气中无O2时引入等离子体NH3产率变化不大,副产物仅有少量N2O和NO,有O2时NH3产率显著降低,且生成较多N2O、NO、NO2及少量NH4NO2和NH4NO3。未来需从优化放电条件和催化剂组成等方面解决引入等离子体导致副产物形成等问题。
中图分类号:
李泽严, 樊星, 李坚. 非热等离子体强化TiO2催化尿素分解副产物水解性能的研究[J]. 化工学报, 2021, 72(9): 4698-4707.
Zeyan LI, Xing FAN, Jian LI. Non-thermal plasma enhanced hydrolysis of urea decomposition by-products over TiO2[J]. CIESC Journal, 2021, 72(9): 4698-4707.

图1 实验装置示意图1—气瓶;2—减压阀;3—质量流量控制器;4—鼓泡塔(水);5—温度控制仪;6—接地极;7—管式炉;8—DBD反应器;9—催化剂;10—交流高压电源;11—冷凝塔;12—FT-IR光谱仪;13—NOx分析仪
Fig.1 Schematic diagram of the experimental set-up

图2 N2+H2O和5%O2+N2+H2O气氛下催化和等离子体强化催化缩二脲水解的曲线(平均放电功率5.6 W;放电时间段:0~85 min)
Fig.2 Hydrolysis curves of biuret by catalysis and plasma-enhanced catalysis in N2+H2O and 5%O2+N2+H2O (average discharge power: 5.6 W; discharge period: 0—85 min)
| 平均放电功率/W | 载气气氛 | CO2开始出现的温度/℃ | CO2达到峰值浓度时的温度/℃ | CO2开始消失的温度/℃ | NH3产率/% | CO2产率/% |
|---|---|---|---|---|---|---|
| 0 | N2+H2O | 43 | 94/161/200 | 261 | 82.1 | 71.0 |
| 0 | 5%O2+N2+H2O | 47 | 77/165/202 | 255 | 79.7 | 62.4 |
| 5.6 | N2+H2O | 36 | 61/136/176 | 221 | 70.3 | 59.8 |
| 5.6 | 5%O2+N2+H2O | 33 | 54/142/183 | 223 | 47.4 | 56.3 |
表1 催化和等离子体强化催化作用下缩二脲水解的结果
Table 1 Results of biuret hydrolysis by catalysis and plasma-enhanced catalysis
| 平均放电功率/W | 载气气氛 | CO2开始出现的温度/℃ | CO2达到峰值浓度时的温度/℃ | CO2开始消失的温度/℃ | NH3产率/% | CO2产率/% |
|---|---|---|---|---|---|---|
| 0 | N2+H2O | 43 | 94/161/200 | 261 | 82.1 | 71.0 |
| 0 | 5%O2+N2+H2O | 47 | 77/165/202 | 255 | 79.7 | 62.4 |
| 5.6 | N2+H2O | 36 | 61/136/176 | 221 | 70.3 | 59.8 |
| 5.6 | 5%O2+N2+H2O | 33 | 54/142/183 | 223 | 47.4 | 56.3 |
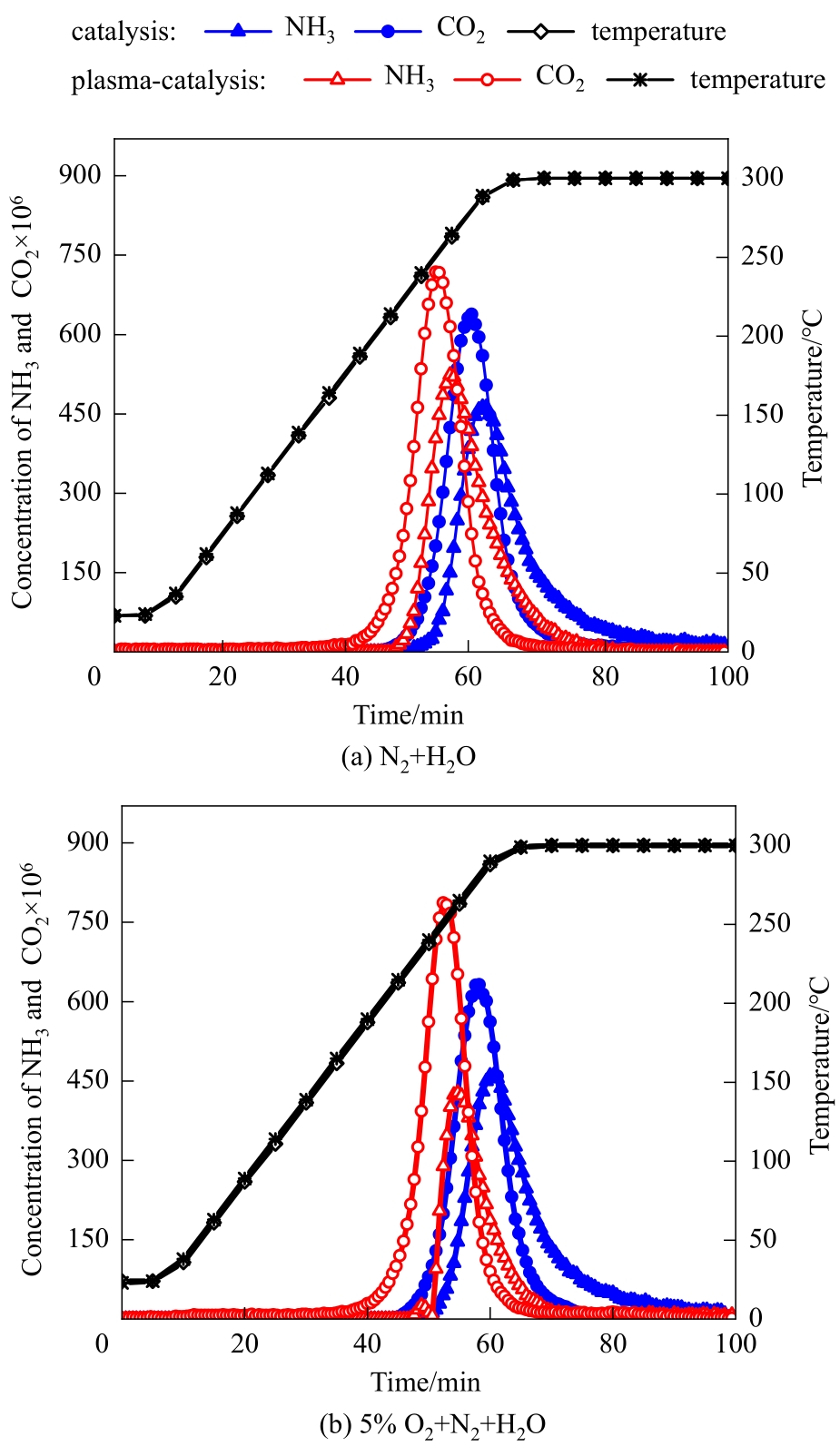
图3 N2+H2O和5%O2+N2+H2O气氛下催化和等离子体强化催化三聚氰酸水解的曲线(平均放电功率:无O2时6.2 W,有O2时5.9 W;放电时间段:0~85 min)
Fig.3 Hydrolysis curves of cyanuric acid by catalysis and plasma-enhanced catalysis in N2+H2O and 5%O2+N2+H2O (average discharge power: 6.2 W and 5.9 W without and with O2, respectively; discharge period: 0—85 min)
| 平均放电功率/W | 载气气氛 | CO2开始出现的 温度/℃ | CO2达到峰值浓度时的温度/℃ | CO2开始消失的 温度/℃ | NH3产率/% | CO2产率/% |
|---|---|---|---|---|---|---|
| 0 | N2+H2O | 217 | 282 | 300 | 54.5 | 57.3 |
| 0 | 5%O2+N2+H2O | 218 | 282 | 300 | 54.8 | 57.3 |
| 6.2 | N2+H2O | 162 | 253 | 300 | 52.6 | 64.0 |
| 5.9 | 5%O2+N2+H2O | 160 | 253 | 300 | 34.1 | 66.8 |
表2 催化和等离子体强化催化作用下三聚氰酸水解的结果
Table 2 Results of cyanuric acid hydrolysis by catalysis and plasma-enhanced catalysis
| 平均放电功率/W | 载气气氛 | CO2开始出现的 温度/℃ | CO2达到峰值浓度时的温度/℃ | CO2开始消失的 温度/℃ | NH3产率/% | CO2产率/% |
|---|---|---|---|---|---|---|
| 0 | N2+H2O | 217 | 282 | 300 | 54.5 | 57.3 |
| 0 | 5%O2+N2+H2O | 218 | 282 | 300 | 54.8 | 57.3 |
| 6.2 | N2+H2O | 162 | 253 | 300 | 52.6 | 64.0 |
| 5.9 | 5%O2+N2+H2O | 160 | 253 | 300 | 34.1 | 66.8 |
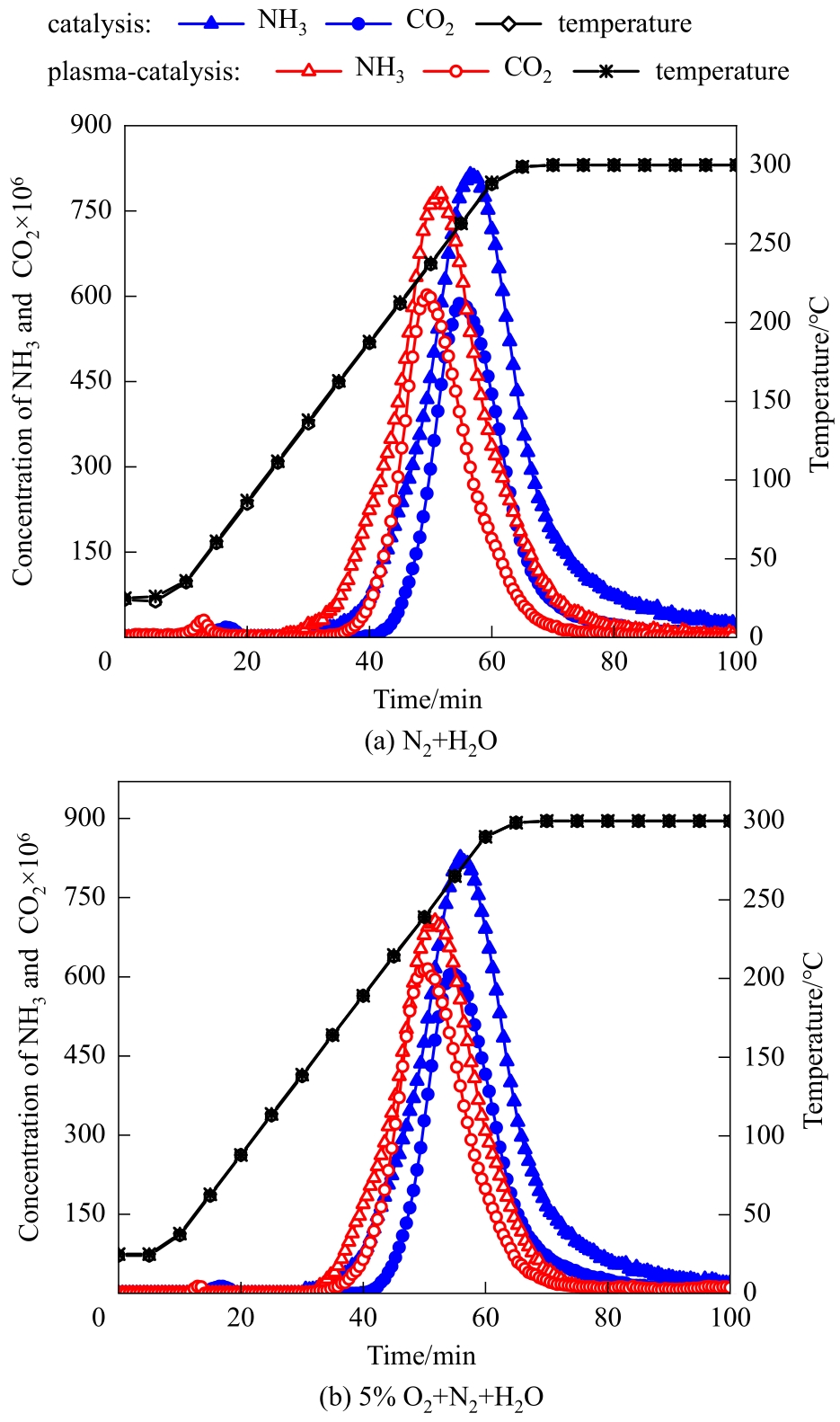
图4 N2+H2O和5%O2+N2+H2O气氛下催化和等离子体强化催化三聚氰胺水解的曲线(平均放电功率:无O2时5.5 W,有O2时5.3 W;放电时间段:0~85 min)
Fig.4 Hydrolysis curves of melamine by catalysis and plasma-enhanced catalysis in N2+H2O and 5%O2+N2+H2O (average discharge power: 5.5 W and 5.3 W without and with O2, respectively; discharge period: 0—85 min)
| 平均放电功率/W | 载气气氛 | CO2开始出现的 温度/℃ | CO2达到峰值浓度时的温度/℃ | CO2开始消失的 温度/℃ | NH3产率/% | CO2产率/% |
|---|---|---|---|---|---|---|
| 0 | N2+H2O | 199 | 263 | 300 | 68.1 | 73.0 |
| 0 | 5%O2+N2+H2O | 201 | 262 | 300 | 66.7 | 74.9 |
| 5.5 | N2+H2O | 171 | 235 | 300 | 60.7 | 71.5 |
| 5.3 | 5%O2+N2+H2O | 166 | 242 | 300 | 50.9 | 77.1 |
表3 催化和等离子体强化催化作用下三聚氰胺水解的结果
Table 3 Results of melamine hydrolysis by catalysis and plasma-enhanced catalysis
| 平均放电功率/W | 载气气氛 | CO2开始出现的 温度/℃ | CO2达到峰值浓度时的温度/℃ | CO2开始消失的 温度/℃ | NH3产率/% | CO2产率/% |
|---|---|---|---|---|---|---|
| 0 | N2+H2O | 199 | 263 | 300 | 68.1 | 73.0 |
| 0 | 5%O2+N2+H2O | 201 | 262 | 300 | 66.7 | 74.9 |
| 5.5 | N2+H2O | 171 | 235 | 300 | 60.7 | 71.5 |
| 5.3 | 5%O2+N2+H2O | 166 | 242 | 300 | 50.9 | 77.1 |
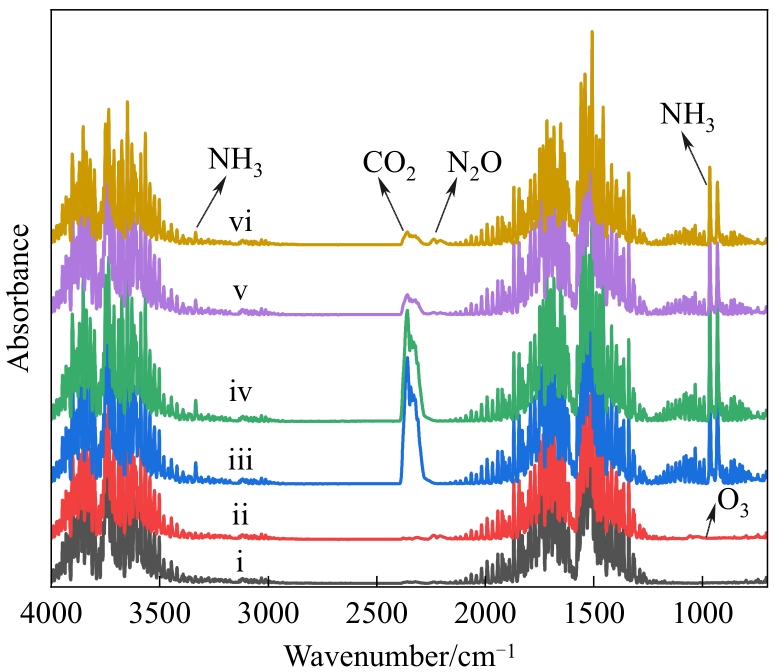
图5 空白放电以及催化和等离子体强化催化作用下缩二脲水解时反应器出口气体典型红外光谱图i—空白放电(N2+H2O);ii—空白放电(5%O2+N2+H2O);iii—催化水解(N2+H2O);iv—催化水解(5%O2+N2+H2O);v—等离子体强化催化水解(N2+H2O);vi—等离子体强化催化水解(5%O2+N2+H2O)
Fig.5 Typical infrared spectra of the outlet gas from discharge with TiO2 alone and from catalytic and plasma-enhanced catalytic hydrolysis of biuret

图6 空白放电以及等离子体强化缩二脲、三聚氰酸和三聚氰胺催化水解生成的N2O、NO和NO2浓度随时间的变化(放电时间段:0~85 min)
Fig.6 Concentration curves of N2O, NO and NO2 generated during discharge with TiO2 alone and during plasma-enhanced catalytic hydrolysis of biuret, cyanuric acid and melamine (discharge period: 0—85 min)
| 1 | Goldbach M, Roppertz A, Langenfeld P, et al. Urea decomposition in selective catalytic reduction on V2O5/WO3/TiO2 catalyst in diesel exhaust[J]. Chemical Engineering & Technology, 2017, 40(11): 2035-2043. |
| 2 | Reşitoğlu İ A, Altinişik K, Keskin A. The pollutant emissions from diesel-engine vehicles and exhaust after treatment systems[J]. Clean Technologies and Environmental Policy, 2015, 17(1): 15-27. |
| 3 | Pronobis M. Reduction of nitrogen oxide emissions[M]//Environmentally Oriented Modernization of Power Boilers. Amsterdam: Elsevier, 2020: 79-133. |
| 4 | Burr M, Gregory C. Vehicular exhausts[M]//Encyclopedia of Environmental Health. 2nd ed. Amsterdam: Elsevier, 2011: 335-343. |
| 5 | Lambert C K. Perspective on SCR NOx control for diesel vehicles[J]. Reaction Chemistry & Engineering, 2019, 4(6): 969-974. |
| 6 | Biswas S, Verma V, Schauer J J, et al. Chemical speciation of PM emissions from heavy-duty diesel vehicles equipped with diesel particulate filter (DPF) and selective catalytic reduction (SCR) retrofits[J]. Atmospheric Environment, 2009, 43(11): 1917-1925. |
| 7 | Yuan X M, Liu H Q, Gao Y. Diesel engine SCR control: current development and future challenges[J]. Emission Control Science and Technology, 2015, 1(2): 121-133. |
| 8 | Bernhard A M, Peitz D, Elsener M, et al. Catalytic urea hydrolysis in the selective catalytic reduction of NOx: catalyst screening and kinetics on anatase TiO2 and ZrO2[J]. Catalysis Science & Technology, 2013, 3(4): 942-951. |
| 9 | Ma Y, Wu X D, Zhang J Y, et al. Urea-related reactions and their active sites over Cu-SAPO-34: formation of NH3 and conversion of HNCO[J]. Applied Catalysis B: Environmental, 2018, 227: 198-208. |
| 10 | Todorova T, Peitz D, Kröcher O, et al. Guanidinium formate decomposition on the (101) TiO2-anatase surface: combined minimum energy reaction pathway calculations and temperature-programmed decomposition experiments[J]. The Journal of Physical Chemistry C, 2011, 115(4): 1195-1203. |
| 11 | Fan X, Kang S J, Li J. Plasma-enhanced hydrolysis of urea and SCR of NOx over V2O5-MoO3/TiO2: decrease of reaction temperature and increase of NOx conversion[J]. Fuel, 2020, 277: 118155. |
| 12 | Bernhard A M, Czekaj I, Elsener M, et al. Adsorption and catalytic thermolysis of gaseous urea on anatase TiO2 studied by HPLC analysis, DRIFT spectroscopy and DFT calculations[J]. Applied Catalysis B: Environmental, 2013, 134/135: 316-323. |
| 13 | Koebel M, Strutz E O. Thermal and hydrolytic decomposition of urea for automotive selective catalytic reduction systems: thermochemical and practical aspects[J]. Industrial & Engineering Chemistry Research, 2003, 42(10): 2093-2100. |
| 14 | Strots V O, Santhanam S, Adelman B J, et al. Deposit formation in urea-SCR systems[J]. SAE International Journal of Fuels and Lubricants, 2010, 2(2): 283-289. |
| 15 | Brack W, Heine B, Birkhold F, et al. Formation of urea-based deposits in an exhaust system: numerical predictions and experimental observations on a hot gas test bench[J]. Emission Control Science and Technology, 2016, 2(3): 115-123. |
| 16 | Ebrahimian V, Nicolle A, Habchi C. Detailed modeling of the evaporation and thermal decomposition of urea-water solution in SCR systems[J]. AIChE Journal, 2012, 58(7): 1998-2009. |
| 17 | Schaber P M, Colson J, Higgins S, et al. Study of the urea thermal decomposition (pyrolysis) reaction and importance to cyanuric acid production[J]. American Laboratory, 1999, 31(16): 13-21. |
| 18 | Schaber P M, Colson J, Higgins S, et al. Thermal decomposition (pyrolysis) of urea in an open reaction vessel[J]. Thermochimica Acta, 2004, 424(1/2): 131-142. |
| 19 | Bann B, Miller S A. Melamine and derivatives of melamine[J]. Chemical Reviews, 1958, 58(1): 131-172. |
| 20 | Eichelbaum M, Siemer A B, Farrauto R J, et al. The impact of urea on the performance of metal-exchanged zeolites for the selective catalytic reduction of NOx(Part Ⅱ): Catalytic, FTIR, and NMR studies[J]. Applied Catalysis B: Environmental, 2010, 97(1/2): 98-107. |
| 21 | Koebel M, Elsener M. Determination of urea and its thermal decomposition products by high-performance liquid chromatography[J]. Journal of Chromatography A, 1995, 689(1): 164-169. |
| 22 | Kröcher O, Elsener M. Materials for thermohydrolysis of urea in a fluidized bed[J]. Chemical Engineering Journal, 2009, 152(1): 167-176. |
| 23 | Xu L F, Watkins W, Snow R, et al. Laboratory and engine study of urea-related deposits in diesel urea-SCR after-treatment systems[R]. SAETechnical Paper Series. Warrendale, PA, United States: SAE International, 2007: 2007-01-1582. |
| 24 | 余俊波, 莫春兰, 黄文君, 等. 柴油机SCR系统尿素沉积物详细反应路径[J]. 内燃机学报, 2020, 38(2): 169-177. |
| Yu J B, Mo C L, Huang W J, et al. Detailed reaction pathways of urea deposit in diesel engine with selective catalytic reduction system[J]. Transactions of CSICE, 2020, 38(2): 169-177. | |
| 25 | Fang H L, DaCosta H F M. Urea thermolysis and NOx reduction with and without SCR catalysts[J]. Applied Catalysis B: Environmental, 2003, 46(1): 17-34. |
| 26 | Zhan Z Q, Müllner M, Lercher J A. Catalytic hydrolysis of s-triazine compounds over Al2O3[J]. Catalysis Today, 1996, 27(1/2): 167-173. |
| 27 | Bernhard A M, Peitz D, Elsener M, et al. Hydrolysis and thermolysis of urea and its decomposition byproducts biuret, cyanuric acid and melamine over anatase TiO2[J]. Applied Catalysis B: Environmental, 2012, 115/116: 129-137. |
| 28 | Mizuno A. Generation of non-thermal plasma combined with catalysts and their application in environmental technology[J]. Catalysis Today, 2013, 211: 2-8. |
| 29 | Chang J S. Physics and chemistry of plasma pollution control technology[J]. Plasma Sources Science and Technology, 2008, 17(4): 045004. |
| 30 | Kim H H, Ogata A. Nonthermal plasma activates catalyst: from current understanding and future prospects[J]. The European Physical Journal Applied Physics, 2011, 55(1): 13806. |
| 31 | Wang H, Cao Y Y, Chen Z W, et al. High-efficiency removal of NOxover natural mordenite using an enhanced plasma-catalytic process at ambient temperature[J]. Fuel, 2018, 224: 323-330. |
| 32 | Wang Y L, Craven M, Yu X T, et al. Plasma-enhanced catalytic synthesis of ammonia over a Ni/Al2O3 catalyst at near-room temperature: insights into the importance of the catalyst surface on the reaction mechanism[J]. ACS Catalysis, 2019, 9(12): 10780-10793. |
| [1] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [2] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [3] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [4] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [5] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [6] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [7] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [8] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [9] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [10] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [11] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [12] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [13] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [14] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [15] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号
