化工学报 ›› 2023, Vol. 74 ›› Issue (11): 4475-4486.DOI: 10.11949/0438-1157.20230902
刘宗鹏1( ), 胡少剑1, 张宇宁1, 马玲2, 李磊2, 武本成1, 朱建华1(
), 胡少剑1, 张宇宁1, 马玲2, 李磊2, 武本成1, 朱建华1( )
)
收稿日期:2023-08-31
修回日期:2023-11-01
出版日期:2023-11-25
发布日期:2024-01-22
通讯作者:
朱建华
作者简介:刘宗鹏(1994—),男,博士研究生,pzuil5201@163.com
Zongpeng LIU1( ), Shaojian HU1, Yuning ZHANG1, Ling MA2, Lei LI2, Bencheng WU1, Jianhua ZHU1(
), Shaojian HU1, Yuning ZHANG1, Ling MA2, Lei LI2, Bencheng WU1, Jianhua ZHU1( )
)
Received:2023-08-31
Revised:2023-11-01
Online:2023-11-25
Published:2024-01-22
Contact:
Jianhua ZHU
摘要:
采用基团贡献法估算了己二酸、2-乙基己醇及三羟甲基丙烷酯化反应体系中各组分的基础热力学数据,探讨了温度对反应焓变、熵变、Gibbs自由能变和平衡常数的影响规律,热力学分析结果表明体系内各反应在所考察的温度范围内均为自发吸热过程,且升高温度有利于反应的正向进行;研究了氧化亚锡催化己二酸与2-乙基己醇以及己二酸单2-乙基己醇酯与三羟甲基丙烷的酯化反应动力学,实验结果表明两步反应均受动力学控制,且在一定的转化率区间内符合二级不可逆反应动力学特征,确定了两步反应的活化能和指前因子,建立的动力学模型能够较准确地描述复合型多元醇酯的合成过程。
中图分类号:
刘宗鹏, 胡少剑, 张宇宁, 马玲, 李磊, 武本成, 朱建华. 复合型多元醇酯合成反应的热力学分析及动力学研究[J]. 化工学报, 2023, 74(11): 4475-4486.
Zongpeng LIU, Shaojian HU, Yuning ZHANG, Ling MA, Lei LI, Bencheng WU, Jianhua ZHU. Thermodynamic analysis and kinetics study on synthesis reaction of complex polyolester[J]. CIESC Journal, 2023, 74(11): 4475-4486.
| Group | Benson method | Ducros method | Rozicka-Domalski method | |||
|---|---|---|---|---|---|---|
| ai | bi /K-1 | ci /K-2 | ||||
| C—(C)4 | 2.09 | -146.96 | 0.00 | -2.9353 | 1.4255 | -0.0853 |
| C—(C)(H)3 | -42.20 | 127.32 | 5.65 | 3.8452 | -0.3400 | 0.1949 |
| C—(C)(O)(H)2 | -33.91 | 41.03 | 4.60 | 1.4596 | 1.4657 | -0.2714 |
| O—(C)(H) | -158.68 | 121.71 | 31.80 | 12.9520 | -10.1450 | 2.6261 |
| O—(CO)(H) | -243.25 | 102.66 | 37.87 | -27.5870 | -0.1649 | 2.7483 |
| C—(C)2(H)2 | -20.72 | 39.44 | 4.98 | 2.7972 | -0.0550 | 0.1068 |
| C—(C)(CO)(H)2 | -21.77 | 40.19 | 2.97 | 6.6782 | -2.4473 | 0.4712 |
| CO—(C)(O) | -146.86 | 20.01 | 9.83 | 29.2460 | 3.4261 | -2.8962 |
| C—(C)3(H) | -7.95 | -50.53 | 3.01 | -0.4287 | 0.9381 | 0.0029 |
| O—(C)(CO) | -185.48 | 35.13 | 8.37 | -21.4340 | -4.0164 | 3.0531 |
表1 不同基团贡献法中各基团的热力学贡献值[14]
Table 1 Thermodynamic contribution value of each group in the different methods[14]
| Group | Benson method | Ducros method | Rozicka-Domalski method | |||
|---|---|---|---|---|---|---|
| ai | bi /K-1 | ci /K-2 | ||||
| C—(C)4 | 2.09 | -146.96 | 0.00 | -2.9353 | 1.4255 | -0.0853 |
| C—(C)(H)3 | -42.20 | 127.32 | 5.65 | 3.8452 | -0.3400 | 0.1949 |
| C—(C)(O)(H)2 | -33.91 | 41.03 | 4.60 | 1.4596 | 1.4657 | -0.2714 |
| O—(C)(H) | -158.68 | 121.71 | 31.80 | 12.9520 | -10.1450 | 2.6261 |
| O—(CO)(H) | -243.25 | 102.66 | 37.87 | -27.5870 | -0.1649 | 2.7483 |
| C—(C)2(H)2 | -20.72 | 39.44 | 4.98 | 2.7972 | -0.0550 | 0.1068 |
| C—(C)(CO)(H)2 | -21.77 | 40.19 | 2.97 | 6.6782 | -2.4473 | 0.4712 |
| CO—(C)(O) | -146.86 | 20.01 | 9.83 | 29.2460 | 3.4261 | -2.8962 |
| C—(C)3(H) | -7.95 | -50.53 | 3.01 | -0.4287 | 0.9381 | 0.0029 |
| O—(C)(CO) | -185.48 | 35.13 | 8.37 | -21.4340 | -4.0164 | 3.0531 |
| Component | Tb/K | ||||
|---|---|---|---|---|---|
| AA | -865.20 | 398.84 | 111.30 | 215.28 | 615.27 |
| EH | -367.82 | 512.11 | 67.99 | 176.09 | 488.39 |
| TMP | -638.60 | 489.75 | 119.83 | 203.31 | 574.37 |
| EHA | -1016.57 | 721.71 | 120.63 | 131.05 | 656.25 |
| DEHA | -1167.94 | 1044.57 | 129.96 | 133.81 | 690.37 |
| TMPME | -1438.72 | 1056.15 | 179.16 | 136.44 | 726.72 |
| TMPDE | -2238.84 | 1575.34 | 238.49 | 142.55 | 813.02 |
| TMPTE | -3038.96 | 2104.43 | 297.82 | 146.46 | 873.51 |
| H2O(g)[ | -241.826 | 188.82 | 40.64 | 108.90 | 373.15 |
表2 酯化体系中各组分的热力学数据
Table 2 Thermodynamic data of each component in the esterification system
| Component | Tb/K | ||||
|---|---|---|---|---|---|
| AA | -865.20 | 398.84 | 111.30 | 215.28 | 615.27 |
| EH | -367.82 | 512.11 | 67.99 | 176.09 | 488.39 |
| TMP | -638.60 | 489.75 | 119.83 | 203.31 | 574.37 |
| EHA | -1016.57 | 721.71 | 120.63 | 131.05 | 656.25 |
| DEHA | -1167.94 | 1044.57 | 129.96 | 133.81 | 690.37 |
| TMPME | -1438.72 | 1056.15 | 179.16 | 136.44 | 726.72 |
| TMPDE | -2238.84 | 1575.34 | 238.49 | 142.55 | 813.02 |
| TMPTE | -3038.96 | 2104.43 | 297.82 | 146.46 | 873.51 |
| H2O(g)[ | -241.826 | 188.82 | 40.64 | 108.90 | 373.15 |
| Group | ΔTbi /K |
|---|---|
 | 0.2878 |
| —CH2O— | 1.6249 |
| —OH | 3.2152 |
| —COOH | 5.8337 |
| —COO— | 2.6446 |
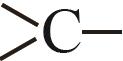 | 0.6033 |
| —CH2— | 0.9225 |
| —CH3 | 0.8894 |
| —CH2COO— | 3.3950 |
表3 Constantinous-Gani法中各基团的热力学贡献值[15]
Table 3 Thermodynamic contribution value of each group in the Constantinous-Gani method[15]
| Group | ΔTbi /K |
|---|---|
 | 0.2878 |
| —CH2O— | 1.6249 |
| —OH | 3.2152 |
| —COOH | 5.8337 |
| —COO— | 2.6446 |
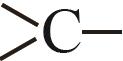 | 0.6033 |
| —CH2— | 0.9225 |
| —CH3 | 0.8894 |
| —CH2COO— | 3.3950 |
| Component | |||
|---|---|---|---|
| a | b×102 | c×104 | |
| AA | 185.14 | 12.62 | 7.15 |
| EH | 273.22 | -71.84 | 26.39 |
| TMP | 390.28 | -207.91 | 60.53 |
| EHA | 401.83 | -6.90 | 14.25 |
| DEHA | 618.52 | -26.42 | 21.34 |
| TMPME | 735.58 | -162.49 | 55.48 |
| TMPDE | 1080.88 | -117.06 | 50.42 |
| TMPTE | 1426.19 | -71.63 | 45.37 |
| H2O(g)[ | 36.54 | -3.48 | 1.17 |
表4 酯化体系中各组分比热容与温度的关系
Table 4 Correlation of specific heat capacity and temperature of each component in the esterification system
| Component | |||
|---|---|---|---|
| a | b×102 | c×104 | |
| AA | 185.14 | 12.62 | 7.15 |
| EH | 273.22 | -71.84 | 26.39 |
| TMP | 390.28 | -207.91 | 60.53 |
| EHA | 401.83 | -6.90 | 14.25 |
| DEHA | 618.52 | -26.42 | 21.34 |
| TMPME | 735.58 | -162.49 | 55.48 |
| TMPDE | 1080.88 | -117.06 | 50.42 |
| TMPTE | 1426.19 | -71.63 | 45.37 |
| H2O(g)[ | 36.54 | -3.48 | 1.17 |
| First esterification step (XA≈0—90%) | Second esterification step (XC≈0—80%) | ||||
|---|---|---|---|---|---|
| T/K | Fitting equation | R2 | T/K | Fitting equation | R2 |
| 413.15 | y=0.0088x-0.0165 | 0.9923 | 423.15 | y=0.0113x+0.0034 | 0.9941 |
| 423.15 | y=0.0198x-0.0337 | 0.9936 | 433.15 | y=0.0163x+0.1692 | 0.9923 |
| 433.15 | y=0.0325x-0.0086 | 0.9915 | 443.15 | y=0.0220x+0.2462 | 0.9850 |
| 443.15 | y=0.0582x+0.1234 | 0.9885 | 453.15 | y=0.0339x+0.3084 | 0.9708 |
表5 不同温度条件下的反应动力学拟合结果
Table 5 Fitting results of reaction kinetic equations at different temperatures
| First esterification step (XA≈0—90%) | Second esterification step (XC≈0—80%) | ||||
|---|---|---|---|---|---|
| T/K | Fitting equation | R2 | T/K | Fitting equation | R2 |
| 413.15 | y=0.0088x-0.0165 | 0.9923 | 423.15 | y=0.0113x+0.0034 | 0.9941 |
| 423.15 | y=0.0198x-0.0337 | 0.9936 | 433.15 | y=0.0163x+0.1692 | 0.9923 |
| 433.15 | y=0.0325x-0.0086 | 0.9915 | 443.15 | y=0.0220x+0.2462 | 0.9850 |
| 443.15 | y=0.0582x+0.1234 | 0.9885 | 453.15 | y=0.0339x+0.3084 | 0.9708 |
| First esterification step(XA≈0—90%) | Second esterification step(XC≈0—80%) | ||
|---|---|---|---|
| A1/(L·mol-1·min-1) | 7.153×109 | A2/(L·mol-1·min-1) | 1.869×105 |
| Ea1/(kJ·mol-1) | 93.99 | Ea2/(kJ·mol-1) | 58.59 |
表6 两步酯化反应的动力学参数
Table 6 Kinetic parameters of the two esterification steps
| First esterification step(XA≈0—90%) | Second esterification step(XC≈0—80%) | ||
|---|---|---|---|
| A1/(L·mol-1·min-1) | 7.153×109 | A2/(L·mol-1·min-1) | 1.869×105 |
| Ea1/(kJ·mol-1) | 93.99 | Ea2/(kJ·mol-1) | 58.59 |
| 1 | Nagendramma P, Kaul S. Development of ecofriendly/biodegradable lubricants: an overview[J]. Renewable and Sustainable Energy Reviews, 2012, 16(1): 764-774. |
| 2 | Pichler J, Eder R M, Besser C, et al. A comprehensive review of sustainable approaches for synthetic lubricant components[J]. Green Chemistry Letters and Reviews, 2023, 16(1): 2185547. |
| 3 | Pettersson A. High-performance base fluids for environmentally adapted lubricants[J]. Tribology International, 2007, 40(4): 638-645. |
| 4 | Raghunanan L, Narine S S. Engineering green lubricants (Ⅰ): Optimizing thermal and flow properties of linear diesters derived from vegetable oils[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(3): 686-692. |
| 5 | Eychenne V, Mouloungui Z. Relationships between structure and lubricating properties of neopentylpolyol esters[J]. Industrial & Engineering Chemistry Research, 1998, 37(12): 4835-4843. |
| 6 | Nagendramma P, Khatri P K, Thakre G D, et al. Lubrication capabilities of amino acid based ionic liquids as green bio-lubricant additives[J]. Journal of Molecular Liquids, 2017, 244: 219-225. |
| 7 | Kamalakar K, Mahesh G, Prasad R B N, et al. A novel methodology for the synthesis of acyloxy castor polyol esters: low pour point lubricant base stocks[J]. Journal of Oleo Science, 2015, 64(12): 1283-1295. |
| 8 | 赵东江. 热力学过程的性质、方向和限度判据的研究[J]. 化工高等教育, 2007, 24(1): 47-49, 63. |
| Zhao D J. Study on the nature, direction and limit criterion of thermodynamic process[J]. Higher Education in Chemical Engineering, 2007, 24(1): 47-49, 63. | |
| 9 | Zanjani N G, Pirzaman A K, Yazdanian E. Biodiesel production in the presence of heterogeneous catalyst of alumina: study of kinetics and thermodynamics[J]. International Journal of Chemical Kinetics, 2020, 52(7): 472-484. |
| 10 | Elmelawy M S, El-Meligy A, Mawgoud H A, et al. Synthesis and kinetics study of trimethylolpropane fatty acid triester from oleic acid methyl ester as potential biolubricant[J]. Biomass Conversion and Biorefinery, 2023, 13(3): 1645-1657. |
| 11 | Altuntepe E, Greinert T, Hartmann F, et al. Thermodynamics of enzyme-catalyzed esterifications(Ⅰ): Succinic acid esterification with ethanol[J]. Applied Microbiology and Biotechnology, 2017, 101(15): 5973-5984. |
| 12 | Aziz N A M, Hamid H A, Yunus R, et al. Kinetics and thermodynamics of synthesis of palm oil-based trimethylolpropane triester using microwave irradiation[J]. Journal of Saudi Chemical Society, 2020, 24(8): 552-566. |
| 13 | Ma L L, Han Y, Sun K A, et al. Kinetic and thermodynamic studies of the esterification of acidified oil catalyzed by sulfonated cation exchange resin[J]. Journal of Energy Chemistry, 2015, 24(4): 456-462. |
| 14 | 董新法, 方利国, 陈砺. 物性估算原理及计算机计算[M]. 北京: 化学工业出版社, 2006: 171-180. |
| Dong X F, Fang L G, Chen L. Principle of Physical Property Estimation and Computer Calculation[M]. Beijing: Chemical Industry Press, 2006: 171-180. | |
| 15 | 马沛生. 化工数据[M]. 北京: 中国石化出版社, 2003: 258-276. |
| Ma P S. Chemical Engineering Data[M]. Beijing: China Petrochemical Press, 2003: 258-276. | |
| 16 | 赵国良, 靳长德. 有机物热力学数据的估算[M]. 北京: 高等教育出版社, 1983: 140-156. |
| Zhao G L, Jin C D. Estimation of Thermodynamic Data of Organic Matter[M]. Beijing: Higher Education Press, 1983: 140-156. | |
| 17 | 马沛生, 夏淑倩, 夏清. 化工物性数据简明手册[M]. 北京: 化学工业出版社, 2013: 258-276. |
| Ma P S, Xia S Q, Xia Q. Concise Handbook of Chemical Physical Property Data[M]. Beijing: Chemical Industry Press, 2013: 258-276. | |
| 18 | 张继龙, 赵志仝, 乔燕, 等. 酯交换制油酸甲酯的基团贡献法热力学分析[J]. 化工学报, 2012, 63(6): 1684-1690. |
| Zhang J L, Zhao Z T, Qiao Y, et al. Thermodynamic analysis on preparation of methyl oleate via transesterification by group-contribution method[J]. CIESC Journal, 2012, 63(6): 1684-1690. | |
| 19 | 刘宏晓, 孙伟振, 赵玲. TOME环化反应热力学分析及反应动力学研究[J]. 化工学报, 2020, 71(2): 500-506. |
| Liu H X, Sun W Z, Zhao L. Thermodynamic analysis and kinetics of cyclization of TOME[J]. CIESC Journal, 2020, 71(2): 500-506. | |
| 20 | 吕全明, 孙伟振, 赵玲. 连四甲苯液相氧化过程热力学分析及动力学模拟[J]. 化工学报, 2021, 72(2): 1009-1017. |
| Lyu Q M, Sun W Z, Zhao L. Thermodynamic analysis and kinetic simulation of liquid phase oxidation of prehnitene to mellophanic acid[J]. CIESC Journal, 2021, 72(2): 1009-1017. | |
| 21 | Forsythe C J. Influence of inert gas sparging on fatty acid lactylate esterification kinetics[J]. Reaction Kinetics, Mechanisms and Catalysis, 2013, 108(2): 263-284. |
| 22 | Tesser R, Casale L, Verde D, et al. Kinetics of free fatty acids esterification: batch and loop reactor modeling[J]. Chemical Engineering Journal, 2009, 154(1/2/3): 25-33. |
| 23 | 付丽丽, 蒋登高. 棕榈酸异丙酯合成反应的热力学分析[J]. 高校化学工程学报, 2016, 30(2): 398-403. |
| Fu L L, Jiang D G. Thermodynamic analysis of isopropyl palmitate synthesis[J]. Journal of Chemical Engineering of Chinese Universities, 2016, 30(2): 398-403. | |
| 24 | 王洪海, 李旭, 李春利, 等. 固定化酶催化制备乙酸正丁酯及动力学[J]. 化工学报, 2017, 68(12): 4685-4690. |
| Wang H H, Li X, Li C L, et al. Kinetics of n-butyl acetate prepared by immobilized enzyme[J]. CIESC Journal, 2017, 68(12): 4685-4690. | |
| 25 | Zhou D, Wang L L, Chen X P, et al. Reaction mechanism investigation on the esterification of rosin with glycerol over annealed Fe3O4/MOF-5 via kinetics and TGA-FTIR analysis[J]. Chemical Engineering Journal, 2020, 401: 126024. |
| 26 | Russo V, Taddeo F, Cogliano T, et al. Investigation of the intrinsic reaction kinetics and the mass transfer phenomena of nonanoic acid esterification with 2-ethylhexanol promoted by sulfuric acid or Amberlite IR120[J]. Chemical Engineering Journal, 2021, 408: 127236. |
| 27 | Wang Y Z, Liu Y P, Liu C G. Kinetics of the esterification of low-concentration naphthenic acids and methanol in oils with or without SnO as a catalyst[J]. Energy & Fuels, 2008, 22(4): 2203-2206. |
| 28 | Kamaruzaman M R, Chin S Y, Pui E C L, et al. Synthesis of biobased polyester polyol through esterification of sorbitol with azelaic acid catalyzed by tin(Ⅱ) oxide: a kinetic modeling study[J]. Industrial & Engineering Chemistry Research, 2019, 58(2): 510-516. |
| 29 | Sánchez-Correa C A, Gil-Chaves I D, Rodríguez-Niño G. Kinetics of acetic acid and isoamyl alcohol liquid esterification over Amberlyst-70[J]. Chemical Engineering Research and Design, 2023, 196: 642-655. |
| 30 | 刘新鹏, 吴天祥. 非酸催化酯化合成对苯二甲酸二异辛酯的反应动力学[J]. 高校化学工程学报, 1994, 8(2): 195-200. |
| Liu X P, Wu T X. Reaction kinetics of esterification of synthetic dioctyl terephthalate with nonacid catalyst[J]. Journal of Chemical Engineering of Chinese Universities, 1994, 8(2): 195-200. | |
| 31 | Salmi T, Paatero E, Nyholm P. Kinetic model for the increase of reaction order during polyesterification[J]. Chemical Engineering and Processing: Process Intensification, 2004, 43(12): 1487-1493. |
| 32 | Tian W Y, Zeng Z X, Xue W L, et al. Kinetics of the mono-esterification between terephthalic acid and 1,4-butanediol[J]. Chinese Journal of Chemical Engineering, 2010, 18(3): 391-396. |
| 33 | Sun L, Zhu L, Xue W L, et al. Kinetics of p-toluene-sulfonic acid catalyzed direct esterification of pentaerythritol with acrylic acid for pentaerythritol diacrylate production[J]. Chemical Engineering Communications, 2020, 207(3): 331-338. |
| 34 | Zhou F, Cai J J, Mao X N, et al. Pseudo-homogeneous kinetic modeling of dioctyl terephthalate (DOTP) production by esterification of terephthalic acid and 2-ethylhexanol over tetrabutyl titanate catalyst[J]. Korean Journal of Chemical Engineering, 2022, 39(9): 2324-2333. |
| 35 | Narayan R C, Madras G. Esterification of sebacic acid in near-critical and supercritical methanol[J]. Industrial & Engineering Chemistry Research, 2017, 56(10): 2641-2649. |
| [1] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [2] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [3] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [4] | 姚晓宇, 沈俊, 李健, 李振兴, 康慧芳, 唐博, 董学强, 公茂琼. 流体气液临界参数测量方法研究进展[J]. 化工学报, 2023, 74(5): 1847-1861. |
| [5] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [6] | 禹进, 余彬彬, 蒋新生. 一种基于虚拟组分的燃烧调控化学作用量化及分析方法研究[J]. 化工学报, 2023, 74(3): 1303-1312. |
| [7] | 毛元敬, 杨智, 莫松平, 郭浩, 陈颖, 罗向龙, 陈健勇, 梁颖宗. C6~C10烷醇的SAFT-VR Mie状态方程参数回归及其热物性研究[J]. 化工学报, 2023, 74(3): 1033-1041. |
| [8] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [9] | 洪小东, 董轩, 林美金, 廖祖维, 任聪静, 杨遥, 蒋斌波, 王靖岱, 阳永荣. 图神经网络预测烃类工质的热力学性质[J]. 化工学报, 2023, 74(11): 4466-4474. |
| [10] | 章蕾, 宋孝辉, 张建庭, 屠美玲, 杨阿三. 氨甲环酸异构化过程的反应动力学研究[J]. 化工学报, 2023, 74(10): 4173-4181. |
| [11] | 陈睿哲, 刘永峰, 殷晨阳, 王龙, 张璐, 宋金瓯. 1-硝基丙烷引发正己烷热解的机理研究[J]. 化工学报, 2023, 74(10): 4319-4329. |
| [12] | 郑直, 郭乃胜, 尤占平, 王家伟. 废木油与石油沥青相容机制的分子动力学研究[J]. 化工学报, 2023, 74(10): 4037-4050. |
| [13] | 杨松涛, 李东洋, 牛玉清, 李鑫钢, 康绍辉, 李洪, 叶开凯, 周志全, 高鑫. 氟化物势能函数和热力学性质的分子模拟研究进展[J]. 化工学报, 2022, 73(9): 3828-3840. |
| [14] | 陈晨, 杨倩, 陈云, 张睿, 刘冬. 不同氧浓度下煤挥发分燃烧的化学动力学研究[J]. 化工学报, 2022, 73(9): 4133-4146. |
| [15] | 何瑞宁, 邹昀, 石萌, 李洋, 徐晶, 童张法. 固载离子液体[HSO3-BMIM][HSO4]/SiO2的制备及其催化乙酸乙醇酯化反应的研究[J]. 化工学报, 2022, 73(9): 3880-3894. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号