化工学报 ›› 2022, Vol. 73 ›› Issue (7): 2996-3006.DOI: 10.11949/0438-1157.20220385
张军1,2,3( ),胡升1,4(
),胡升1,4( ),顾菁1,2,3,袁浩然1,2,3(
),顾菁1,2,3,袁浩然1,2,3( ),陈勇1,2,3
),陈勇1,2,3
收稿日期:2022-03-17
修回日期:2022-06-22
出版日期:2022-07-05
发布日期:2022-08-01
通讯作者:
袁浩然
作者简介:张军(1987—),男,博士,副研究员,基金资助:
Jun ZHANG1,2,3( ),Sheng HU1,4(
),Sheng HU1,4( ),Jing GU1,2,3,Haoran YUAN1,2,3(
),Jing GU1,2,3,Haoran YUAN1,2,3( ),Yong CHEN1,2,3
),Yong CHEN1,2,3
Received:2022-03-17
Revised:2022-06-22
Online:2022-07-05
Published:2022-08-01
Contact:
Haoran YUAN
摘要:
以电镀行业废弃物电镀污泥为前体合成磁性多金属催化材料,考察其在甲醇供氢体系生物基糠醛加氢转化制备糠醇和2-甲基呋喃的催化性能。通过X射线衍射(XRD)、液氮吸脱附、NH3程序升温脱附(NH3-TPD)、扫描电镜(SEM)等手段对煅烧后电镀污泥进行表征,并研究了煅烧温度和各反应工艺条件对甲醇供氢体系糠醛转化的影响。结果表明,电镀污泥衍生磁性多金属材料均具有强酸性位点和部分介孔结构,以铜组分为主的催化活性中心在反应过程中部分被还原为零价,有助于促进甲醇重整产氢和糠醛加氢转化;以700℃煅烧的电镀污泥为催化剂,在240℃反应2 h以上,糠醛几乎完全转化,产物中糠醇和2-甲基呋喃最高收率(摩尔分数)分别为70.9%和31.9%,反应过程副产物以2-呋喃甲基甲醚和2-(二甲氧基甲基)呋喃为主。此外,基于甲醇重整产氢、铜镍组分原位还原以及糠醛加氢反应之间的耦合作用,推测出甲醇体系电镀污泥衍生磁性多金属材料催化糠醛加氢转化可能的反应机制。
中图分类号:
张军, 胡升, 顾菁, 袁浩然, 陈勇. 甲醇体系电镀污泥衍生磁性多金属材料催化糠醛加氢转化[J]. 化工学报, 2022, 73(7): 2996-3006.
Jun ZHANG, Sheng HU, Jing GU, Haoran YUAN, Yong CHEN. Catalytic hydrogenation of furfural over magnetic polymetallic materials derived from electroplating sludge in methanol[J]. CIESC Journal, 2022, 73(7): 2996-3006.
| 组成 | 质量分数/% |
|---|---|
| Fe | 44.57 |
| Cu | 30.28 |
| O | 14.28 |
| Ni | 2.51 |
| Sn | 2.35 |
| S | 2.04 |
| Si | 1.04 |
| Ca | 0.96 |
| 其他 | 1.97 |
表1 电镀污泥主要元素成分
Table 1 Main elemental compositions of electroplating sludge
| 组成 | 质量分数/% |
|---|---|
| Fe | 44.57 |
| Cu | 30.28 |
| O | 14.28 |
| Ni | 2.51 |
| Sn | 2.35 |
| S | 2.04 |
| Si | 1.04 |
| Ca | 0.96 |
| 其他 | 1.97 |
| 样品 | 比表面积/(m2/g) | 平均孔径/nm | 总孔容/(cm3/g) |
|---|---|---|---|
| CES-400 | 82.53 | 29.41 | 0.34 |
| CES-550 | 48.13 | 30.20 | 0.21 |
| CES-700 | 41.10 | 54.05 | 0.17 |
| CES-850 | 10.14 | 3.41 | 0.02 |
| CES-700R | 25.47 | 3.86 | 0.10 |
表2 CES-T催化剂结构特性
Table 2 Structural properties of as-prepared CES-T
| 样品 | 比表面积/(m2/g) | 平均孔径/nm | 总孔容/(cm3/g) |
|---|---|---|---|
| CES-400 | 82.53 | 29.41 | 0.34 |
| CES-550 | 48.13 | 30.20 | 0.21 |
| CES-700 | 41.10 | 54.05 | 0.17 |
| CES-850 | 10.14 | 3.41 | 0.02 |
| CES-700R | 25.47 | 3.86 | 0.10 |
| 样品 | 弱酸性/(μmol/g) | 中等酸性/(μmol/g) | 强酸性/(μmol/g) | 总酸量/(μmol/g) |
|---|---|---|---|---|
| CES-400 | 1.8 | 2.4 | 8.1 | 12.3 |
| CES-550 | 1.3 | 2.2 | 6.8 | 10.3 |
| CES-700 | — | — | 24.6 | 24.6 |
| CES-850 | 1.9 | — | 18.5 | 20.4 |
表3 CES-T样品酸性位点分布
Table 3 Acid site distribution of as-prepared CES-T
| 样品 | 弱酸性/(μmol/g) | 中等酸性/(μmol/g) | 强酸性/(μmol/g) | 总酸量/(μmol/g) |
|---|---|---|---|---|
| CES-400 | 1.8 | 2.4 | 8.1 | 12.3 |
| CES-550 | 1.3 | 2.2 | 6.8 | 10.3 |
| CES-700 | — | — | 24.6 | 24.6 |
| CES-850 | 1.9 | — | 18.5 | 20.4 |
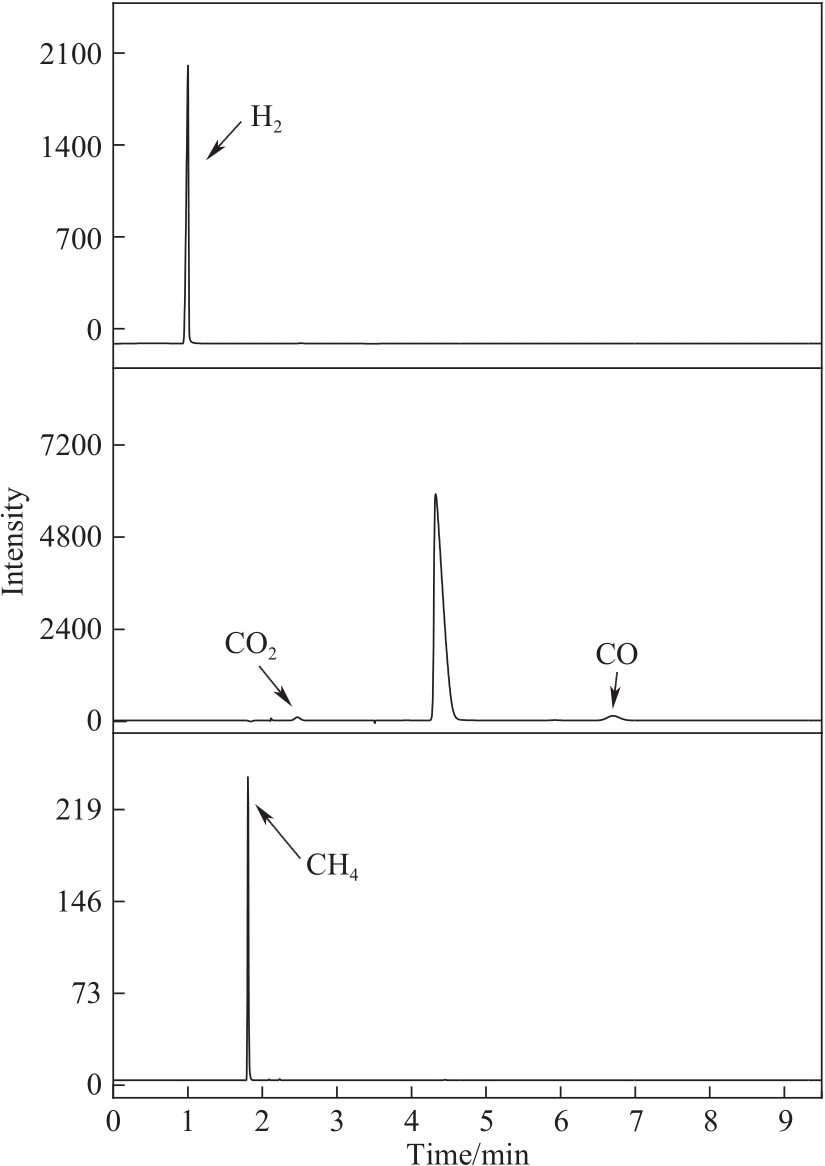
图6 CES-700催化甲醇重整气相色谱图(反应条件:甲醇12 ml,CES-700 30 mg,220℃, 2 h)
Fig.6 GC graph for methanol reforming over CES-700 (reaction conditions: methanol 12 ml, CES-700 30 mg, 220℃, 2 h)
| 保留时间/min | 组分 | 含量/%(体积分数) |
|---|---|---|
| 1.001 | H2 | 11.41 |
| 1.807 | CH4 | 0.20 |
| 2.092 | C2H6 | 0.0003 |
| 2.323 | C2H4 | 0.0005 |
| 2.469 | CO2 | 0.74 |
| 6.702 | CO | 2.57 |
表4 CES-700催化甲醇重整气相产物分布
Table 4 Gas phase product distribution from methanol reforming over CES-700
| 保留时间/min | 组分 | 含量/%(体积分数) |
|---|---|---|
| 1.001 | H2 | 11.41 |
| 1.807 | CH4 | 0.20 |
| 2.092 | C2H6 | 0.0003 |
| 2.323 | C2H4 | 0.0005 |
| 2.469 | CO2 | 0.74 |
| 6.702 | CO | 2.57 |
| 样品 | FFR转化率/% | FA收率/% | MF收率/% |
|---|---|---|---|
| CES-400 | 58.4 | 3.7 | 0.8 |
| CES-550 | 60.9 | 4.1 | 2.9 |
| CES-700 | 96.8 | 40.4 | 5.9 |
| CES-850 | 75.0 | 35.5 | 0.5 |
表5 CES-T对FFR转移加氢反应的催化活性
Table 5 Catalytic performance of CES-T on transfer hydrogenation of FFR
| 样品 | FFR转化率/% | FA收率/% | MF收率/% |
|---|---|---|---|
| CES-400 | 58.4 | 3.7 | 0.8 |
| CES-550 | 60.9 | 4.1 | 2.9 |
| CES-700 | 96.8 | 40.4 | 5.9 |
| CES-850 | 75.0 | 35.5 | 0.5 |
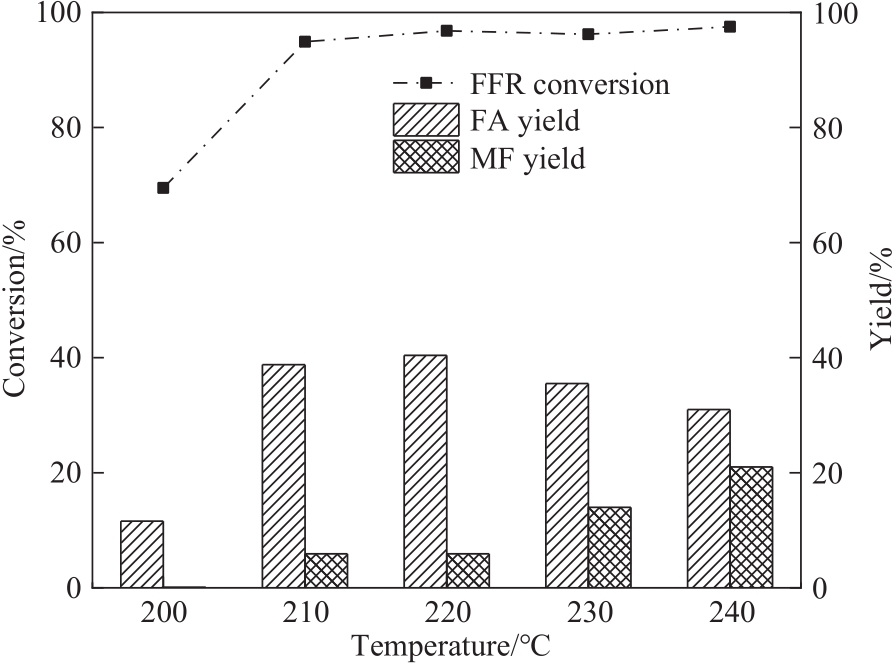
图7 反应温度对FFR加氢转化的影响(反应条件:FFR 0.6 mmol,甲醇12 ml,CES-700 30 mg,2 h)
Fig.7 Effect of reaction temperature on FFR hydrogenation (reaction conditions: FFR 0.6 ml, methanol 12 ml, CES-700 30 mg, 2 h)
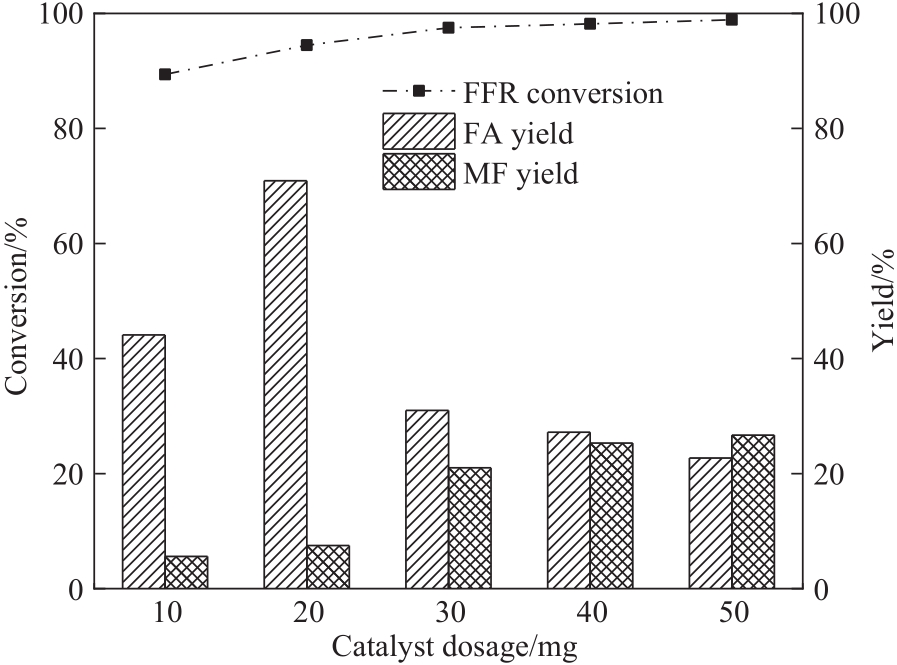
图9 催化剂用量对FFR转移加氢的影响(反应条件:FFR 0.6 mmol,甲醇12 ml,240℃,2 h)
Fig.9 Effect of catalyst dosage on the transfer hydrogenation of FFR (reaction conditions: FFR 0.6 mmol, methanol 12 ml, 240℃, 2 h)
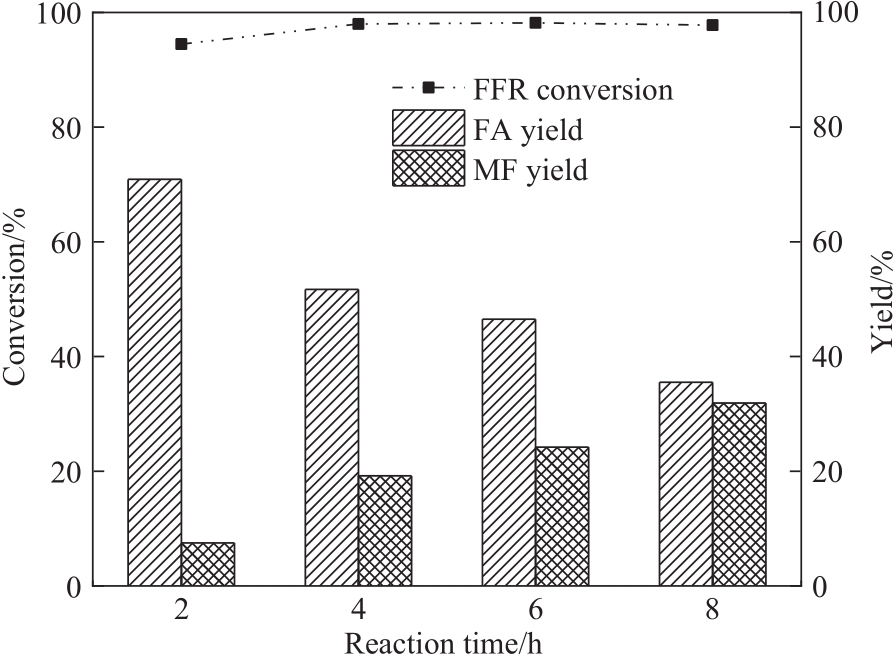
图10 反应时间对FFR转移加氢的影响(反应条件:FFR 0.6 mmol,甲醇12 ml,CES-700 20 mg,240℃)
Fig.10 Effect of reaction time on the transfer hydrogenation of FFR (reaction conditions: FFR 0.6 mmol, methanol 12 ml, CES-700 20 mg, 240℃)
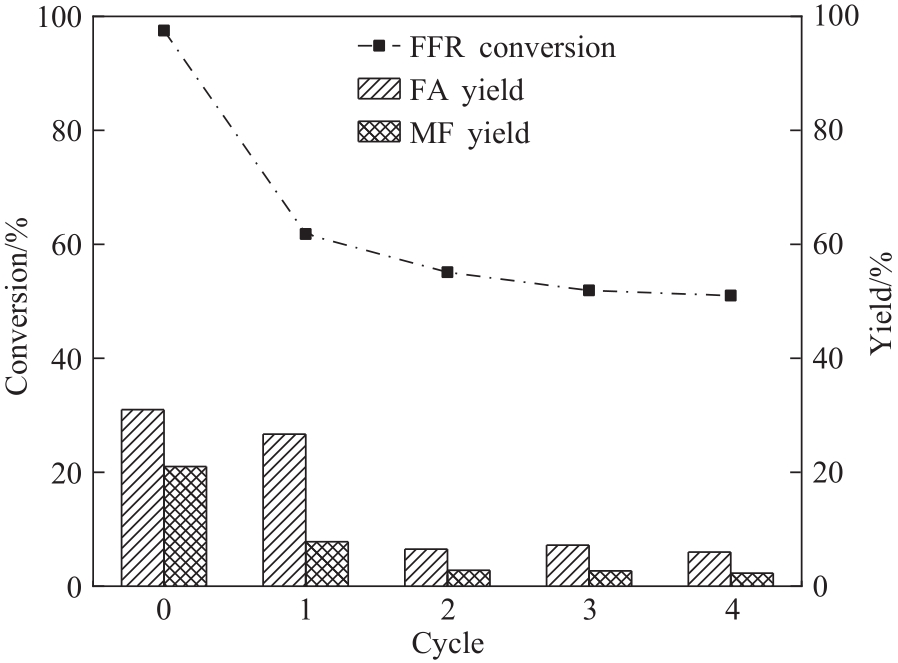
图11 催化剂循环使用性能(反应条件:FFR 0.6 mmol,甲醇12 ml,CES-700 30 mg,240℃,2 h)
Fig.11 Catalyst recycling (reaction conditions: FFR 0.6 mmol, methanol 12 ml, CES-700 30 mg, 240℃, 2 h)
| 1 | Fan L L, Ruan R, Li J, et al. Aromatics production from fast co-pyrolysis of lignin and waste cooking oil catalyzed by HZSM-5 zeolite[J]. Applied Energy, 2020, 263: 114629. |
| 2 | 郭海军, 张海荣, 丁帅, 等. 木质纤维素多元醇液化及液化产物提质的研究进展[J]. 化工学报, 2021, 72(6): 3228-3238. |
| Guo H J, Zhang H R, Ding S, et al. Research progress on lignocellulose liquefaction in polyhydric alcohol and upgrading of liquefaction product[J]. CIESC Journal, 2021, 72(6): 3228-3238. | |
| 3 | He Y F, Bie Y W, Lehtonen J, et al. Hydrodeoxygenation of guaiacol as a model compound of lignin-derived pyrolysis bio-oil over zirconia-supported Rh catalyst: process optimization and reaction kinetics[J]. Fuel, 2019, 239: 1015-1027. |
| 4 | Yu Z Z, Wu H G, Li Y, et al. Advances in heterogeneously catalytic degradation of biomass saccharides with ordered-nanoporous materials[J]. Industrial & Engineering Chemistry Research, 2020, 59(39): 16970-16986. |
| 5 | Zhou K, Chen J X, Cheng Y J, et al. Enhanced catalytic transfer hydrogenation of biomass-based furfural into 2-methylfuran over multifunctional Cu-Re bimetallic catalysts[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(44): 16624-16636. |
| 6 | Chen L F, Ye J Y, Yang Y S, et al. Catalytic conversion furfuryl alcohol to tetrahydrofurfuryl alcohol and 2-methylfuran at terrace, step, and corner sites on Ni[J]. ACS Catalysis, 2020, 10(13): 7240-7249. |
| 7 | Khemthong P, Yimsukanan C, Narkkun T, et al. Advances in catalytic production of value-added biochemicals and biofuels via furfural platform derived lignocellulosic biomass[J]. Biomass and Bioenergy, 2021, 148: 106033. |
| 8 | Weerachawanasak P, Krawmanee P, Inkamhaeng W, et al. Development of bimetallic Ni-Cu/SiO2 catalysts for liquid phase selective hydrogenation of furfural to furfuryl alcohol[J]. Catalysis Communications, 2021, 149:106221. |
| 9 | Jiménez-Gómez C P, Cecilia J A, Moreno-Tost R, et al. Selective production of 2-methylfuran by gas-phase hydrogenation of furfural on copper incorporated by complexation in mesoporous silica catalysts[J]. ChemSusChem, 2017, 10(7): 1448-1459. |
| 10 | Durndell L J, Zou G C, Shangguan W F, et al. Structure-reactivity relations in ruthenium catalysed furfural hydrogenation[J]. ChemCatChem, 2019, 11(16): 3927-3932. |
| 11 | Pirmoradi M, Kastner J R. A kinetic model of multi-step furfural hydrogenation over a Pd-TiO2 supported activated carbon catalyst[J]. Chemical Engineering Journal, 2021, 414: 128693. |
| 12 | Tolek W, Khruechao K, Pongthawornsakun B, et al. Flame spray-synthesized Pt-Co/TiO2 catalysts for the selective hydrogenation of furfural to furfuryl alcohol[J]. Catalysis Communications, 2021, 149: 106246. |
| 13 | Gao X, Tian S Y, Jin Y Y, et al. Bimetallic PtFe-catalyzed selective hydrogenation of furfural to furfuryl alcohol: solvent effect of isopropanol and hydrogen activation[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(33): 12722-12730. |
| 14 | Zhang H G, Tong X L, Gao Y Q, et al. Highly efficient catalytic valorization of biomass-derived furfural in methanol and ethanol[J]. Journal of Industrial and Engineering Chemistry, 2019, 70: 152-159. |
| 15 | Wang T, Du J, Sun Y, et al. Catalytic transfer hydrogenation of biomass-derived furfural to furfuryl alcohol with formic acid as hydrogen donor over CuCs-MCM catalyst[J]. Chinese Chemical Letters, 2021, 32(3): 1186-1190. |
| 16 | Wang Y T, Zhao D Y, Liang R, et al. Transfer hydrogenation of furfural to furfuryl alcohol over modified Zr-based catalysts using primary alcohols as H-donors[J]. Molecular Catalysis, 2021, 499: 111199. |
| 17 | Wang Y C, Hong Z Y, Mei D Q. A thermally autonomous methanol steam reforming microreactor with porous copper foam as catalyst support for hydrogen production[J]. International Journal of Hydrogen Energy, 2021, 46(9): 6734-6744. |
| 18 | Zhang S Q, Yang X, Zheng K, et al. In-situ hydrogenation of furfural conversion to furfuryl alcohol via aqueous-phase reforming of methanol[J]. Applied Catalysis A: General, 2019, 581: 103-110. |
| 19 | Zhang J, Chen J Z. Selective transfer hydrogenation of biomass-based furfural and 5-hydroxymethylfurfural over hydrotalcite-derived copper catalysts using methanol as a hydrogen donor[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(7): 5982-5993. |
| 20 | Shen S Y, Liu Y, Zhai D, et al. Electroplating sludge-derived spinel catalysts for NO removal via NH3 selective catalysis reduction[J]. Applied Surface Science, 2020, 528: 146969. |
| 21 | Bai H, Wang Z F, Zhang J, et al. Synthesis of a perovskite-type catalyst from Cr electroplating sludge for effective catalytic oxidization of VOC[J]. Journal of Environmental Management, 2021, 294: 113025. |
| 22 | Zhang C, Song J, Zhang J, et al. Understanding and application of an electroplating sludge-derived catalyst with an active texture for improved NO reduction[J]. Science of the Total Environment, 2018, 631/632: 308-316. |
| 23 | Chen D, Hou J, Yao L H, et al. Ferrite materials prepared from two industrial wastes: electroplating sludge and spent pickle liquor[J]. Separation and Purification Technology, 2010, 75(2): 210-217. |
| 24 | Li C Y, Zhang J, Gu J, et al. Insight into the role of varied acid-base sites on fast pyrolysis kinetics and mechanism of cellulose[J]. Waste Management, 2021, 135: 140-149. |
| 25 | Qi S C, Liu X Y, Zhu R R, et al. Causation of catalytic activity of Cu-ZnO for CO2 hydrogenation to methanol[J]. Chemical Engineering Journal, 2022, 430: 132784. |
| 26 | Xia H H, Li J, Chen C Z, et al. Selective aqueous-phase hydrogenation of furfural to cyclopentanol over Ni-based catalysts prepared from Ni-MOF composite[J]. Inorganic Chemistry Communications, 2021, 133: 108894. |
| 27 | Singh G, Khan T S, Samanta C, et al. Single-step synthesis of 2-pentanone from furfural over Cu-Ni @SBA-15[J]. Biomass and Bioenergy, 2022, 156: 106321. |
| 28 | Chubar N, Gerda V, Szlachta M, et al. Effect of Fe oxidation state (+2 versus +3) in precursor on the structure of Fe oxides/carbonates-based composites examined by XPS, FTIR and EXAFS[J]. Solid State Sciences, 2021, 121: 106752. |
| 29 | Chen Y P, Ma L X, Zhang R G, et al. Carbon-supported Fe catalysts with well-defined active sites for highly selective alcohol production from Fischer-Tropsch synthesis[J]. Applied Catalysis B: Environmental, 2022, 312: 121393. |
| 30 | Winoto H P, Ahn B S, Jae J. Production of γ-valerolactone from furfural by a single-step process using Sn-Al-Beta zeolites: optimizing the catalyst acid properties and process conditions[J]. Journal of Industrial and Engineering Chemistry, 2016, 40: 62-71. |
| 31 | Li F, Lu C S, Li X N. The effect of the amount of ammonia on the Cu0/Cu+ ratio of Cu/SiO2 catalyst for the hydrogenation of dimethyl oxalate to ethylene glycol[J]. Chinese Chemical Letters, 2014, 25(11): 1461-1465. |
| 32 | Ma F, Li H L, Jiang J X. Furfural reduction via hydrogen transfer from supercritical methanol[J]. Chemical Research in Chinese Universities, 2019, 35(3): 498-503. |
| 33 | Pasini T, Lolli A, Albonetti S, et al. Methanol as a clean and efficient H-transfer reactant for carbonyl reduction: scope, limitations, and reaction mechanism[J]. Journal of Catalysis, 2014, 317: 206-219. |
| 34 | Gilkey M J, Panagiotopoulou P, Mironenko A V, et al. Mechanistic insights into metal lewis acid-mediated catalytic transfer hydrogenation of furfural to 2-methylfuran[J]. ACS Catalysis, 2015, 5(7): 3988-3994. |
| 35 | Chen H, Ruan H H, Lu X L, et al. Catalytic conversion of furfural to methyl levulinate in a single-step route over Zr/SBA-15 in near-critical methanol[J]. Chemical Engineering Journal, 2018, 333: 434-442. |
| [1] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [2] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [3] | 曹跃, 余冲, 李智, 杨明磊. 工业数据驱动的加氢裂化装置多工况切换过渡状态检测[J]. 化工学报, 2023, 74(9): 3841-3854. |
| [4] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [5] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [6] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [7] | 吴文涛, 褚良永, 张玲洁, 谭伟民, 沈丽明, 暴宁钟. 腰果酚生物基自愈合微胶囊的高效制备工艺研究[J]. 化工学报, 2023, 74(7): 3103-3115. |
| [8] | 李贵贤, 曹阿波, 孟文亮, 王东亮, 杨勇, 周怀荣. 耦合固体氧化物电解槽的CO2制甲醇过程设计与评价研究[J]. 化工学报, 2023, 74(7): 2999-3009. |
| [9] | 董茂林, 陈李栋, 黄六莲, 吴伟兵, 戴红旗, 卞辉洋. 酸性助水溶剂制备木质纳米纤维素及功能应用研究进展[J]. 化工学报, 2023, 74(6): 2281-2295. |
| [10] | 杨峥豪, 何臻, 常玉龙, 靳紫恒, 江霞. 生物质快速热解下行式流化床反应器研究进展[J]. 化工学报, 2023, 74(6): 2249-2263. |
| [11] | 葛泽峰, 吴雨青, 曾名迅, 查振婷, 马宇娜, 侯增辉, 张会岩. 灰化学成分对生物质气化特性的影响规律[J]. 化工学报, 2023, 74(5): 2136-2146. |
| [12] | 吕阳光, 左培培, 杨正金, 徐铜文. 三嗪框架聚合物膜用于有机纳滤甲醇/正己烷分离[J]. 化工学报, 2023, 74(4): 1598-1606. |
| [13] | 刘海芹, 李博文, 凌喆, 刘亮, 俞娟, 范一民, 勇强. 羟基-炔点击化学改性半乳甘露聚糖薄膜的制备及性能研究[J]. 化工学报, 2023, 74(3): 1370-1378. |
| [14] | 张江淮, 赵众. 碳三加氢装置鲁棒最小协方差约束控制及应用[J]. 化工学报, 2023, 74(3): 1216-1227. |
| [15] | 祖凌鑫, 胡荣庭, 李鑫, 陈余道, 陈广林. 木质生物质化学组分的碳释放产物特征和反硝化利用程度[J]. 化工学报, 2023, 74(3): 1332-1342. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号