化工学报 ›› 2023, Vol. 74 ›› Issue (1): 365-379.DOI: 10.11949/0438-1157.20221183
李沐紫1( ), 贾国伟2, 赵砚珑1, 张鑫1(
), 贾国伟2, 赵砚珑1, 张鑫1( ), 李建荣1(
), 李建荣1( )
)
收稿日期:2022-08-30
修回日期:2022-11-11
出版日期:2023-01-05
发布日期:2023-03-20
通讯作者:
张鑫,李建荣
作者简介:李沐紫(1998—),女,硕士研究生,limuzi@bjut.edu.cn
基金资助:
Muzi LI1( ), Guowei JIA2, Yanlong ZHAO1, Xin ZHANG1(
), Guowei JIA2, Yanlong ZHAO1, Xin ZHANG1( ), Jianrong LI1(
), Jianrong LI1( )
)
Received:2022-08-30
Revised:2022-11-11
Online:2023-01-05
Published:2023-03-20
Contact:
Xin ZHANG, Jianrong LI
摘要:
随着全球暖化研究的深入,非二氧化碳温室气体(非二气体)日益受到重视,其温升效应大,寿命长,能够带来巨大的温室效应,因而成为近年来的研究热点。其中对非二气体的高效捕捉是目前面临的难点和新挑战。多孔材料吸附捕捉是一种低能耗的气体捕集分离技术,关键在于高效的吸附剂开发。金属-有机框架材料(MOFs)因其结构多样,孔道的高度可调控性能,以及具有丰富的开放金属位点等特点为非二气体的分离和捕捉提供了新的机遇。以此为主题,总结了近年来MOF材料在非二气体分离方面的研究成果,分析了各材料的分离机理与性能,展望了未来MOFs材料在非二气体分离领域的挑战与机遇。
中图分类号:
李沐紫, 贾国伟, 赵砚珑, 张鑫, 李建荣. 金属有机框架材料对非二氧化碳温室气体捕捉研究进展[J]. 化工学报, 2023, 74(1): 365-379.
Muzi LI, Guowei JIA, Yanlong ZHAO, Xin ZHANG, Jianrong LI. The progress of metal-organic frameworks for non-CO2 greenhouse gases capture[J]. CIESC Journal, 2023, 74(1): 365-379.
| 气体名称 | 分子式 | 寿命/a | GWP 20 | GWP 100 | GWP 500 | GTP 50 | GTP 100 |
|---|---|---|---|---|---|---|---|
| 二氧化碳 | CO2 | — | 1 | 1 | 1 | 1 | 1 |
| 甲烷 | CH4 | 11.8 | 81.2 | 27.9 | 7.95 | 11 | 5.38 |
| 氧化亚氮 | N2O | 109 | 273 | 273 | 130 | 290 | 233 |
| 氢氟化碳(HFCs) | |||||||
| HFC-23 | CHF3 | 228 | 12400 | 14600 | 10500 | 15400 | 15100 |
| HFC-32 | CH2F2 | 5.4 | 2690 | 771 | 220 | 181 | 142 |
| HFC-125 | CHF2CF3 | 30 | 6740 | 3740 | 1110 | 3300 | 1300 |
| HFC-134a | CH2FCF3 | 147 | 4140 | 1530 | 436 | 733 | 306 |
| HFC-143a | CH3CF3 | 51 | 7840 | 5810 | 1940 | 5910 | 3250 |
| HFC-152a | CH3CHF2 | 1.6 | 591 | 164 | 46.8 | 36.5 | 29.8 |
| HFC-227ea | CF3CHFCF3 | 36 | 5850 | 3600 | 1100 | 3400 | 1490 |
| HFC-236fa | CF3CH2CF3 | 213 | 7450 | 8690 | 6040 | 9200 | 88700 |
| HFC-245fa | CHF2CH2CF3 | 7.9 | 3170 | 962 | 274 | 262 | 180 |
| 全氟化碳(PFCs) | |||||||
| PFC-14 | CF4 | 50000 | 5300 | 7380 | 34100 | 7660 | 9050 |
| PFC-116 | C2F6 | 10000 | 8940 | 12400 | 10600 | 12900 | 15200 |
| 六氟化硫 | SF6 | 3200 | 18300 | 25200 | 17500 | 26200 | 30600 |
| 三氟化氮 | NF3 | 569 | 13400 | 17400 | 18200 | 18200 | 20000 |
表1 温室气体的GWP值/GTP值及寿命[8]
Table 1 Global warming potential (GWP), global temperature potential (GTP) and lifetime of green house gases[8]
| 气体名称 | 分子式 | 寿命/a | GWP 20 | GWP 100 | GWP 500 | GTP 50 | GTP 100 |
|---|---|---|---|---|---|---|---|
| 二氧化碳 | CO2 | — | 1 | 1 | 1 | 1 | 1 |
| 甲烷 | CH4 | 11.8 | 81.2 | 27.9 | 7.95 | 11 | 5.38 |
| 氧化亚氮 | N2O | 109 | 273 | 273 | 130 | 290 | 233 |
| 氢氟化碳(HFCs) | |||||||
| HFC-23 | CHF3 | 228 | 12400 | 14600 | 10500 | 15400 | 15100 |
| HFC-32 | CH2F2 | 5.4 | 2690 | 771 | 220 | 181 | 142 |
| HFC-125 | CHF2CF3 | 30 | 6740 | 3740 | 1110 | 3300 | 1300 |
| HFC-134a | CH2FCF3 | 147 | 4140 | 1530 | 436 | 733 | 306 |
| HFC-143a | CH3CF3 | 51 | 7840 | 5810 | 1940 | 5910 | 3250 |
| HFC-152a | CH3CHF2 | 1.6 | 591 | 164 | 46.8 | 36.5 | 29.8 |
| HFC-227ea | CF3CHFCF3 | 36 | 5850 | 3600 | 1100 | 3400 | 1490 |
| HFC-236fa | CF3CH2CF3 | 213 | 7450 | 8690 | 6040 | 9200 | 88700 |
| HFC-245fa | CHF2CH2CF3 | 7.9 | 3170 | 962 | 274 | 262 | 180 |
| 全氟化碳(PFCs) | |||||||
| PFC-14 | CF4 | 50000 | 5300 | 7380 | 34100 | 7660 | 9050 |
| PFC-116 | C2F6 | 10000 | 8940 | 12400 | 10600 | 12900 | 15200 |
| 六氟化硫 | SF6 | 3200 | 18300 | 25200 | 17500 | 26200 | 30600 |
| 三氟化氮 | NF3 | 569 | 13400 | 17400 | 18200 | 18200 | 20000 |
| 材料 | 选择性 | 吸附容量/ (cm3 CH4/g) | 压力/bar | 温度/ K | 文献 |
|---|---|---|---|---|---|
| Ni(ina)2 | 15.8① | 40.8 | 1 | 298 | [ |
| Al-CDC | 13.0① | 29.24 | 1 | 298 | [ |
| Co(C4O2)2(OH)2 | 12.5① | 8.26 | 1 | 298 | [ |
| Al-Fum | 11.7① | 25.54 | 1 | 298 | [ |
| SB-MOF | 11.5① | 26.15 | 1 | 298 | [ |
| Cu-MOF | 11.0① | 13.70 | 1 | 298 | [ |
| ATC-Cu | 9.0① | 62.5 | 1 | 298 | [ |
| Ni(3-ain)2 | 9.0① | 46.7 | 1 | 298 | [ |
| MIL-160 | 8.9① | 10.53 | 1 | 298 | [ |
| Cu(INA)2 | 8.3① | 16.07 | 1 | 298 | [ |
| CAU-10-H | 7.2① | 16.58 | 1 | 298 | [ |
| MIL-53 | 7.1① | 12.77 | 1 | 298 | [ |
| Zn(CH3COO)2·H2O | 7.0① | 24.64 | 1 | 298 | [ |
| Ni-BPZ | 6.6① | 34.94 | 1 | 298 | [ |
| [Ni(HCOO)6] | 6.1① | 17.47 | 1 | 298 | [ |
| ZIF-8 | 5.2 | 90.05 | 1 | 196 | [ |
| UiO-66-Br2 | 5.1① | 11.41 | 1 | 298 | [ |
| [Co(HCOO)6] | 5.1① | 10.98 | 1 | 298 | [ |
| Cu(OTf) 2D | 4.8① | 5.7 | 1 | 298 | [ |
| Ni(2-ain)2 | 4.2① | 6.8 | 1 | 298 | [ |
| MOF-177 | 4.0① | 12.28 | 1 | 298 | [ |
| Ni-MOF-74 | 3.8① | 31.74 | 1 | 298 | [ |
| HKUST-1 | 3.7① | 18.35 | 1 | 298 | [ |
| Cu(OTf) 3D | 3.2① | 7.9 | 1 | 298 | [ |
| MIL-100(Cr) | 3① | 12.32 | 1 | 298 | [ |
| MIL-100(V) | 3① | 4.98 | 1 | 298 | [ |
| Co-MOF-74 | 3① | 28.57 | 1 | 298 | [ |
| Mg-MOF-74 | 1.5① | 33.86 | 1 | 298 | [ |
| Ni(pba)2 | 1.6① | 17.1 | 1 | 298 | [ |
| MOF-5 | 1.1① | 1.72 | 1 | 298 | [ |
| V2Cl2.8(btdd) | >20② | 42.56 | 1 | 298 | [ |
| MIL-101(Cr) | 5~10② | 21.28(1 bar) | 0.1~10 | 283 | [ |
| MIL-100(Cr) | 8② | 35.84 | 1 | 293 | [ |
| TYUT-96Cr | 4.6② | 约25 | 1 | 298 | [ |
表2 MOFs对CH4/N2的分离性能
Table 2 MOFs separation performance for CH4/N2
| 材料 | 选择性 | 吸附容量/ (cm3 CH4/g) | 压力/bar | 温度/ K | 文献 |
|---|---|---|---|---|---|
| Ni(ina)2 | 15.8① | 40.8 | 1 | 298 | [ |
| Al-CDC | 13.0① | 29.24 | 1 | 298 | [ |
| Co(C4O2)2(OH)2 | 12.5① | 8.26 | 1 | 298 | [ |
| Al-Fum | 11.7① | 25.54 | 1 | 298 | [ |
| SB-MOF | 11.5① | 26.15 | 1 | 298 | [ |
| Cu-MOF | 11.0① | 13.70 | 1 | 298 | [ |
| ATC-Cu | 9.0① | 62.5 | 1 | 298 | [ |
| Ni(3-ain)2 | 9.0① | 46.7 | 1 | 298 | [ |
| MIL-160 | 8.9① | 10.53 | 1 | 298 | [ |
| Cu(INA)2 | 8.3① | 16.07 | 1 | 298 | [ |
| CAU-10-H | 7.2① | 16.58 | 1 | 298 | [ |
| MIL-53 | 7.1① | 12.77 | 1 | 298 | [ |
| Zn(CH3COO)2·H2O | 7.0① | 24.64 | 1 | 298 | [ |
| Ni-BPZ | 6.6① | 34.94 | 1 | 298 | [ |
| [Ni(HCOO)6] | 6.1① | 17.47 | 1 | 298 | [ |
| ZIF-8 | 5.2 | 90.05 | 1 | 196 | [ |
| UiO-66-Br2 | 5.1① | 11.41 | 1 | 298 | [ |
| [Co(HCOO)6] | 5.1① | 10.98 | 1 | 298 | [ |
| Cu(OTf) 2D | 4.8① | 5.7 | 1 | 298 | [ |
| Ni(2-ain)2 | 4.2① | 6.8 | 1 | 298 | [ |
| MOF-177 | 4.0① | 12.28 | 1 | 298 | [ |
| Ni-MOF-74 | 3.8① | 31.74 | 1 | 298 | [ |
| HKUST-1 | 3.7① | 18.35 | 1 | 298 | [ |
| Cu(OTf) 3D | 3.2① | 7.9 | 1 | 298 | [ |
| MIL-100(Cr) | 3① | 12.32 | 1 | 298 | [ |
| MIL-100(V) | 3① | 4.98 | 1 | 298 | [ |
| Co-MOF-74 | 3① | 28.57 | 1 | 298 | [ |
| Mg-MOF-74 | 1.5① | 33.86 | 1 | 298 | [ |
| Ni(pba)2 | 1.6① | 17.1 | 1 | 298 | [ |
| MOF-5 | 1.1① | 1.72 | 1 | 298 | [ |
| V2Cl2.8(btdd) | >20② | 42.56 | 1 | 298 | [ |
| MIL-101(Cr) | 5~10② | 21.28(1 bar) | 0.1~10 | 283 | [ |
| MIL-100(Cr) | 8② | 35.84 | 1 | 293 | [ |
| TYUT-96Cr | 4.6② | 约25 | 1 | 298 | [ |
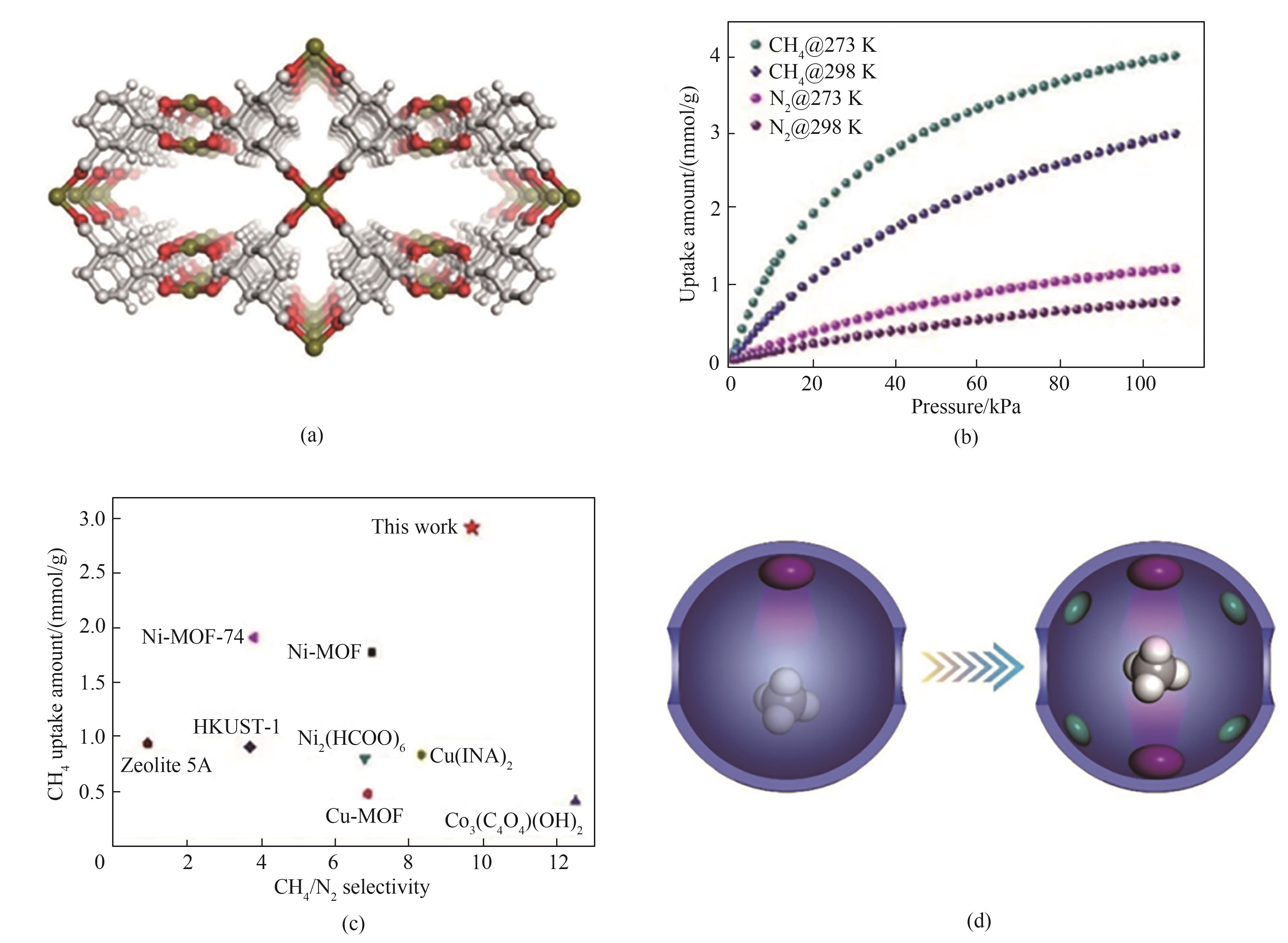
图1 ATC-Cu的晶体结构(a);ATC-Cu对CH4和N2的吸附等温线(b);在298 K、1 bar下各材料与ATC-Cu的吸附容量与选择性对比(c);普通CH4吸附剂与纳米阱的对比(d)[33]
Fig.1 Illustration of the crystal structure of ATC-Cu (a); The methane and nitrogen adsorption isotherm for ATC-Cu at 273 and 298 K (b); The CH4/N2 selectivity for high-performance materials at 1 bar and 298 K (c); The comparison of traditional methane adsorbent and nano-trap (d)[33]
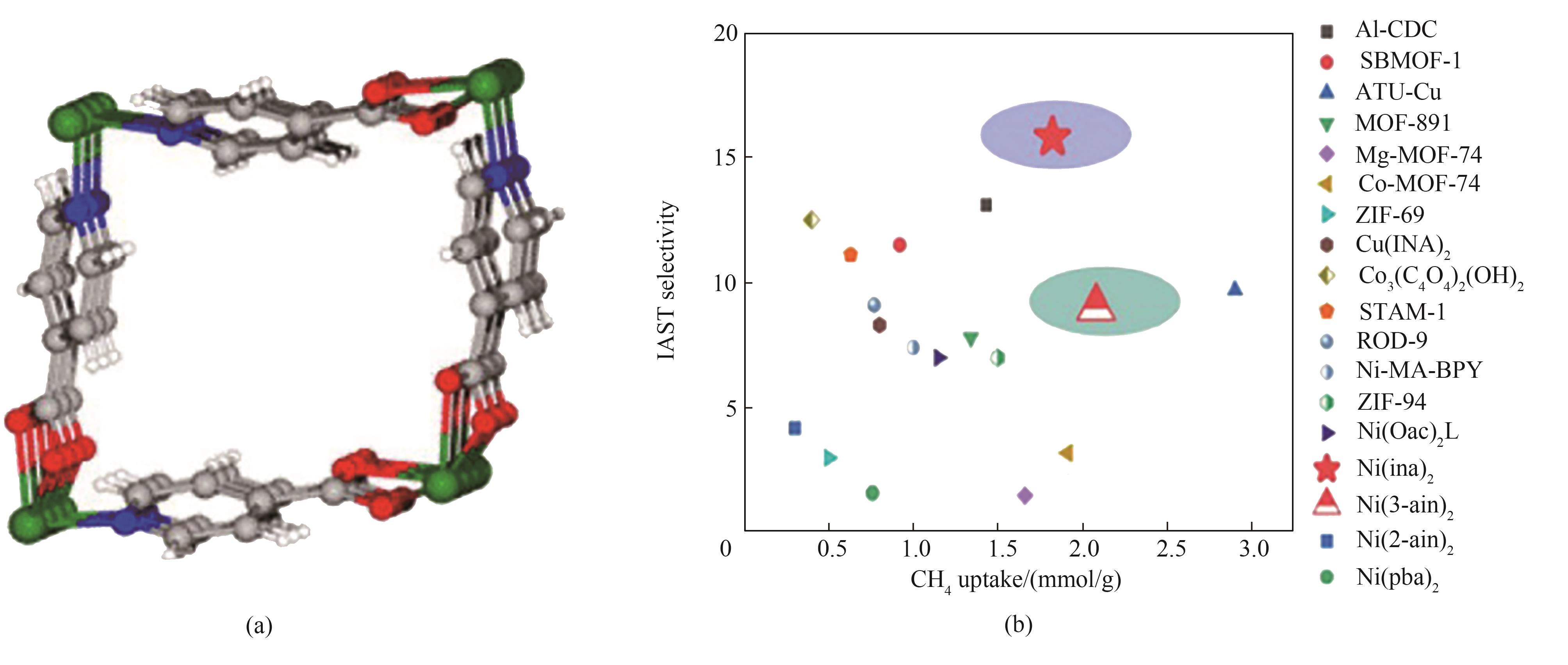
图 2 Ni(ina)2的配体与结构模型(a);在298 K、1 bar下各材料与Al-CDC的吸附容量与选择性对比(b) [27]
Fig.2 Illustration of the crystal structure of Ni(ina)2 (a); Comparison of IAST selectivity of CH4 uptake for previously reported MOFs with Al-CDC (b)[27]
| 分子 | 动力学直径/Å | 偶极矩/ | 极化率/(C·m²/V) | 四极矩/m2 |
|---|---|---|---|---|
| N2O | 3.30 | 0.17 | 3.08 | 3 |
| CO2 | 3.30 | 0 | 2.93 | 4.3 |
| N2 | 3.64 | 0 | 1.74 | 1.4 |
表3 N2O排放组分的物理性质[56]
Table 3 Physical properties of N2O emission components[56]
| 分子 | 动力学直径/Å | 偶极矩/ | 极化率/(C·m²/V) | 四极矩/m2 |
|---|---|---|---|---|
| N2O | 3.30 | 0.17 | 3.08 | 3 |
| CO2 | 3.30 | 0 | 2.93 | 4.3 |
| N2 | 3.64 | 0 | 1.74 | 1.4 |
| 材料 | N2O/CO2选择性 | N2O吸附容量/(ml/g) | 压力/bar | 温度/K | 文献 |
|---|---|---|---|---|---|
| MIL-101(Cr)-NH2 | 1.91 | 113.76 | 1 | 298 | [ |
| MIL-100(Fe) | 1.84 | 105.27 | 1 | 298 | [ |
| ZIF-7 | 1.4~1.7 | 56 | 1 | 298 | [ |
| MIL-101(Cr) | 1.49 | 122 | 1 | 298 | [ |
| ZIF-8 | 1.30 | 31.1 | 1 | 298 | [ |
| MIL-101(Cr)-Br | 1.30 | 57.78 | 1 | 298 | [ |
| UiO-66 | 1.29 | 96.9 | 1 | 298 | [ |
| HKUST-1 | 1.20 | 87.6 | 1 | 298 | [ |
| NU-1000-PhTz | 1.10 | 35.8 | 1 | 298 | [ |
| MIL-101(Cr)-NO2 | 1.02 | 53.31 | 1 | 298 | [ |
| MIL-100(Cr) | 1 | 129.4 | 1 | 298 | [ |
| MOF-5 | — | 20.4 | 1 | 298 | [ |
| Ni-MOF | — | 63 | 1 | 298 | [ |
| ELM-11 | — | 1.46 | 1 | 298 | [ |
| ELM-12 | — | 19.26 | 1 | 298 | [ |
| MIL-53(Al) | — | 60.48 | 1 | 298 | [ |
表4 MOFs对N2O/CO2的分离性能
Table 4 MOFs separation performance for N2O/CO2
| 材料 | N2O/CO2选择性 | N2O吸附容量/(ml/g) | 压力/bar | 温度/K | 文献 |
|---|---|---|---|---|---|
| MIL-101(Cr)-NH2 | 1.91 | 113.76 | 1 | 298 | [ |
| MIL-100(Fe) | 1.84 | 105.27 | 1 | 298 | [ |
| ZIF-7 | 1.4~1.7 | 56 | 1 | 298 | [ |
| MIL-101(Cr) | 1.49 | 122 | 1 | 298 | [ |
| ZIF-8 | 1.30 | 31.1 | 1 | 298 | [ |
| MIL-101(Cr)-Br | 1.30 | 57.78 | 1 | 298 | [ |
| UiO-66 | 1.29 | 96.9 | 1 | 298 | [ |
| HKUST-1 | 1.20 | 87.6 | 1 | 298 | [ |
| NU-1000-PhTz | 1.10 | 35.8 | 1 | 298 | [ |
| MIL-101(Cr)-NO2 | 1.02 | 53.31 | 1 | 298 | [ |
| MIL-100(Cr) | 1 | 129.4 | 1 | 298 | [ |
| MOF-5 | — | 20.4 | 1 | 298 | [ |
| Ni-MOF | — | 63 | 1 | 298 | [ |
| ELM-11 | — | 1.46 | 1 | 298 | [ |
| ELM-12 | — | 19.26 | 1 | 298 | [ |
| MIL-53(Al) | — | 60.48 | 1 | 298 | [ |
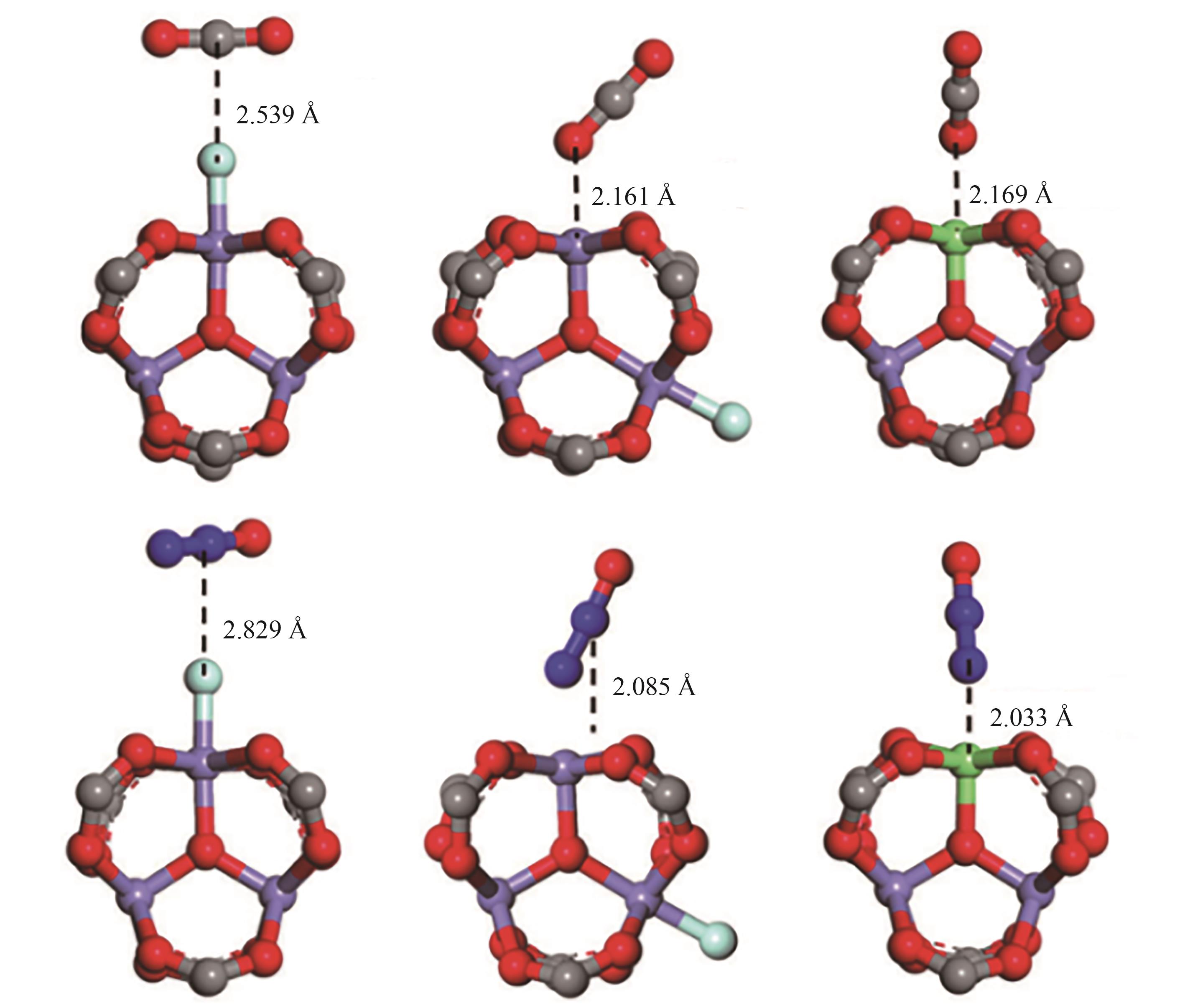
图3 DFT计算的CO2与N2O在Fe3+-F-,开放Fe3+位点和开放Fe2+位点上的作用模型[60]紫色—三价铁; 绿色—二价铁; 灰色—碳; 深蓝色—氮; 红色—氧; 浅蓝色—氟
Fig.3 The DFT-calculated adsorption configurations of CO2 and N2O on the Fe3+-F–, open-Fe3+ site and open Fe2+ sites in MIL-100(Fe), using a cluster model[60]
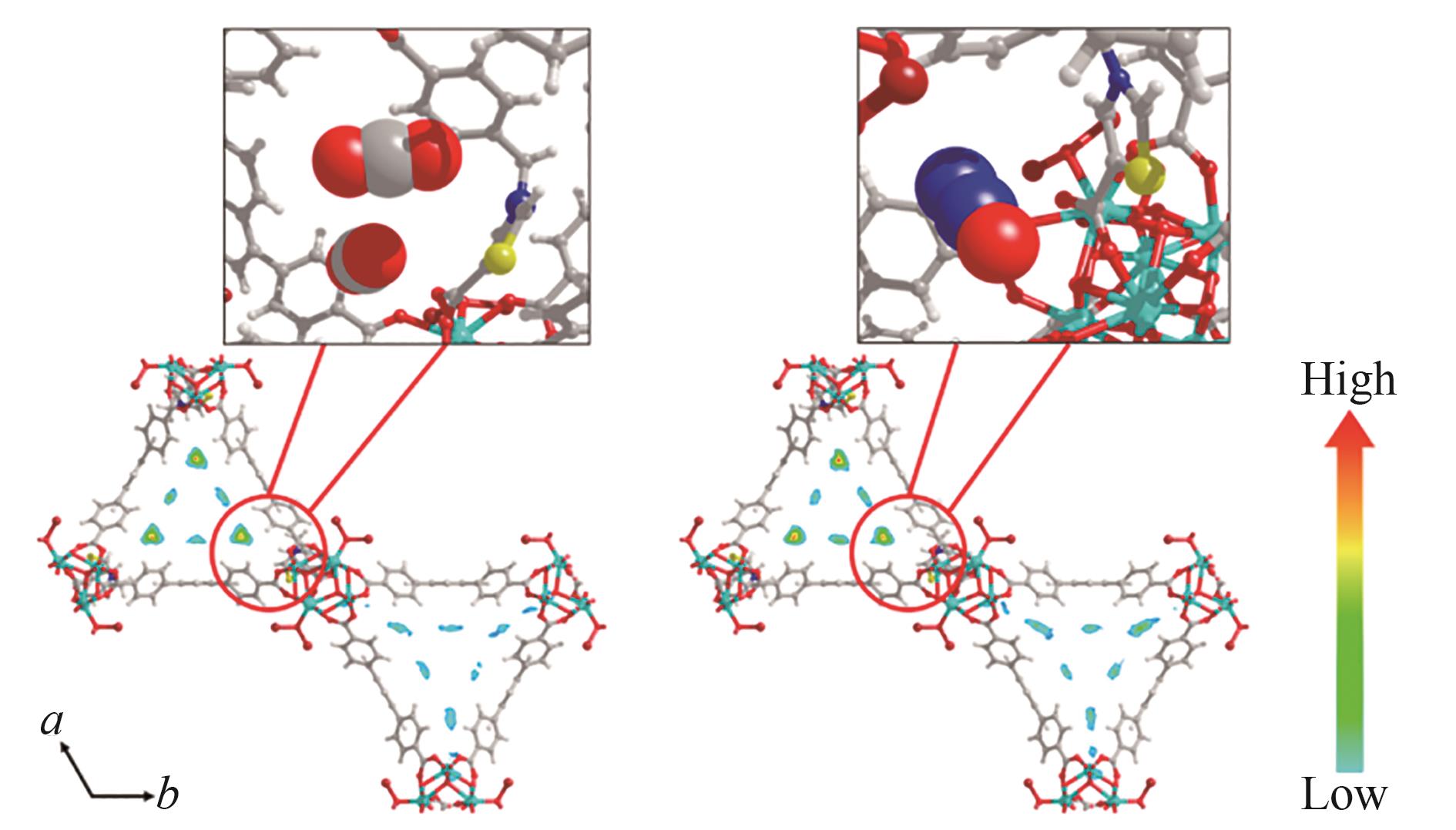
图4 在298 K和1 bar时,NU-1000-PhTz中吸附的CO2和N2的质量中心概率密度等值线图[57]
Fig.4 Contour plots of the center-of-mass probability densities of adsorbed CO2 and N2O in NU-1000-PhTz at 298 K and 1 bar[57]

图5 通过pcu拓扑基本单元的孔径和关键参数的变化来说明LIFM-W2中骨架的3D变化对不同芳香族客体的响应(a);R22和R134a在273 K和298 K时的吸附等温线[(b)、(c)];使用LIFM-W2在298 K时N2/R22/R134a混合气体(99.8∶0.01∶0.01,体积比)的柱穿透曲线(d)[23]
Fig.5 Representative 3D variations of the framework in LIFM-W2 responsive to different aromatic guests, illustrated by the changes of pore size and key parameters of the basic unit of the topologically simplified pcu net (a);R22 and R134a uptakes at 273 K and 298 K [(b),(c)];Column breakthrough curves of N2/R22/R134a mixed gases (99.8 : 0.01 : 0.01, volume ratio) at 298 K by using LIFM-W2 (d) [23]
| 材料 | SF6/N2选择性 | SF6吸附容量/(cm3/g) | 压力/bar | 温度/K | 文献 |
|---|---|---|---|---|---|
| Ni(NDC)(TED)0.5 | 750 | 61.86 | 1 | 298 | [ |
| SBMOF-1 | 727 | 22.8 | 1 | 298 | [ |
| Ni(ina)2 | 375.1 | 63.7 | 1 | 298 | [ |
| Zn-MOF-74 | 313 | 85.12 | 1 | 298 | [ |
| Cu-MOF-NH2 | 266 | 176.51 | 1 | 298 | [ |
| Ni(pba)2 | 200.6 | 78.5 | 1 | 298 | [ |
| Co-MOF-74 | 52.9 | 116.48 | 1 | 298 | [ |
| UU-200 | 44.81 | 24.69 | 1 | 298 | [ |
| UiO-67 | 37 | 133.6 | 1 | 298 | [ |
| Mg-MOF-74 | 32.9 | 134.4 | 1 | 298 | [ |
| Cu(peba)2 | 18.2 | 52.8 | 1 | 298 | [ |
| MIL-101 | — | 276 | 18 | 298 | [ |
| Cu3(BTC)2 | — | 107.07 | 1 | 298 | [ |
| Co2(1,4-bdc)2(dabco) | — | 75.94 | 1 | 298 | [ |
| Zn4O(btb) | — | 69.89 | 1 | 298 | [ |
| MIL-100(Fe) | — | 65.86 | 1 | 298 | [ |
| Zn4O(dmcpz)3 | — | 56.9 | 1 | 298 | [ |
| DUT-9 | — | 52 | 18 | 298 | [ |
表5 MOFs对SF6/N2分离性能
Table 5 MOFs separation performance for SF6/N2
| 材料 | SF6/N2选择性 | SF6吸附容量/(cm3/g) | 压力/bar | 温度/K | 文献 |
|---|---|---|---|---|---|
| Ni(NDC)(TED)0.5 | 750 | 61.86 | 1 | 298 | [ |
| SBMOF-1 | 727 | 22.8 | 1 | 298 | [ |
| Ni(ina)2 | 375.1 | 63.7 | 1 | 298 | [ |
| Zn-MOF-74 | 313 | 85.12 | 1 | 298 | [ |
| Cu-MOF-NH2 | 266 | 176.51 | 1 | 298 | [ |
| Ni(pba)2 | 200.6 | 78.5 | 1 | 298 | [ |
| Co-MOF-74 | 52.9 | 116.48 | 1 | 298 | [ |
| UU-200 | 44.81 | 24.69 | 1 | 298 | [ |
| UiO-67 | 37 | 133.6 | 1 | 298 | [ |
| Mg-MOF-74 | 32.9 | 134.4 | 1 | 298 | [ |
| Cu(peba)2 | 18.2 | 52.8 | 1 | 298 | [ |
| MIL-101 | — | 276 | 18 | 298 | [ |
| Cu3(BTC)2 | — | 107.07 | 1 | 298 | [ |
| Co2(1,4-bdc)2(dabco) | — | 75.94 | 1 | 298 | [ |
| Zn4O(btb) | — | 69.89 | 1 | 298 | [ |
| MIL-100(Fe) | — | 65.86 | 1 | 298 | [ |
| Zn4O(dmcpz)3 | — | 56.9 | 1 | 298 | [ |
| DUT-9 | — | 52 | 18 | 298 | [ |
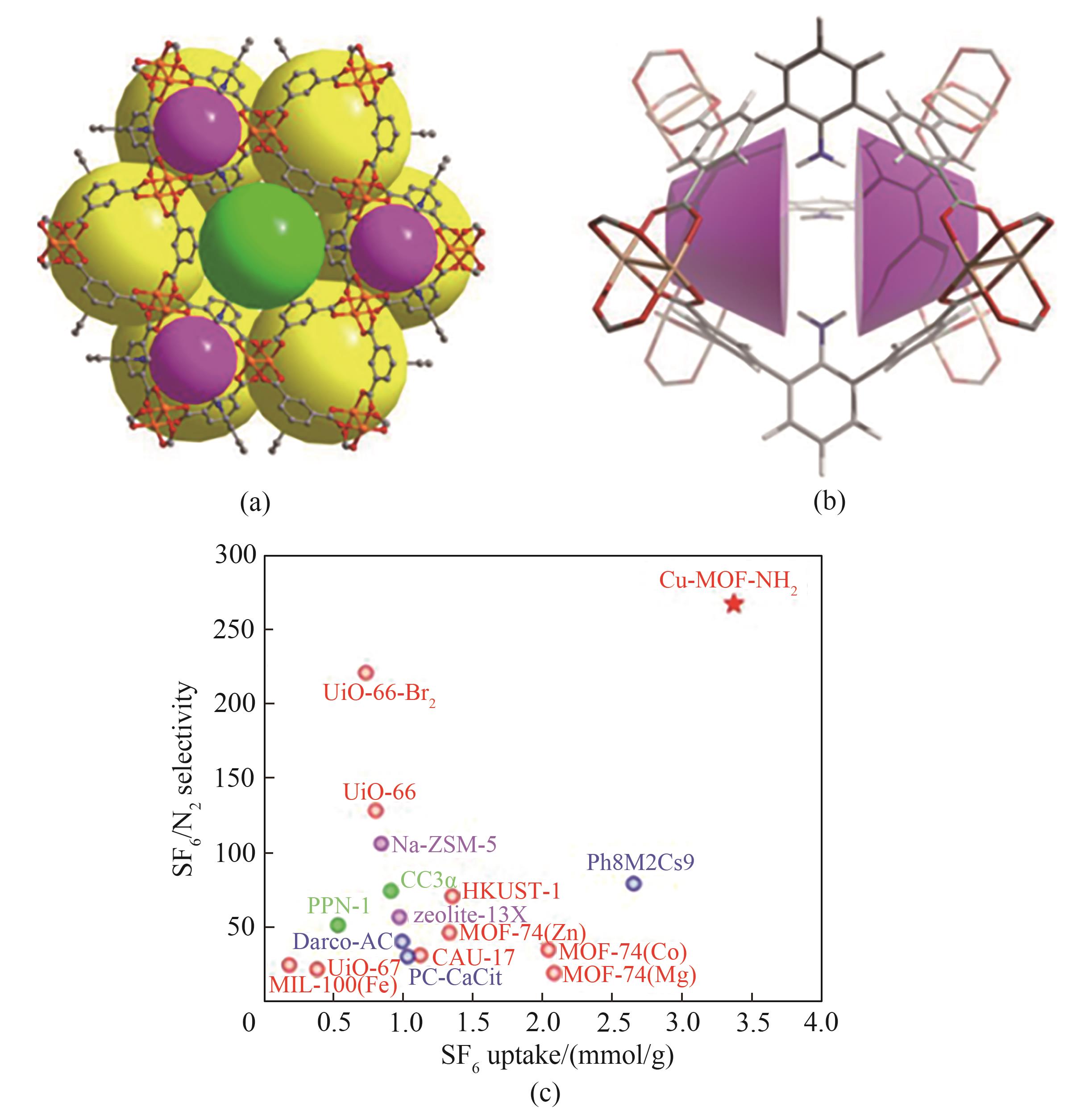
图6 Cu-MOF-NH2的结构模型,金属桨轮单元[Cu2(CO2)4]和弯曲的NH2-TPTC连接器组成(a);Cu-MOF-NH2空腔中杯芳烃类似微环境的表征(b);0.1 bar下SF6吸附容量及298 K和1.0 bar下预测的气体混合物(SF6∶N2=10∶90)的选择性(c)[87]
Fig.6 Illustration of the identified top-performers MOF hereafter labeled Cu-MOF-NH2 consisting of dimeric metal-paddlewheel units [Cu2(CO2)4] and bent NH2-TPTC linkers (a);Representation of the calix arene-analogous microenvironments present in the cavity of Cu-MOF-NH2 (b); The SF6 uptake as a single component at 0.1 bar and the IAST selectivity predicted at 298 K and 1.0 bar for the gas mixture (SF6∶N2=10∶90), respectively (c)[87]

图7 Cu(peba)2、Ni(pba)2和Ni(ina)2的结构模型(a); 三种材料在298 K下的SF6和N2吸附等温线[(b)~(d)]; 使用IAST计算三种MOF在298 K下(SF6/N2体积比1/9)的SF6/N2选择性(e); 比较298 K和1 bar下的SF6/N2 (1/9) IAST选择性,以及Ni(ina)2、Ni(pba)2、Cu(peba)2和其他高性能材料在298 K和0.1 bar下的SF6吸附容量(f); Ni(ina)2中SF6吸附结合位点的计算(g) [79]
Fig.7 The pore structures of Cu(peba)2, Ni(pba)2 and Ni(ina)2 (a); SF6 and N2 adsorption isotherms of the three materials at 298 K [(b)-(d)]; SF6/N2 selectivity of the three MOFs calculated at 298 K (SF6/N2 volume ratio 1/9) using IAST (e); Comparison of SF6/N2 (1/9) IAST selectivities at 298 K and 1 bar and SF6 uptakes at 298 K and 0.1 bar in Ni(ina)2, Ni(pba)2, Cu(peba)2 and other top-performance materials (f); The calculated adsorption binding sites of SF6 in Ni(ina)2 (g)[79]
| 1 | Sesana E, Gagnon A S, Ciantelli C, et al. Climate change impacts on cultural heritage: a literature review[J]. WIREs Climate Change, 2021, 12(4): e710. |
| 2 | Hansen J, Sato M, Hearty P, et al. Ice melt, sea level rise and superstorms: evidence from paleoclimate data, climate modeling, and modern observations that 2℃ global warming could be dangerous[J]. Atmospheric Chemistry and Physics, 2016, 16(6): 3761-3812. |
| 3 | Zarch A E, Ahmadi H, Moeini A, et al. Impacts of environmental and human factors on desertification-induced land degradation in arid areas[J]. Arabian Journal of Geosciences, 2021, 14(22): 2447-2458. |
| 4 | Grünig M, Calanca P, Mazzi D, et al. Inflection point in climatic suitability of insect pest species in Europe suggests non‐linear responses to climate change[J]. Global Change Biology, 2020, 26(11): 6338-6349. |
| 5 | 章诚, 郑玉洁, 凌红. 中国的“双碳”目标与实践: 形成逻辑、现实挑战、社会风险及推进进路[J]. 河海大学学报(哲学社会科学版), 2022, 24(6): 78-87. |
| Zhang C, Zheng Y J, Ling H. China’s “dual carbon” goal and practices: forming logic, realistic challenges, social risks and advancing route[J]. Journal of Hohai University (Philosophy and Social Sciences), 2022, 24(6): 78-87. | |
| 6 | United Nations framework convention on climate change Kyoto Protocol [EB/OL]. 1997.[2022-07-30]. . |
| 7 | Cao J J, Zeng N, Liu Y, et al. Preface to the special issue on carbon neutrality: important roles of renewable energies, carbon sinks, NETs, and non-CO2 GHGs[J]. Advances in Atmospheric Science, 2022, 39: 1207-1208. |
| 8 | Intergovernmental Panel on Climate Change. AR6 synthesis report: climate change 2022[R]. IPCC, 2022. |
| 9 | Duda A, Valverde G F. Environmental and safety risks related to methane emissions in underground coal mine closure processes[J]. Energies, 2020, 13(23): 6312-6328. |
| 10 | Hönemann C, Kim S C. Please stop using nitrous oxide in routine clinical practice (comment on: use of nitrous oxide in contemporary anesthesia—an ongoing tug of war)[J]. Canadian Journal of Anaesthesia, 2021, 69(2): 271-272. |
| 11 | Liu X, Zhu Y, Liu T, et al. Exploring toxicity of perfluorinated compounds through complex network and pathway modeling[J]. Journal of Biomolecular Structure and Dynamics, 2020, 38(9): 2604-2612. |
| 12 | Lin R B, Wu H, Li L, et al. Boosting ethane/ethylene separation within isoreticular ultramicroporous metal-organic frameworks[J]. Journal of the American Chemical Society, 2018, 140(40): 12940-12946. |
| 13 | Zhu B, Cao J W, Mukherjee S, et al. Pore engineering for one-step ethylene purification from a three-component hydrocarbon mixture[J]. Journal of the American Chemical Society, 2021, 143(3): 1485-1492. |
| 14 | Fracaroli A M, Furukawa H, Suzuki M, et al. Metal-organic frameworks with precisely designed interior for carbon dioxide capture in the presence of water[J]. Journal of the American Chemical Society, 2014, 136(25): 8863-8866. |
| 15 | Herm Z R, Swisher J A, Smit B, et al. Metal-organic frameworks as adsorbents for hydrogen purification and precombustion carbon dioxide capture[J]. Journal of the American Chemical Society, 2011, 133(15): 5664-5667. |
| 16 | D’alessandro D M, Smit B, Long J R. Carbon dioxide capture: prospects for new materials[J]. Angewandte Chemie International Edition, 2010, 49(35): 6058-6082. |
| 17 | Borchardt L, Zhu Q L, Casco M E, et al. Toward a molecular design of porous carbon materials[J]. Materials Today, 2017, 20(10): 592-610. |
| 18 | Zhang X, Lin R B, Wang J, et al. Optimization of the pore structures of MOFs for record high hydrogen volumetric working capacity[J]. Advanced Materials, 2020, 32(17) :1907995. |
| 19 | Wang B, Lv X L, Feng D, et al. Highly stable Zr(Ⅳ)-based metal-organic frameworks for the detection and removal of antibiotics and organic explosives in water[J]. Journal of the American Chemical Society, 2016, 138(19): 6204-6216. |
| 20 | Wang H T, Chen Q, Zhang X, et al. Two isostructural metal-organic frameworks with unique nickel clusters for C2H2/C2H6/C2H4 mixture separation[J]. Journal of Materials Chemistry A, 2022, 10(23): 12497-12502. |
| 21 | Xin Z, Wang Y R, Chen Y, et al. Metallocene implanted metalloporphyrin organic framework for highly selective CO2 electroreduction[J]. Nano Energy, 2020, 67: 30940-30941. |
| 22 | Wang M, Guo L, Cao D. Amino-functionalized luminescent metal-organic framework test paper for rapid and selective sensing of SO2 gas and its derivatives by luminescence turn-on effect[J]. Analytical Chemistry, 2018, 90(5): 3608-3614. |
| 23 | Wang W, Xiong X H, Zhu N X, et al. A rare flexible metal-organic framework based on a tailorable Mn8-cluster showing smart responsiveness to aromatic guests and capacity for gas separation[J]. Angewandte Chemie International Edition, 2022, 61(26): e202201766. |
| 24 | Cui X L, Xing H B, Yang Q W, et al. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene[J]. Science, 2016, 353(6295): 141-144. |
| 25 | Lin J B, Ramanathan V, Jake B, et al. A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture[J]. Science 2021, 374(6574): 1464-1469. |
| 26 | Zhang X, Li J R. Recovery of greenhouse gas as cleaner fossil fuel contributes to carbon neutrality[J]. Green Energy & Environment, 2022, doi:10.1016/j.gee.2022.06.002 . |
| 27 | Wang S M, Shivanna M, Yang Q Y. Nickel-based metal-organic frameworks for coal-bed methane purification with record CH4/N2 selectivity[J]. Angewandte Chemie International Edition, 2022, 61(15): e202201017. |
| 28 | Chang M, Zhao Y, Liu D, et al. Methane-trapping metal-organic frameworks with an aliphatic ligand for efficient CH4/N2 separation[J]. Sustainable Energy & Fuels, 2020, 4(1): 138-142. |
| 29 | Li L, Yang L, Wang J, et al. Highly efficient separation of methane from nitrogen on a squarate-based metal-organic framework[J]. AIChE Journal, 2018, 64(10): 3681-3689. |
| 30 | Huang Z, Hu P, Liu J, et al. Enhancing CH4/N2 separation performance within aluminum-based metal-organic frameworks: influence of the pore structure and linker polarity[J]. Separation and Purification Technology, 2022, 286: 120446. |
| 31 | Chang M, Ren J, Yang Q, et al. A robust calcium-based microporous metal-organic framework for efficient CH4/N2 separation[J]. Chemical Engineering Journal, 2021, 408: 127294-127301. |
| 32 | Chang M, Zhao Y, Yang Q, et al. Microporous metal-organic frameworks with hydrophilic and hydrophobic pores for efficient separation of CH4/N2 mixture[J]. ACS Omega, 2019, 4(11): 14511-14516. |
| 33 | Niu Z, Cui X, Pham T, et al. A metal-organic framework based methane nano-trap for the capture of coal-mine methane[J]. Angewandte Chemie International Edition, 2019, 58(30): 10138-10141. |
| 34 | Hu J, Sun T, Liu X, et al. Separation of CH4/N2 mixtures in metal-organic frameworks with 1D micro-channels[J]. RSC Advances, 2016, 6(68): 64039-64046. |
| 35 | Kim T H, Kim S Y, Yoon T U, et al. Improved methane/nitrogen separation properties of zirconium-based metal-organic framework by incorporating highly polarizable bromine atoms[J]. Chemical Engineering Journal, 2020, 399: 125717. |
| 36 | Chen Y, Wu H, Yuan Y, et al. Highly rapid mechanochemical synthesis of a pillar-layer metal-organic framework for efficient CH4/N2 separation[J]. Chemical Engineering Journal, 2020, 385:123836. |
| 37 | Tu S, Yu L, Lin D, et al. Robust nickel-based metal-organic framework for highly efficient methane purification and capture[J]. ACS Applied Materials Interfaces, 2022, 14(3): 4242-4250. |
| 38 | Ren X, Sun T, Hu J, et al. Highly enhanced selectivity for the separation of CH4 over N2 on two ultra-microporous frameworks with multiple coordination modes[J]. Microporous and Mesoporous Materials, 2014, 186: 137-145. |
| 39 | Eyer S, Stadie N P, Borgschulte A, et al. Methane preconcentration by adsorption: a methodology for materials and conditions selection[J]. Adsorption, 2014, 20(5/6): 657-666. |
| 40 | Wang X, Li L, Yang J, et al. CO2/CH4 and CH4/N2 separation on isomeric metal organic frameworks[J]. Chinese Journal of Chemical Engineering, 2016, 24(12): 1687-1694. |
| 41 | Dipeneu S, Fengjia, Deng S G. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A[J]. Environmental Science Technology, 2010, 44(5): 1820-1826. |
| 42 | Li L, Yang J, Li J, et al. Separation of CO2 /CH4 and CH4 /N2 mixtures by M/DOBDC: a detailed dynamic comparison with MIL-100(Cr) and activated carbon[J]. Microporous and Mesoporous Materials, 2014, 198: 236-246. |
| 43 | Wang Q M, Martin B, Lau M L, et al. Metallo-organic molecular sieve for gas separation and purification[J]. Microporous and Mesoporous Materials, 2002, 55: 217-230. |
| 44 | Yang J, Wang Y, Li L, et al. Protection of open-metal V(Ⅲ) sites and their associated CO2/CH4/N2/O2/H2O adsorption properties in mesoporous V-MOFs[J]. Journal of Colloid Interface Science, 2015, 456: 197-205. |
| 45 | Jaramillo D E, Reed D A, Jiang H Z H, et al. Selective nitrogen adsorption via backbonding in a metal-organic framework with exposed vanadium sites[J]. Nature Materials, 2020, 19(5): 517-521. |
| 46 | Pillai R S, Yoon J W, Lee S J, et al. N2 capture performances of the hybrid porous MIL-101(Cr): from prediction toward experimental testing[J]. The Journal of Physical Chemistry C, 2017, 121(40): 22130-22138. |
| 47 | Yoon J W, Chang H, Lee S J, et al. Selective nitrogen capture by porous hybrid materials containing accessible transition metal ion sites[J]. Nature Materials, 2017, 16(5): 526-531. |
| 48 | Zhang F, Li K, Chen J, et al. Efficient N2/CH4 separation in a stable metal-organic framework with high density of open Cr sites[J]. Separation and Purification Technology, 2022, 281: 119951. |
| 49 | Möllmer J, Lange M, Möller A, et al. Pure and mixed gas adsorption of CH4 and N2 on the metal-organic framework Basolite® A100 and a novel copper-based 1,2,4-triazolyl isophthalate MOF[J]. Journal of Materials Chemistry, 2012, 22(20): 10274-10286. |
| 50 | Wu X, Yuan B, Bao Z, et al. Adsorption of carbon dioxide, methane and nitrogen on an ultramicroporous copper metal-organic framework[J]. Journal of Colloid Interface Science, 2014, 430: 78-84. |
| 51 | Li J R, Li M, Zhou P X, et al. ROD-8, a rod MOF with a pyrene-cored tetracarboxylate linker: framework disorder, derived nets and selective gas adsorption[J]. CrystEngComm, 2014, 16(28): 6291-6295. |
| 52 | Lv D, Wu Y, Chen J, et al. Improving CH4/N2 selectivity within isomeric Al‐based MOFs for the highly selective capture of coal‐mine methane[J]. AIChE Journal, 2020, 66(9): e16287. |
| 53 | Wu Y, Yuan D, He D, et al. Decorated traditional zeolites with subunits of metal-organic frameworks for CH4 /N2 separation[J]. Angewandte Chemie International Edition, 2019, 58(30): 10241-10244. |
| 54 | Qu Z G, Wang H, Zhang W. Highly efficient adsorbent design using a Cu-BTC/CuO/carbon fiber paper composite for high CH4/N2 selectivity[J]. RSC Advances, 2017, 7(23): 14206-14218. |
| 55 | Buhre W, Disma N, Hendrickx J, et al. European society of anaesthesiology task force on nitrous oxide: a narrative review of its role in clinical practice[J]. British Journal of Anaesthesia, 2019, 122(5): 587-604. |
| 56 | Li J R, Kuppler R J, Zhou H C. Selective gas adsorption and separation in metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1477-1504. |
| 57 | Mercuri G, Moroni M, Galli S, et al. Temperature-dependent nitrous oxide/carbon dioxide preferential adsorption in a thiazolium-functionalized NU-1000 metal-organic framework[J]. ACS Applied Materials Interfaces, 2021, 13(49): 58982-58993. |
| 58 | Ma L, Zhang F, Li K, et al. Improved N2O capture performance of chromium terephthalate MIL-101 via substituent engineering[J]. Journal of Solid State Chemistry, 2022, 309: 122951. |
| 59 | Yang J, Du B, Liu J, et al. MIL-100Cr with open Cr sites for a record N2O capture[J]. Chemical Communications, 2018, 54(100): 14061-14064. |
| 60 | Wang L, Li Y, Wang Y, et al. Research on CO2-N2O separation using flexible metal organic frameworks[J]. Separation and Purification Technology, 2020, 251: 117311. |
| 61 | Chen D L, Wang N, Wang F F, et al. Utilizing the gate-opening mechanism in ZIF-7 for adsorption discrimination between N2O and CO2 [J]. The Journal of Physical Chemistry C, 2014, 118(31): 17831-17837. |
| 62 | Wang L, Zhang F, Wang C, et al. Ethylenediamine-functionalized metal organic frameworks MIL-100(Cr) for efficient CO2/N2O separation[J]. Separation and Purification Technology, 2020, 235: 116219. |
| 63 | Zhang X, Chen W, Shi W, et al. Highly selective sorption of CO2 and N2O and strong gas-framework interactions in a nickel(Ⅱ) organic material[J]. Journal of Materials Chemistry A, 2016, 4(41): 16198-16204. |
| 64 | Wang L, Zhang F, Yang J, et al. The efficient separation of N2O/CO2 using unsaturated Fe2+ sites in MIL-100(Fe)[J]. Chemical Communications, 2021, 57(54): 6636-6639. |
| 65 | Denysenko D, Jelic J, Magdysyuk O V, et al. Elucidating Lewis acidity of metal sites in MFU-4l metal-organic frameworks: N2O and CO2 adsorption in MFU-4l, CuI-MFU-4l and Li-MFU-4l[J]. Microporous and Mesoporous Materials, 2015, 216: 146-150. |
| 66 | Pan B, Wang G, Shi H, et al. Green gas for grid as an eco-friendly alternative insulation gas to SF6: a review[J]. Applied Sciences, 2020, 10(7): 2526-2539. |
| 67 | Motkuri R K, Annapureddy H V, Vijaykumar M, et al. Fluorocarbon adsorption in hierarchical porous frameworks[J]. Nature Communications, 2014, 5: 4368-4373. |
| 68 | Annapureddy H V, Nune S K, Motkuri R K, et al. A combined experimental and computational study on the stability of nanofluids containing metal organic frameworks[J]. Journal Physical Chemistry B, 2015, 119(29): 8992-8899. |
| 69 | Wanigarathna D, Gao J, Liu B. Fluorocarbon separation in a thermally robust zirconium carboxylate metal-organic framework[J]. Chemistry—An Asian Journal, 2018, 13(8): 977-981. |
| 70 | Chen T H, Popov I, Kaveevivitchai W, et al. Mesoporous fluorinated metal-organic frameworks with exceptional adsorption of fluorocarbons and CFCs[J]. Angewandte Chemie International Edition, 2015, 54(47): 13902-13906. |
| 71 | Xie J, Sun F, Wang C, et al. Stability and hydrocarbon/fluorocarbon sorption of a metal-organic framework with fluorinated channels[J]. Materials (Basel), 2016, 9(5): 327-332. |
| 72 | Wang H, Yu L, Lin Y, et al. Adsorption of fluorocarbons and chlorocarbons by highly porous and robust fluorinated zirconium metal-organic frameworks[J]. Inorganic Chemistry, 2020, 59(7): 4167-4171. |
| 73 | Lee H M, Chang M B, Wu K Y. Abatement of sulfur hexafluoride emissions from the semiconductor manufacturing process by atmospheric-pressure plasmas[J]. Journal of the Air & Waste Management Association, 2004, 54(8): 960-970. |
| 74 | Maiss M, Brenninkmeijer C A M. Atmospheric SF6: trends, sources, and prospects[J]. Environmental Science and Technology, 1998, 32(20): 3077-3086. |
| 75 | Seeger M. Perspectives on research on high voltage gas circuit breakers[J]. Plasma Chemistry and Plasma Processing, 2014, 35(3): 527-541. |
| 76 | Montzka S A, Dlugokencky E J, Butler J H. Non-CO2 greenhouse gases and climate change[J]. Nature, 2011, 476(7358): 43-50. |
| 77 | Yang M, Chang M, Yan T, et al. A nickel-based metal-organic framework for efficient SF6/N2 separation with record SF6 uptake and SF6/N2 selectivity[J]. Separation and Purification Technology, 2022, 295: 121340. |
| 78 | Wang T, Chang M, Yan T, et al. Calcium-based metal-organic framework for efficient capture of sulfur hexafluoride at low concentrations[J]. Industrial & Engineering Chemistry Research, 2021, 60(16): 5976-5983. |
| 79 | Wang S M, Mu X T, Liu H R, et al. Pore-structure control in metal-organic frameworks (MOFs) for capture of the greenhouse gas SF6 with record separation[J]. Angewandte Chemie International Edition, 2022, 61(33): e202207066. |
| 80 | Kim M B, Lee S J, Lee C Y, et al. High SF6 selectivities and capacities in isostructural metal-organic frameworks with proper pore sizes and highly dense unsaturated metal sites[J]. Microporous and Mesoporous Materials, 2014, 190: 356-361. |
| 81 | Ren J, Chang M, Zeng W, et al. Computer-aided discovery of MOFs with calixarene-analogous microenvironment for exceptional SF6 capture[J]. Chemistry of Materials, 2021, 33(13): 5108-5114. |
| 82 | Åhlén M, Kapaca E, Hedbom D, et al. Gas sorption properties and kinetics of porous bismuth-based metal-organic frameworks and the selective CO2 and SF6 sorption on a new bismuth trimesate-based structure UU-200[J]. Microporous and Mesoporous Materials, 2022, 329: 111548. |
| 83 | Kim M B, Kim T H, Yoon T U, et al. Efficient SF6/N2 separation at high pressures using a zirconium-based mesoporous metal-organic framework[J]. Journal of Industrial and Engineering Chemistry, 2020, 84: 179-184. |
| 84 | Senkovska I, Barea E, Navarro J, et al. Adsorptive capturing and storing greenhouse gases such as sulfur hexafluoride and carbon tetrafluoride using metal-organic frameworks[J]. Microporous and Mesoporous Materials, 2012, 156: 115-120. |
| 85 | Chowdhury P, Bikkina C, Meister D, et al. Comparison of adsorption isotherms on Cu-BTC metal organic frameworks synthesized from different routes[J]. Microporous and Mesoporous Materials, 2009, 117(1/2): 406-413. |
| 86 | Chowdhury P, Bikkina C, Sasidhar G. Gas adsorption properties of the chromium-based metal organic framework MIL-101[J]. The Journal of Physical Chemistry C, 2009, 113: 6616-6621. |
| 87 | Cha J, Ga S, Lee S J, et al. Integrated material and process evaluation of metal-organic frameworks database for energy-efficient SF6/N2 separation[J]. Chemical Engineering Journal, 2021, 426: 131787. |
| [1] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [2] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [3] | 朱兴驰, 郭志远, 纪志永, 汪婧, 张盼盼, 刘杰, 赵颖颖, 袁俊生. 选择性电渗析镁锂分离过程模拟优化[J]. 化工学报, 2023, 74(6): 2477-2485. |
| [4] | 顾浩, 张福建, 刘珍, 周文轩, 张鹏, 张忠强. 力电耦合作用下多孔石墨烯膜时间维度的脱盐性能及机理研究[J]. 化工学报, 2023, 74(5): 2067-2074. |
| [5] | 张正, 何永平, 孙海东, 张荣子, 孙正平, 陈金兰, 郑一璇, 杜晓, 郝晓刚. 蛇形流场电控离子交换装置用于选择性提锂[J]. 化工学报, 2023, 74(5): 2022-2033. |
| [6] | 王子健, 柯明, 李佳涵, 李舒婷, 孙巾茹, 童燕兵, 赵治平, 刘加英, 任璐. 短b轴ZSM-5分子筛制备方法及应用研究进展[J]. 化工学报, 2023, 74(4): 1457-1473. |
| [7] | 许万, 陈振斌, 张慧娟, 牛昉昉, 火婷, 刘兴盛. 线性温敏性聚合物嵌段调控的 |
| [8] | 余后川, 任腾, 张宁, 姜晓滨, 代岩, 张晓鹏, 鲍军江, 贺高红. 二维氧化石墨烯膜离子选择性传递调控的研究进展[J]. 化工学报, 2023, 74(1): 303-312. |
| [9] | 闫军营, 王皝莹, 李瑞瑞, 符蓉, 蒋晨啸, 汪耀明, 徐铜文. 选择性电渗析:机遇与挑战[J]. 化工学报, 2023, 74(1): 224-236. |
| [10] | 孙嘉辰, 裴春雷, 陈赛, 赵志坚, 何盛宝, 巩金龙. 化学链低碳烷烃氧化脱氢技术进展[J]. 化工学报, 2023, 74(1): 205-223. |
| [11] | 杨宏欣, 李兴亚, 葛亮, 徐铜文. 含哌啶阳离子侧长链型一/二价阴离子选择性分离膜的制备[J]. 化工学报, 2022, 73(8): 3739-3748. |
| [12] | 王佳铭, 阮雪华, 贺高红. 面向不同工业二氧化碳分离体系的膜材料研究进展[J]. 化工学报, 2022, 73(8): 3417-3432. |
| [13] | 郭丹, 方雨洁, 许一寒, 李致远, 黄守莹, 王胜平, 马新宾. 乙烷和二氧化碳催化转化的研究进展[J]. 化工学报, 2022, 73(8): 3406-3416. |
| [14] | 苏晨昱, 杨颖, 宋兴福. 岩盐矿提钾老卤中溴离子选择性电氧化过程研究[J]. 化工学报, 2022, 73(7): 3007-3017. |
| [15] | 朱江伟, 马鹏飞, 杜晓, 杨言言, 郝晓刚, 罗善霞. 基于可变价NiFe-LDH/rGO对磷酸根离子的特异性电控分离[J]. 化工学报, 2022, 73(7): 3057-3067. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号