化工学报 ›› 2022, Vol. 73 ›› Issue (9): 3929-3939.DOI: 10.11949/0438-1157.20220501
李承威1( ), 骆华勇1(
), 骆华勇1( ), 张铭轩1, 廖鹏2, 方茜1, 荣宏伟1, 王竞茵1
), 张铭轩1, 廖鹏2, 方茜1, 荣宏伟1, 王竞茵1
收稿日期:2022-05-04
修回日期:2022-06-09
出版日期:2022-09-05
发布日期:2022-10-09
通讯作者:
骆华勇
作者简介:李承威(1996—),男,硕士研究生,1287377003@qq.com
基金资助:
Chengwei LI1( ), Huayong LUO1(
), Huayong LUO1( ), Mingxuan ZHANG1, Peng LIAO2, Qian FANG1, Hongwei RONG1, Jingyin WANG1
), Mingxuan ZHANG1, Peng LIAO2, Qian FANG1, Hongwei RONG1, Jingyin WANG1
Received:2022-05-04
Revised:2022-06-09
Online:2022-09-05
Published:2022-10-09
Contact:
Huayong LUO
摘要:
基于微流控技术制备了氢氧化镧[La(OH)3]交联壳聚糖(CS)微球(La-CS-M),并对其组成和结构进行表征,探究了微球吸附去除水中磷酸盐的性能和机理。结果表明,La-CS-M成功负载了La(OH)3,与传统滴落法制备的氢氧化镧交联壳聚糖球(La-CS)相比,其表面和内部具有疏松多孔结构,其体积平均粒径为415.8 μm,孔隙率为89.22%,平均孔径为960.0 nm,pHpzc约为6.5。La-CS-M在宽pH范围内(3.0~10.0)均保持较高吸附量,
中图分类号:
李承威, 骆华勇, 张铭轩, 廖鹏, 方茜, 荣宏伟, 王竞茵. 氢氧化镧交联壳聚糖微球的微流控制备及其除磷性能[J]. 化工学报, 2022, 73(9): 3929-3939.
Chengwei LI, Huayong LUO, Mingxuan ZHANG, Peng LIAO, Qian FANG, Hongwei RONG, Jingyin WANG. Microfludically-generated lanthanum hydroxide cross-linked chitosan microspheres for phosphate removal[J]. CIESC Journal, 2022, 73(9): 3929-3939.
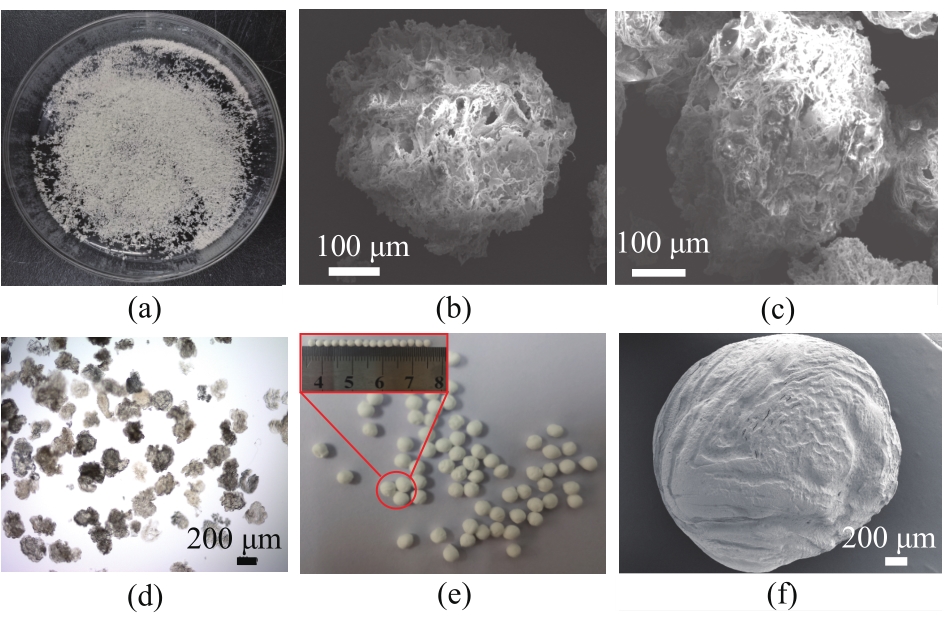
图2 冷冻干燥后的La-CS-M实物照片(a),La-CS-M吸附磷酸盐前(b)、后(c)的SEM照片,La-CS-M在光学显微镜下的照片(d),冷冻干燥后的La-CS实物照片(e)和SEM照片(f)
Fig.2 Digital image of freeze-dried La-CS-M (a), SEM images of La-CS-M before (b) and after (c) phosphate adsorption, optical image of La-CS-M (d), digital (e) and SEM (f) images of freeze-dried La-CS
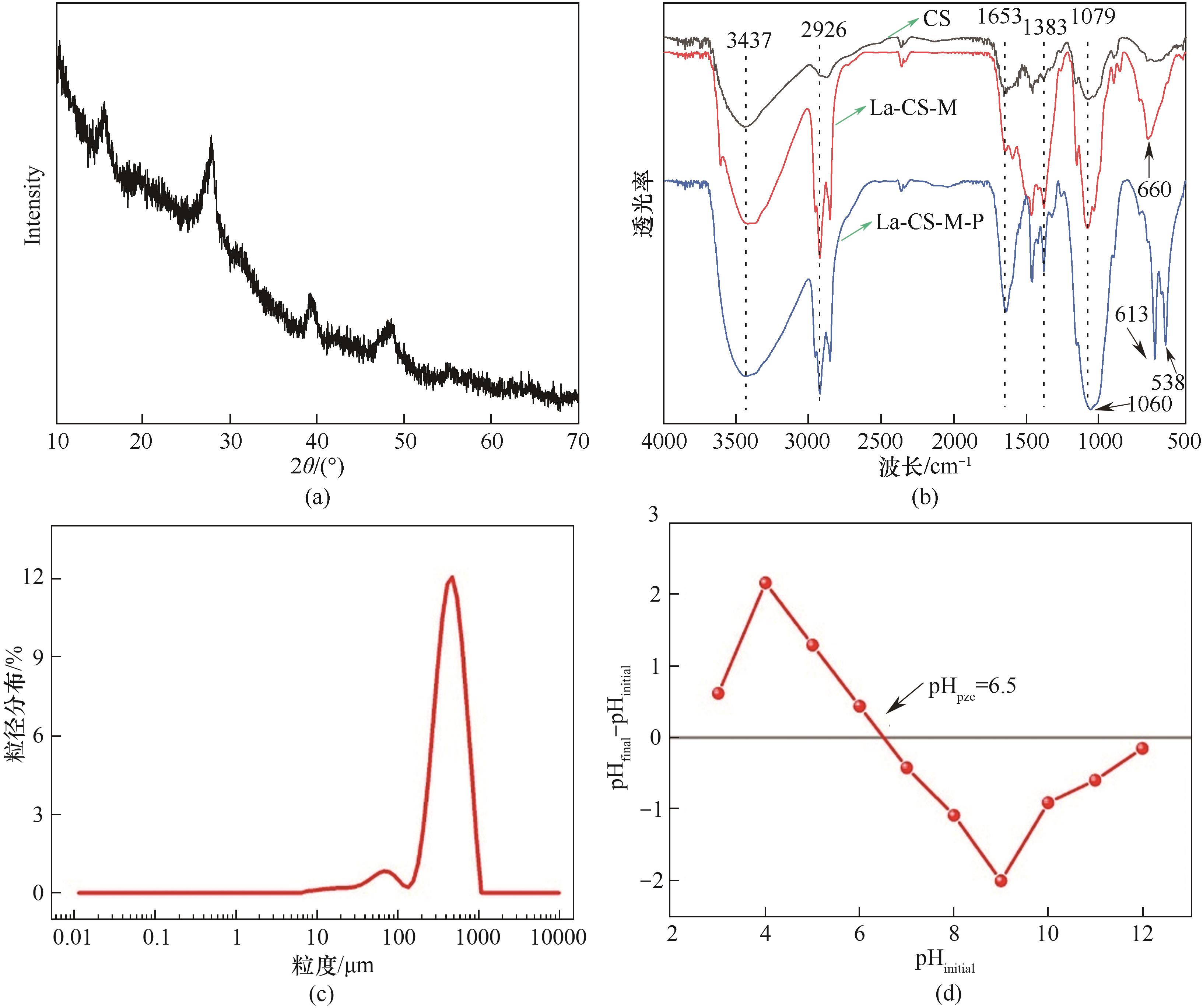
图3 La-CS-M的XRD谱图(a),CS、La-CS-M和吸附磷后La-CS-M(La-CS-M-P)的红外光谱(b),La-CS-M的粒径分布(c)以及La-CS-M在零点电荷时的pH(pHpzc)(d)
Fig.3 XRD pattern of La-CS-M (a), FTIR spectra of CS and La-CS-M before and after phosphate adsorption (b), particle size distribution of La-CS-M (c) and the pH at point of zero charge (pHpzc) of La-CS-M (d)
| 吸附剂 | 孔隙度/% | 平均孔径/nm | La负载量/%(质量) |
|---|---|---|---|
| La-CS-M | 89.22 | 960.0 | 36.26 |
| La-CS | 87.86 | 473.9 | 37.99 |
表1 壳聚糖微球的孔隙特征和La负载量
Table 1 The pore characteristics and La loading capacities of chitosan microspheres
| 吸附剂 | 孔隙度/% | 平均孔径/nm | La负载量/%(质量) |
|---|---|---|---|
| La-CS-M | 89.22 | 960.0 | 36.26 |
| La-CS | 87.86 | 473.9 | 37.99 |
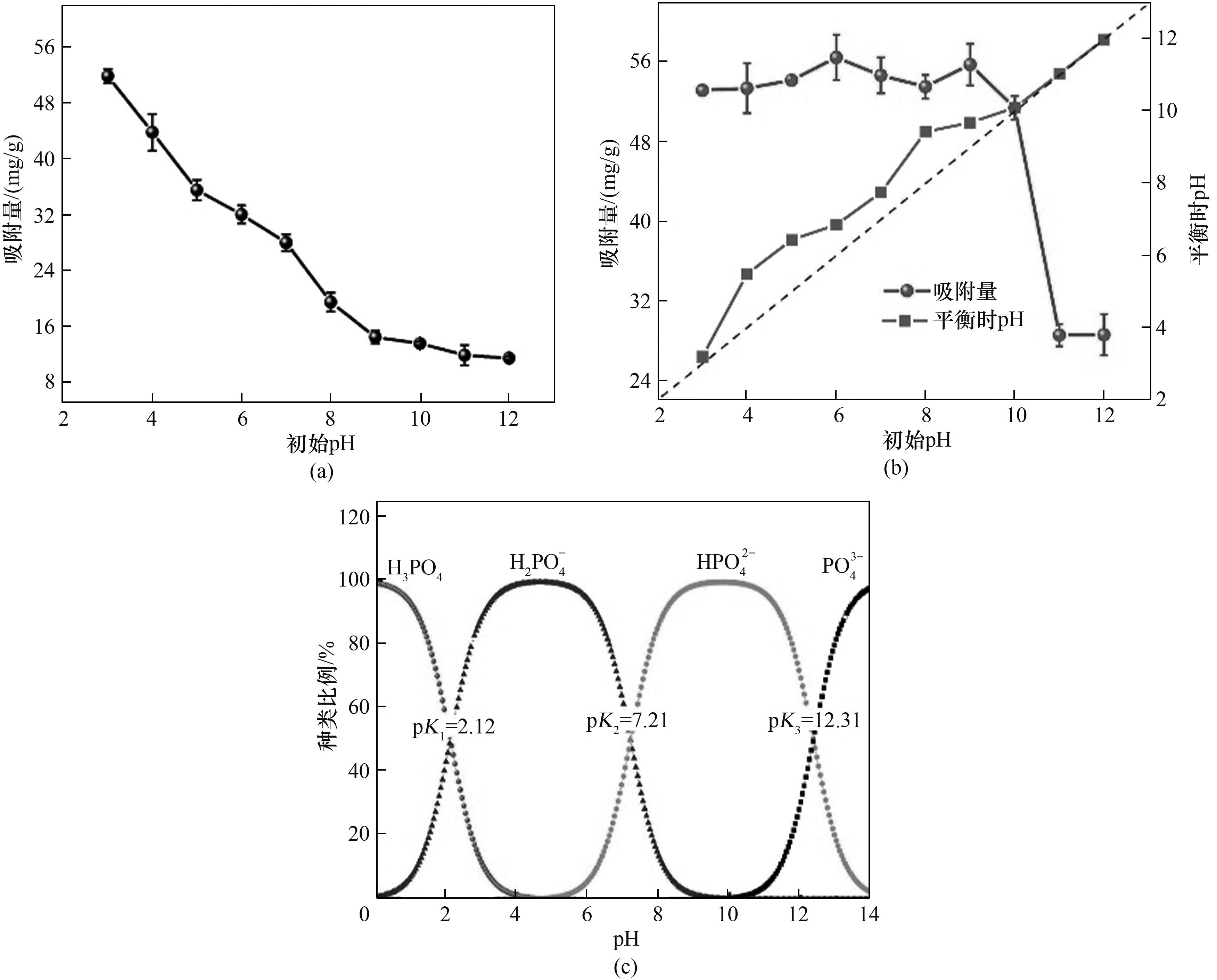
图4 pH对La-CS吸附磷酸盐性能的影响(a),pH对La-CS-M吸附磷酸盐性能的影响(b)及磷酸盐种类在不同pH下的分布(c)
Fig.4 Effect of pH on phosphate adsorption onto La-CS (a), effect of pH on phosphate adsorption onto La-CS-M (b), and species of phosphate in different pH (c)

图6 La-CS-M对磷酸盐的吸附动力学拟合曲线(a),颗粒内扩散模型拟合曲线(b)以及吸附等温线(c)
Fig.6 Fitting curves of kinetic models (a) and intraparticle diffusion model (b) for phosphate adsorption onto La-CS-M,and adsorption isotherms (c)
| 模型 | 参数 | 数值 |
|---|---|---|
| 准一级动力学模型 | qe/(mg/g) | 50.95 |
| k1/min-1 | 6.75×10-3 | |
| R2 | 0.923 | |
| 准二级动力学模型 | qe/(mg/g) | 57.43 |
| k2/(g/(mg·min)) | 1.52×10-4 | |
| R2 | 0.969 | |
| 颗粒内扩散模型 | k1d/(mg/(g·min0.5)) | 1.89 |
| C1 | 4.82 | |
| R2 | 0.989 | |
| k2d/(mg/(g·min0.5)) | 0.57 | |
| C2 | 33.97 | |
| R2 | 0.918 | |
| Langmuir模型 | qmax/(mg/g) | 56.48 |
| kL/(L/mg) | 0.40 | |
| R2 | 0.980 | |
| Freundlich模型 | kF/(mg/g)(L/mg)1/n | 23.15 |
| n | 4.11 | |
| R2 | 0.962 |
表2 La-CS-M对磷酸盐的吸附动力学模型和等温吸附模型拟合参数
Table 2 Kinetic and isotherm modeling parameters for phosphate adsorption onto La-CS-M
| 模型 | 参数 | 数值 |
|---|---|---|
| 准一级动力学模型 | qe/(mg/g) | 50.95 |
| k1/min-1 | 6.75×10-3 | |
| R2 | 0.923 | |
| 准二级动力学模型 | qe/(mg/g) | 57.43 |
| k2/(g/(mg·min)) | 1.52×10-4 | |
| R2 | 0.969 | |
| 颗粒内扩散模型 | k1d/(mg/(g·min0.5)) | 1.89 |
| C1 | 4.82 | |
| R2 | 0.989 | |
| k2d/(mg/(g·min0.5)) | 0.57 | |
| C2 | 33.97 | |
| R2 | 0.918 | |
| Langmuir模型 | qmax/(mg/g) | 56.48 |
| kL/(L/mg) | 0.40 | |
| R2 | 0.980 | |
| Freundlich模型 | kF/(mg/g)(L/mg)1/n | 23.15 |
| n | 4.11 | |
| R2 | 0.962 |
| 吸附剂 | 磷酸盐浓度/(mg/L) | pH | 温度/℃ | qmax/(mg/g) | 文献 |
|---|---|---|---|---|---|
| La-CS-M | 5~50 | 6.0±0.1 | 25 | 56.48 | 本研究 |
| 包埋滑石粉的海藻酸镧凝胶 | 2.5~50.0 | 4 | 25 | 16.034 | [ |
| 水合氧化镧改性的硅藻土 | 10~100 | 5.60 | 25 | 58.70 | [ |
| 氢氧化镧改性介孔稻壳生物炭 | 5~100 | 6.6 | 25 | 41.22~45.62 | [ |
| MgFe2O4-生物炭基海藻酸镧珠 | 5~50 | 5.3±0.3 | 25±1 | 27.68 | [ |
| 包埋氢氧化镧的聚乙烯醇/海藻酸铝凝胶球 | 0~50 | 4.0 | 25 | 7.86 | [ |
| 掺杂氢氧化镧的活性炭纤维 | 10~70 | — | 室温 | 15.3 | [ |
表3 La-CS-M与其他载镧吸附剂对磷酸盐基于Langmuir模型最大吸附容量的比较
Table 3 Comparison of maximum phosphate adsorption capacity (qmax) based on Langmuir model between La-CS-Mand other La-loaded adsorbents
| 吸附剂 | 磷酸盐浓度/(mg/L) | pH | 温度/℃ | qmax/(mg/g) | 文献 |
|---|---|---|---|---|---|
| La-CS-M | 5~50 | 6.0±0.1 | 25 | 56.48 | 本研究 |
| 包埋滑石粉的海藻酸镧凝胶 | 2.5~50.0 | 4 | 25 | 16.034 | [ |
| 水合氧化镧改性的硅藻土 | 10~100 | 5.60 | 25 | 58.70 | [ |
| 氢氧化镧改性介孔稻壳生物炭 | 5~100 | 6.6 | 25 | 41.22~45.62 | [ |
| MgFe2O4-生物炭基海藻酸镧珠 | 5~50 | 5.3±0.3 | 25±1 | 27.68 | [ |
| 包埋氢氧化镧的聚乙烯醇/海藻酸铝凝胶球 | 0~50 | 4.0 | 25 | 7.86 | [ |
| 掺杂氢氧化镧的活性炭纤维 | 10~70 | — | 室温 | 15.3 | [ |

图8 La-CS-M吸附磷酸盐前后的XPS全谱图(a)及O 1s (b)、La 3d (c)和P 2p (d)的高分辨率谱图
Fig.8 XPS full spectrum of La-CS-M before and after phosphate adsorption (a), and the high resolution spectra of O 1s (b),La 3d (c) and P 2p (d)
| 1 | Zhi Y, Zhang C, Hjorth R, et al. Emerging lanthanum (Ⅲ)-containing materials for phosphate removal from water: a review towards future developments[J]. Environment International, 2020, 145: 106-115. |
| 2 | Razanajatovo M R, Gao W, Song Y, et al. Selective adsorption of phosphate in water using lanthanum-based nanomaterials: a critical review[J]. Chinese Chemical Letters, 2021, 32(9): 2637-2647. |
| 3 | Zhao Y, Guo L, Shen W, et al. Function integrated chitosan-based beads with throughout sorption sites and inherent diffusion network for efficient phosphate removal[J]. Carbohydrate Polymers, 2020, 230: 115639. |
| 4 | Xu W, Zheng W, Wang F, et al. Using iron ion-loaded aminated polyacrylonitrile fiber to efficiently remove wastewater phosphate[J]. Chemical Engineering Journal, 2021, 403: 126349. |
| 5 | Wang B, Zhang W, Li L, et al. Novel talc encapsulated lanthanum alginate hydrogel for efficient phosphate adsorption and fixation[J]. Chemosphere, 2020, 256: 127124. |
| 6 | Xie J, Wang Z, Lu S, et al. Removal and recovery of phosphate from water by lanthanum hydroxide materials[J]. Chemical Engineering Journal, 2014, 254: 163-170. |
| 7 | 罗元, 谢坤, 张克强, 等. 镧(La)改性吸附材料脱除水体磷酸盐研究进展[J]. 化工进展, 2019, 38(11): 5005-5014. |
| Luo Y, Xie K, Zhang K Q, et al. Research progress on removal phosphate in aqueous solution by lanthanum modified adsorption materials[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 5005-5014. | |
| 8 | Qiu H, Liang C, Yu J, et al. Preferable phosphate sequestration by nano-La (Ⅲ)(hydr)oxides modified wheat straw with excellent properties in regeneration[J]. Chemical Engineering Journal, 2017, 315: 345-354. |
| 9 | Dong S, Wang Y, Zhao Y, et al. La3+/La(OH)3 loaded magnetic cationic hydrogel composites for phosphate removal: effect of lanthanum species and mechanistic study[J]. Water Research, 2017, 126: 433-441. |
| 10 | Zhou A, Zhu C, Chen W, et al. Phosphorus recovery from water by lanthanum hydroxide embedded interpenetrating network poly (vinyl alcohol)/sodium alginate hydrogel beads[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 554: 237-244. |
| 11 | Zhang Y, Pan B, Shan C, et al. Enhanced phosphate removal by nanosized hydrated La(Ⅲ) oxide confined in cross-linked polystyrene networks[J]. Environmental Science & Technology, 2016, 50(3): 1447-1454. |
| 12 | Wang B, Bai Z, Jiang H, et al. Selective heavy metal removal and water purification by microfluidically-generated chitosan microspheres: characteristics, modeling and application[J]. Journal of Hazardous Materials, 2019, 364: 192-205. |
| 13 | Dong S, Ji Q, Wang Y, et al. Enhanced phosphate removal using zirconium hydroxide encapsulated in quaternized cellulose[J]. Journal of Environmental Sciences, 2020, 89: 102-112. |
| 14 | Xu J H, Zhao H, Lan W J, et al. A novel microfluidic approach for monodispersed chitosan microspheres with controllable structures[J]. Advanced Healthcare Materials, 2012, 1, 106-111. |
| 15 | 肖艾. 基于微流控技术一步制备磁性聚乙烯醇微球及其在介入栓塞治疗中的应用[D]. 武汉: 华中科技大学, 2016. |
| Xiao A. Microfluidic one-step preparation of magnetic poly(vinyl alcohol) microspheres and their applications in interventional embolization therapy[D]. Wuhan: Huazhong University of Science & Technology, 2016. | |
| 16 | Chen Z, Luo H, Rong H. Development of polyaminated chitosan-zirconium (Ⅳ) complex bead adsorbent for highly efficient removal and recovery of phosphorus in aqueous solutions[J]. International Journal of Biological Macromolecules, 2020, 164: 1183-1193. |
| 17 | 骆华勇, 荣宏伟, 曾学阳, 等. 全互穿网络温敏海藻酸锆凝胶球的磷吸附性能[J].高等学校化学学报, 2018, 39(10): 2289-2297. |
| Luo H Y, Rong H W, Zeng X Y, et al. Performance of phosphorus sorption on thermosensitive zirconium alginate hydrogel beads with full-interpenetrating network[J]. Chemical Journal of Chinese Universities, 2018, 39(10): 2289-2297. | |
| 18 | Luo H, Liu Y, Lu H, et al. Efficient adsorption of tetracycline from aqueous solutions by modified alginate beads after the removal of Cu (Ⅱ) ions[J]. ACS omega, 2021, 6(9): 6240-6251. |
| 19 | 陆瀚兴, 李明, 骆华勇, 等. 水合氧化锆改性污泥生物炭对磷酸盐的吸附特性研究[J]. 水处理技术, 2022, 48(4): 65-70. |
| Lu H X, Li M, Luo H Y, et al. Performance of phosphate adsorption on hydrous zirconium oxide-modified biochars derived from sewage sludge[J]. Technology of Water Treatment, 2022, 48(4): 65-70. | |
| 20 | Li H, Zhao Y, Xiao Z, et al. Analysis on approximate site energy distribution and adsorption behaviors unveils reasons for highly efficient phosphorus removal by a novel sludge-based magnetic gel bead[J]. Chemical Engineering Journal, 2021, 422: 130028. |
| 21 | Bansiwal A, Thakre D, Labhshetwar N, et al. Fluoride removal using lanthanum incorporated chitosan beads[J]. Colloids and Surfaces B: Biointerfaces, 2009, 74(1): 216-224. |
| 22 | Xu Q, Chen Z, Wu Z, et al. Novel lanthanum doped biochars derived from lignocellulosic wastes for efficient phosphate removal and regeneration[J]. Bioresource Technology, 2019, 289: 121600. |
| 23 | 华熠, 潘蒙晓, 丁冬东, 等. 四乙烯五胺功能化纳米高分子材料对Cr(Ⅵ)与磷酸盐共存体系的吸附机理[J]. 中国科学: 化学, 2014, 44(11): 1776-1787. |
| Hua Y, Pan M X, Ding D D, et al. Adsorption mechanism of tetraethylenepentamine-functionalized nano polymers in Cr(Ⅵ), phosphate co-existing water system[J]. Scientia Sinica Chimica, 2014, 44(11): 1776-1787. | |
| 24 | 许润, 石程好, 唐倩, 等. 氢氧化镧改性介孔稻壳生物炭除磷性能[J]. 环境科学, 2019, 40(4): 1834-1841. |
| Xu R, Shi C H, Tang Q, et al. Phosphate removal using rice husk biochars modified with lanthanum hydroxide[J]. Environmental Science, 2019, 40(4): 1834-1841. | |
| 25 | Wu Y, Li X, Yang Q, et al. Hydrated lanthanum oxide-modified diatomite as highly efficient adsorbent for low-concentration phosphate removal from secondary effluents[J]. Journal of Environmental Management, 2019, 231: 370-379. |
| 26 | Zhang L, Zhou Q, Liu J, et al. Phosphate adsorption on lanthanum hydroxide-doped activated carbon fiber[J]. Chemical Engineering Journal, 2012, 185: 160-167. |
| 27 | Spears B M, Lürling M, Yasseri S, et al. Lake responses following lanthanum-modified bentonite clay (Phoslock®) application: an analysis of water column lanthanum data from 16 case study lakes[J]. Water Research, 2013, 47(15): 5930-5942. |
| 28 | Acelas N Y, Martin B D, López D, et al. Selective removal of phosphate from wastewater using hydrated metal oxides dispersed within anionic exchange media[J]. Chemosphere, 2015, 119: 1353-1360. |
| 29 | Wang L, Wang J, Yan W, et al. MgFe2O4-biochar based lanthanum alginate beads for advanced phosphate removal[J]. Chemical Engineering Journal, 2020, 387: 123305. |
| 30 | Wang B, Hu X, Zhou D, et al. Highly selective and sustainable clean-up of phosphate from aqueous phase by eco-friendly lanthanum cross-linked polyvinyl alcohol/alginate/palygorskite composite hydrogel beads[J]. Journal of Cleaner Production, 2021, 298: 126878. |
| 31 | 宋小宝, 何世颖, 冯彦房, 等. 载镧磁性水热生物炭的制备及其除磷性能[J]. 环境科学, 2020, 41(2): 773-783. |
| Song X B, He S Y, Feng Y F, et al. Fabrication of La-MHTC composites for phosphate removal: adsorption behavior and mechanism[J]. Environmental Science, 2020, 41(2): 773-783. | |
| 32 | Shan S, Wang W, Liu D, et al. Remarkable phosphate removal and recovery from wastewater by magnetically recyclable La2O2CO3/γ-Fe2O3 nanocomposites[J]. Journal of Hazardous Materials, 2020, 397: 122597. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [5] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [6] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [7] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [8] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [9] | 吴文涛, 褚良永, 张玲洁, 谭伟民, 沈丽明, 暴宁钟. 腰果酚生物基自愈合微胶囊的高效制备工艺研究[J]. 化工学报, 2023, 74(7): 3103-3115. |
| [10] | 王志龙, 杨烨, 赵真真, 田涛, 赵桐, 崔亚辉. 搅拌时间和混合顺序对锂离子电池正极浆料分散特性的影响[J]. 化工学报, 2023, 74(7): 3127-3138. |
| [11] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [12] | 朱风, 陈凯琳, 黄小凤, 鲍银珠, 李文斌, 刘嘉鑫, 吴玮强, 高王伟. KOH改性电石渣脱除羰基硫的性能研究[J]. 化工学报, 2023, 74(6): 2668-2679. |
| [13] | 张艳梅, 袁涛, 李江, 刘亚洁, 孙占学. 高效SRB混合菌群构建及其在酸胁迫条件下的性能研究[J]. 化工学报, 2023, 74(6): 2599-2610. |
| [14] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| [15] | 张兰河, 赖青燚, 王铁铮, 关潇卓, 张明爽, 程欣, 徐小惠, 贾艳萍. H2O2对SBR脱氮效率和污泥性能的影响[J]. 化工学报, 2023, 74(5): 2186-2196. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号