化工学报 ›› 2021, Vol. 72 ›› Issue (11): 5590-5597.DOI: 10.11949/0438-1157.20210721
收稿日期:2021-05-27
修回日期:2021-09-10
出版日期:2021-11-05
发布日期:2021-11-12
通讯作者:
陆安慧
作者简介:丁鼎(1996—),男,硕士研究生,基金资助:
Ding DING( ),Wenduo LU,Lu HOU,Anhui LU(
),Wenduo LU,Lu HOU,Anhui LU( )
)
Received:2021-05-27
Revised:2021-09-10
Online:2021-11-05
Published:2021-11-12
Contact:
Anhui LU
摘要:
通过静电纺丝法制备了一种高温焙烧后具有纤维状结构的磷酸硼/二氧化硅(BPO4/SiO2)催化剂,并考察了BPO4负载量和焙烧温度对该催化剂的结构和催化丙烷氧化脱氢性能的影响。研究发现焙烧过程使纤维直径减小。随着BPO4负载量的增加,丙烷氧化脱氢活性增高。当BPO4负载量为7%(质量分数),焙烧温度为600℃时,BPO4/SiO2催化剂具有最佳的催化性能;在反应温度为480℃下,丙烷转化率和丙烯产率分别达到17.0%和13.0%,且催化剂稳定性良好。当焙烧温度较低时(550℃),催化剂中的有机物分子未被除尽,导致烯烃的选择性偏低;当焙烧温度较高时(700℃),SiO2结构收缩紧密,抑制了活性相的暴露。由于纤维结构可暴露更多活性位点,该催化剂较相同条件下粉末状BPO4催化剂有着更高的催化活性。
中图分类号:
丁鼎, 陆文多, 侯璐, 陆安慧. 纤维状BPO4/SiO2催化剂的制备及其丙烷氧化脱氢性能[J]. 化工学报, 2021, 72(11): 5590-5597.
Ding DING, Wenduo LU, Lu HOU, Anhui LU. Preparation of fibrous BPO4/SiO2 catalyst for oxidative dehydrogenation of propane[J]. CIESC Journal, 2021, 72(11): 5590-5597.

图3 未焙烧和不同焙烧温度的BPO4(3%)/SiO2纤维状催化剂的FT IR谱图
Fig.3 FT IR spectra of BPO4(3%)/SiO2 nanofibrous catalysts before calcined and at different calcination temperatures
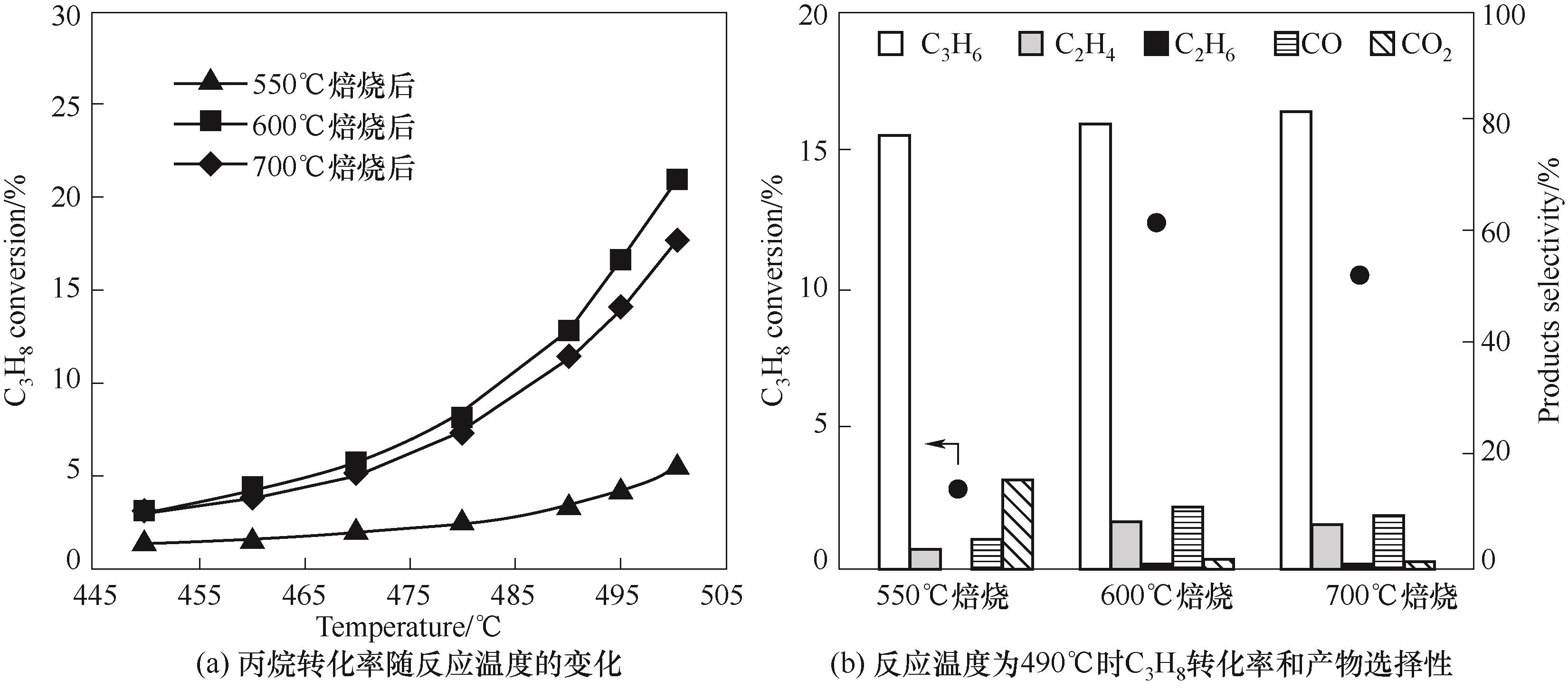
图5 经过不同温度焙烧的BPO4(3%)/SiO2的催化性能 (反应条件: 16.7%(体积分数) C3H8, 25.0%(体积分数) O2, 58.3% (体积分数)N2; V总流量 = 12 ml·min-1)
Fig.5 Catalytic performance of BPO4(3%)/SiO2 nanofibrous catalysts calcined at different temperatures (reaction conditions: 16.7%(vol) C3H8, 25.0%(vol) O2, 58.3%(vol) N2; total flow rate 12 ml·min-1)
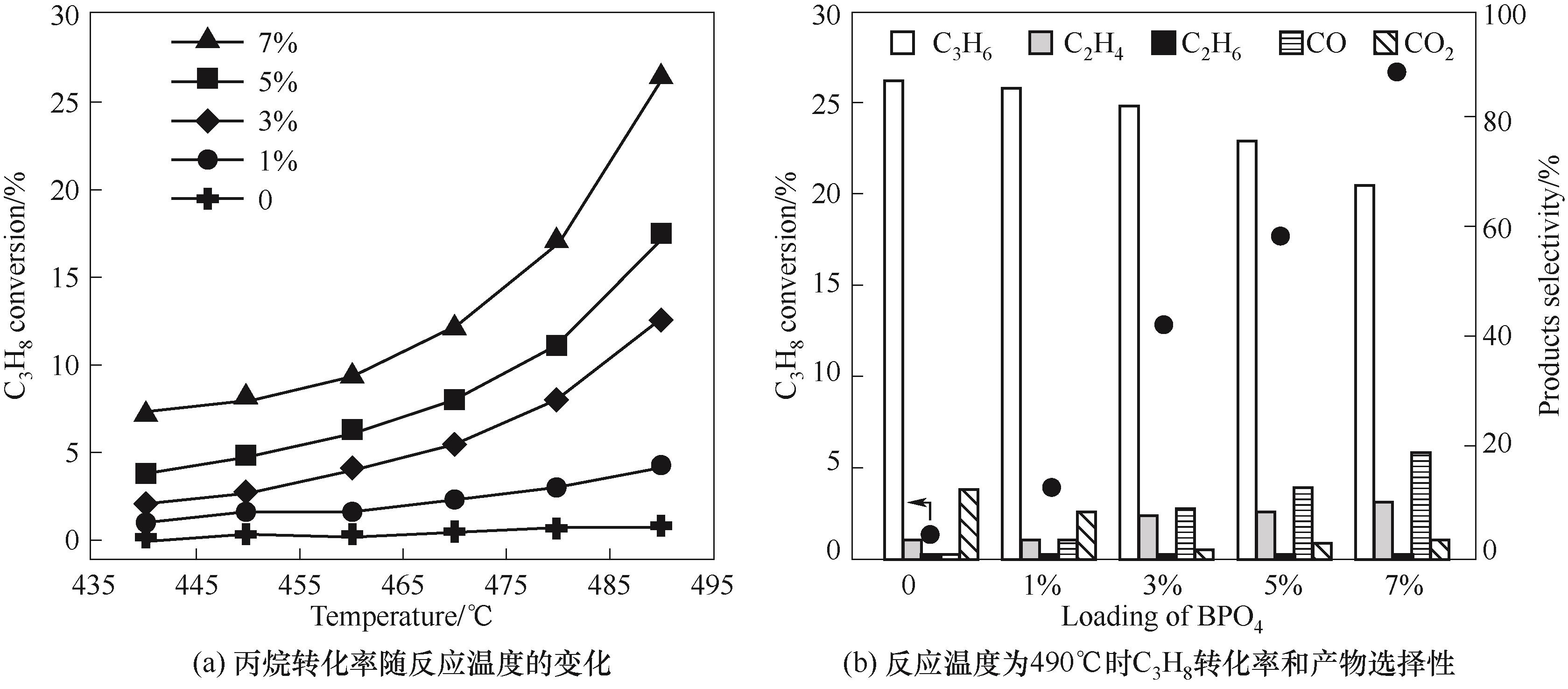
图6 不同BPO4负载量的BPO4/SiO2纤维状催化剂的催化性能 (反应条件: 16.7%(体积分数) C3H8, 25.0%(体积分数) O2, 58.3%(体积分数) N2; V总流量 = 12 ml·min-1)
Fig.6 Catalytic performance of BPO4/SiO2 nanofibrous catalysts with different BPO4 loadings (reaction conditions: 16.7%(vol) C3H8, 25.0%(vol) O2, 58.3%(vol) N2; total flow rate 12 ml·min-1)
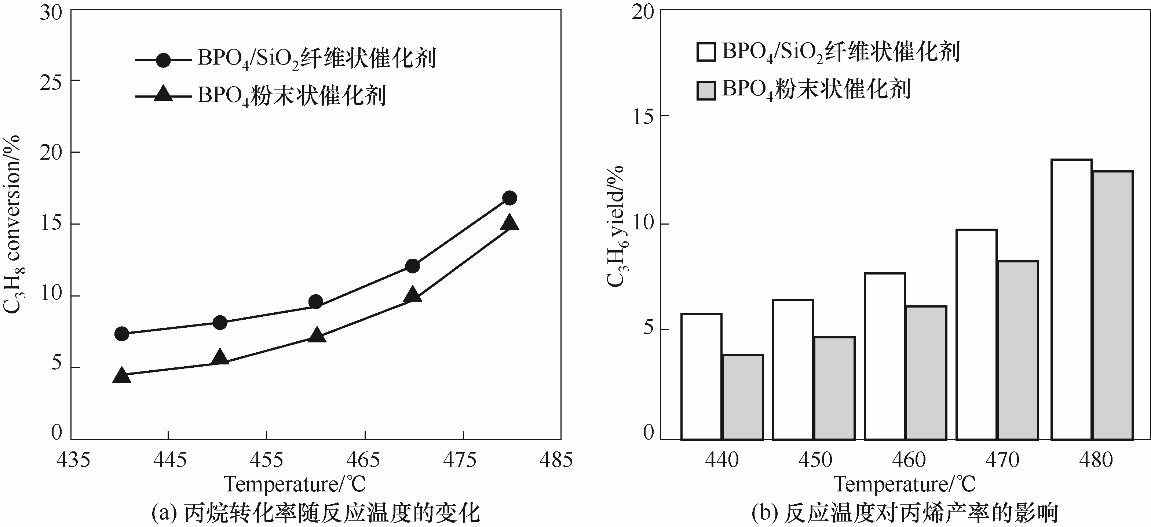
图7 BPO4(7%)/SiO2纤维状和BPO4体相催化剂性能比较 (反应条件: 16.7% C3H8, 25.0% O2, 58.3% N2; V总流量 = 12 ml·min-1)
Fig.7 Comparison of the catalytic property of BPO4/SiO2 nanofibrous catalyst and the bulk BPO4 catalyst (reaction conditions: 16.7% C3H8, 25.0% O2, 58.3% N2; total flow rate 12 ml·min-1)
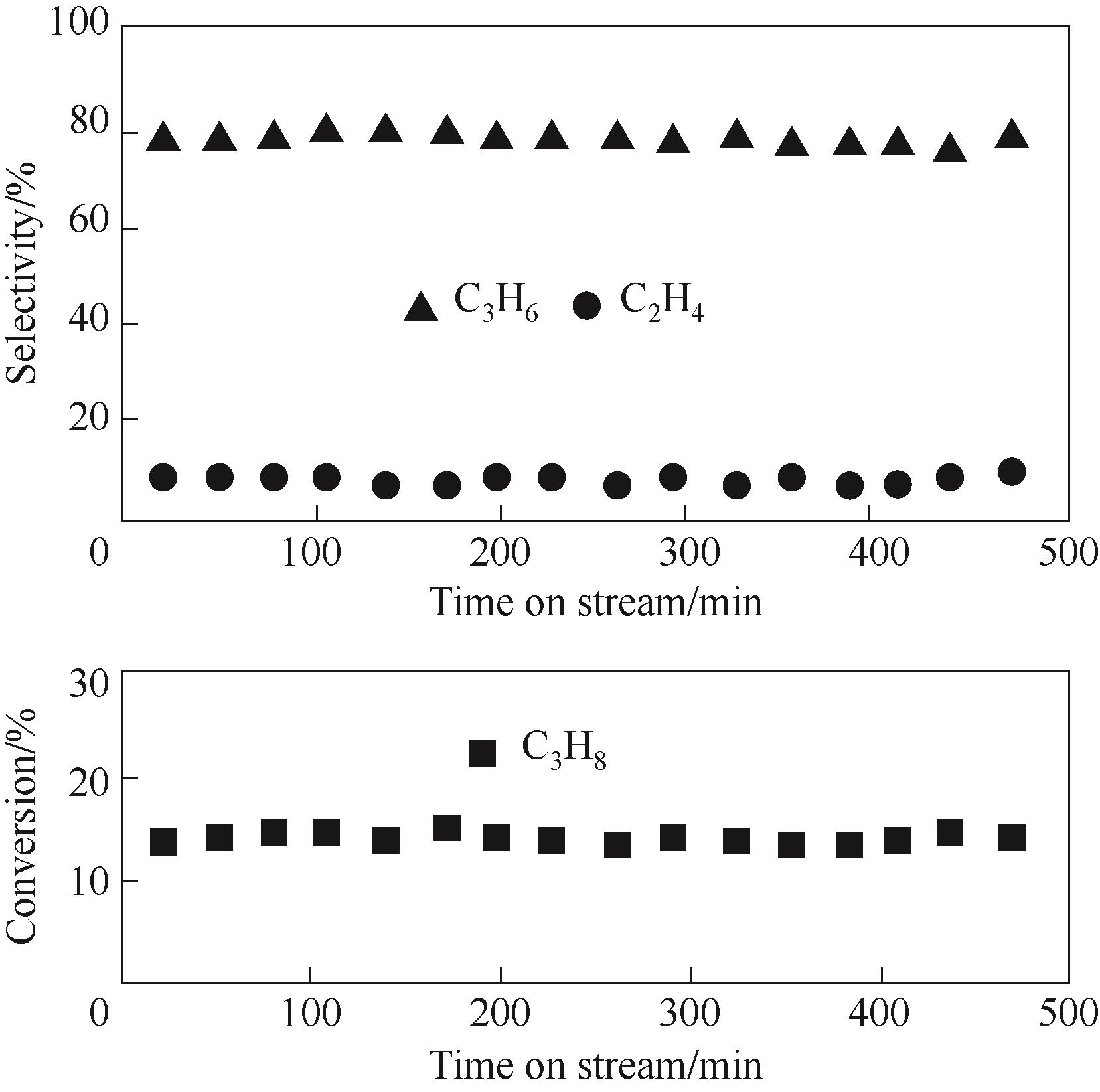
图8 480℃下BPO4(7%)/SiO2纤维状催化剂的稳定性测试 (反应条件: 反应气, 16.7% C3H8, 25.0% O2, 58.3% N2; V总流量 = 12 ml·min-1)
Fig.8 Catalytic stability tests over BPO4(7%)/SiO2 nanofibrous catalyst at 480℃ (reaction conditions: gas feed, 16.7% C3H8, 25.0% O2, 58.3% N2; total flow rate 12 ml·min-1)
| 1 | Sattler J J, Ruiz-Martinez J, Santillan-Jimenez E, et al. Catalytic dehydrogenation of light alkanes on metals and metal oxides[J]. Chemical Reviews, 2014, 114(20): 10613-10653. |
| 2 | Sheng J, Yan B, Lu W D, et al. Oxidative dehydrogenation of light alkanes to olefins on metal-free catalysts[J]. Chemical Society Reviews, 2021, 50(2): 1438-1468. |
| 3 | 戴伟, 罗晴, 王定博. 烯烃转化生产丙烯的研究进展[J]. 石油化工, 2008, 37(5): 425-433. |
| Dai W, Luo Q, Wang D B. Review of propylene production through olefins conversion[J]. Petrochemical Technology, 2008, 37(5): 425-433. | |
| 4 | 林少波, 单玉领, 隋志军, 等. 氧对丙烷脱氢反应体系影响的热力学分析[J]. 化工进展, 2015, 34(4): 970-975, 1006. |
| Lin S B, Shan Y L, Sui Z J, et al. Thermodynamic analysis of effects of oxygen addition on dehydrogenation of propane[J]. Chemical Industry and Engineering Progress, 2015, 34(4): 970-975, 1006. | |
| 5 | Shi L, Wang Y, Yan B, et al. Progress in selective oxidative dehydrogenation of light alkanes to olefins promoted by boron nitride catalysts[J]. Chemical Communications (Cambridge, England), 2018, 54(78): 10936-10946. |
| 6 | 张海娟, 高杰, 张浩楠, 等. 低碳烷烃深加工制烯烃技术的研究进展[J]. 石油化工, 2016, 45(12): 1411-1419. |
| Zhang H J, Gao J, Zhang H N, et al. Progresses in processes and catalysts for dehydrogenation of light paraffins to olefins[J]. Petrochemical Technology, 2016, 45(12): 1411-1419. | |
| 7 | Cavani F, Ballarini N, Cericola A. Oxidative dehydrogenation of ethane and propane: how far from commercial implementation? [J]. Catalysis Today, 2007, 127(1/2/3/4): 113-131. |
| 8 | De León M A, De Los Santos C, Latrónica L, et al. High catalytic activity at low temperature in oxidative dehydrogenation of propane with Cr-Al pillared clay[J]. Chemical Engineering Journal, 2014, 241: 336-343. |
| 9 | Grant J T, Carrero C A, Goeltl F, et al. Selective oxidative dehydrogenation of propane to propene using boron nitride catalysts[J]. Science, 2016, 354(6319): 1570-1573. |
| 10 | Tian J S, Tan J Q, Xu M L, et al. Propane oxidative dehydrogenation over highly selective hexagonal boron nitride catalysts: the role of oxidative coupling of methyl[J]. Science Advances, 2019, 5(3): eaav8063. |
| 11 | Zhang X Y, You R, Wei Z Y, et al. Radical chemistry and reaction mechanisms of propane oxidative dehydrogenation over hexagonal boron nitride catalysts[J]. Angewandte Chemie International Edition, 2020, 59(21): 8042-8046. |
| 12 | Venegas J M, Zhang Z S, Agbi T O, et al. Why boron nitride is such a selective catalyst for the oxidative dehydrogenation of propane[J]. Angewandte Chemie International Edition, 2020, 59(38): 16527-16535. |
| 13 | Shi L, Yan B, Shao D, et al. Selective oxidative dehydrogenation of ethane to ethylene over a hydroxylated boron nitride catalyst[J]. Chinese Journal of Catalysis, 2017, 38(2): 389-395. |
| 14 | Zhou Y L, Lin J, Li L, et al. Enhanced performance of boron nitride catalysts with induction period for the oxidative dehydrogenation of ethane to ethylene[J]. Journal of Catalysis, 2018, 365: 14-23. |
| 15 | Si C W, Lian Z, Olanrele S O, et al. Revealing the origin of the reactivity of metal-free boron nitride catalysts in oxidative dehydrogenation of propane[J]. Applied Surface Science, 2020, 519: 146241. |
| 16 | Lu W D, Gao X Q, Wang Q G, et al. Ordered macroporous boron phosphate crystals as metal-free catalysts for the oxidative dehydrogenation of propane[J]. Chinese Journal of Catalysis, 2020, 41(12): 1837-1845. |
| 17 | Xue J J, Wu T, Dai Y Q, et al. Electrospinning and electrospun nanofibers: methods, materials, and applications[J]. Chemical Reviews, 2019, 119(8): 5298-5415. |
| 18 | Dai Y Q, Liu W Y, Formo E, et al. Ceramic nanofibers fabricated by electrospinning and their applications in catalysis, environmental science, and energy technology[J]. Polymers for Advanced Technologies, 2011, 22(3): 326-338. |
| 19 | Yang G, Li X L, He Y, et al. From nano to micro to macro: Electrospun hierarchically structured polymeric fibers for biomedical applications[J]. Progress in Polymer Science, 2018, 81: 80-113. |
| 20 | 丁春立, 林帝出, 王德武, 等. 电纺及疏水改性制备CA/SiNPs-FAS超疏水复合膜及膜蒸馏脱盐研究[J]. 化工学报, 2018, 69(4): 1774-1782. |
| Ding C L, Lin D C, Wang D W, et al. Preparation of superhydrophobic CA/SiNPs-FAS electrospun nanofibrous membranes for direct contact membrane distillation[J]. CIESC Journal, 2018, 69(4): 1774-1782. | |
| 21 | 何璐铭, 辛忠, 高文莉, 等. 静电纺丝法制备高活性多孔Ni/SiO2甲烷化催化剂[J]. 化工学报, 2020, 71(11): 5007-5015. |
| He L M, Xin Z, Gao W L, et al. Highly efficient porous Ni/SiO2 catalysts prepared by electrospinning method for CO methanation[J]. CIESC Journal, 2020, 71(11): 5007-5015. | |
| 22 | Liu G, Zhang T D, Feng Y, et al. Sandwich-structured polymers with electrospun boron nitrides layers as high-temperature energy storage dielectrics[J]. Chemical Engineering Journal, 2020, 389: 124443. |
| 23 | Li S, Cui Z M, Li D M, et al. Hierarchically structured electrospinning nanofibers for catalysis and energy storage[J]. Composites Communications, 2019, 13: 1-11. |
| 24 | Kang H G, Zhu Y H, Yang X L, et al. A novel catalyst based on electrospun silver-doped silica fibers with ribbon morphology[J]. Journal of Colloid and Interface Science, 2010, 341(2): 303-310. |
| 25 | Zhu Y F, Ikoma T, Hanagata N, et al. Rattle-type Fe3O4@SiO2 hollow mesoporous spheres as carriers for drug delivery[J]. Small, 2010, 6(3): 471-478. |
| 26 | Yan B, Lu W D, Sheng J, et al. Electrospinning synthesis of porous boron-doped silica nanofibers for oxidative dehydrogenation of light alkanes[J]. Chinese Journal of Catalysis, 2021, 42(10): 1782-1789. |
| 27 | Ishigami M, Chen J H, Cullen W G, et al. Atomic structure of graphene on SiO2[J]. Nano Letters, 2007, 7(6): 1643-1648. |
| 28 | Li X, Zhi C Y, Hanagata N, et al. Boron nitride nanotubes functionalized with mesoporous silica for intracellular delivery of chemotherapy drugs[J]. Chemical Communications, 2013, 49(66): 7337-7339. |
| 29 | Feng S N, Zhang H J, Xu S, et al. Folate-conjugated, mesoporous silica functionalized boron nitride nanospheres for targeted delivery of doxorubicin[J]. Materials Science and Engineering: C, 2019, 96: 552-560. |
| 30 | Lu W D, Wang D Q, Zhao Z C, et al. Supported boron oxide catalysts for selective and low-temperature oxidative dehydrogenation of propane[J]. ACS Catalysis, 2019, 9(9): 8263-8270. |
| 31 | Wang Y, Li W C, Zhou Y X, et al. Boron nitride wash-coated cordierite monolithic catalyst showing high selectivity and productivity for oxidative dehydrogenation of propane[J]. Catalysis Today, 2020, 339: 62-66. |
| 32 | Zhou Y X, Wang Y, Lu W D, et al. A high propylene productivity over B2O3/SiO2@honeycomb cordierite catalyst for oxidative dehydrogenation of propane[J]. Chinese Journal of Chemical Engineering, 2020, 28(11): 2778-2784. |
| 33 | Sun G X, Bi J Q, Wang W L, et al. Enhancing mechanical properties of fused silica composites by introducing well-dispersed boron nitride nanosheets[J]. Ceramics International, 2018, 44(5): 5002-5009. |
| 34 | Li D, Zhang C R, Li B, et al. Preparation and mechanical properties of unidirectional boron nitride fibre reinforced silica matrix composites[J]. Materials & Design, 2012, 34: 401-405. |
| 35 | Namba S, Takagaki A, Jimura K, et al. Effects of ball-milling treatment on physicochemical properties and solid base activity of hexagonal boron nitrides[J]. Catalysis Science & Technology, 2019, 9(2): 302-309. |
| 36 | Lee D, Lee B, Park K H, et al. Scalable exfoliation process for highly soluble boron nitride nanoplatelets by hydroxide-assisted ball milling[J]. Nano Letters, 2015, 15(2): 1238-1244. |
| 37 | Chang M J, Cui W N, Liu J. Facile preparation of porous inorganic SiO2 nanofibrous membrane by electrospinning method[J]. Journal of Nanomaterials, 2017, 2017: 1-8. |
| 38 | Otomo R, Yamaguchi C, Iwaisako D, et al. Selective dehydration of 1, 2-propanediol to propanal over boron phosphate catalyst in the presence of steam[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(3): 3027-3033. |
| 39 | Wu Y J, Xu Y Z, Wang D J, et al. FT-IR spectroscopic investigation on the interaction between nylon 66 and lithium salts[J]. Journal of Applied Polymer Science, 2004, 91(5): 2869-2875. |
| 40 | Wu F, He L, Li W C, et al. Highly dispersed boron-nitride/CuOx-supported Au nanoparticles for catalytic CO oxidation at low temperatures[J]. Chinese Journal of Catalysis, 2021, 42(3): 388-395. |
| 41 | Qiu B, Jiang F, Lu W D, et al. Oxidative dehydrogenation of propane using layered borosilicate zeolite as the active and selective catalyst[J]. Journal of Catalysis, 2020, 385: 176-182. |
| 42 | Shi L, Wang D Q, Song W, et al. Edge-hydroxylated boron nitride for oxidative dehydrogenation of propane to propylene[J]. ChemCatChem, 2017, 9(10): 1720. |
| 43 | Belgamwar R, Rankin A G M, Maity A, et al. Boron nitride and oxide supported on dendritic fibrous nanosilica for catalytic oxidative dehydrogenation of propane[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(43): 16124-16135. |
| [1] | 吴延鹏, 李晓宇, 钟乔洋. 静电纺丝纳米纤维双疏膜油性细颗粒物过滤性能实验分析[J]. 化工学报, 2023, 74(S1): 259-264. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [4] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [5] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [6] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [7] | 胡兴枝, 张皓焱, 庄境坤, 范雨晴, 张开银, 向军. 嵌有超小CeO2纳米粒子的碳纳米纤维的制备及其吸波性能[J]. 化工学报, 2023, 74(8): 3584-3596. |
| [8] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [9] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [10] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [11] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [12] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [13] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [14] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [15] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号