化工学报 ›› 2024, Vol. 75 ›› Issue (3): 956-966.DOI: 10.11949/0438-1157.20231357
王佳琪( ), 魏皓琦, 苟阿静, 刘佳兴, 周昕霖, 葛坤(
), 魏皓琦, 苟阿静, 刘佳兴, 周昕霖, 葛坤( )
)
收稿日期:2023-12-21
修回日期:2024-03-05
出版日期:2024-03-25
发布日期:2024-05-11
通讯作者:
葛坤
作者简介:王佳琪(1988—),女,博士,副教授,jiaqiwang@hrbeu.edu.cn
基金资助:
Jiaqi WANG( ), Haoqi WEI, Ajing GOU, Jiaxing LIU, Xinlin ZHOU, Kun GE(
), Haoqi WEI, Ajing GOU, Jiaxing LIU, Xinlin ZHOU, Kun GE( )
)
Received:2023-12-21
Revised:2024-03-05
Online:2024-03-25
Published:2024-05-11
Contact:
Kun GE
摘要:
气体水合物技术在海水淡化、水合物蓄冷、CO2封存等领域有着广阔的应用前景,水合物快速生成是制约水合物技术应用的关键问题之一。利用自主搭建的CO2水合物可视化生成实验装置进行了纳米流体中CO2水合物生成特性的实验研究,分析了纳米粒子对CO2水合物生成特性的影响。结果表明,与纯水相比,纳米流体体系中气体消耗量增加了2.17 mmol/mol,且诱导时间缩短了277.5 min。对不同种类的纳米流体中CO2水合物生成特性的研究发现,氧化铜纳米流体中水合物生成的诱导时间最短,只有179 min。氧化铜纳米流体对CO2水合物的促进存在一个最佳浓度,CO2水合物耗气量随着氧化铜纳米流体质量分数的增加先增加后减少。不同种类的纳米流体中CO2水合物生成过程中形态学图像存在较大差异。
中图分类号:
王佳琪, 魏皓琦, 苟阿静, 刘佳兴, 周昕霖, 葛坤. 纳米粒子作用下CO2水合物生成机理研究[J]. 化工学报, 2024, 75(3): 956-966.
Jiaqi WANG, Haoqi WEI, Ajing GOU, Jiaxing LIU, Xinlin ZHOU, Kun GE. Study on the formation mechanism of CO2 hydrate under the action of nanoparticles[J]. CIESC Journal, 2024, 75(3): 956-966.
| 纳米流体体系 | 去离子水体积/ml | 纳米粒子种类 | 纳米粒子质量/g |
|---|---|---|---|
| 0.10%石墨纳米流体 | 50 | 石墨纳米粒子 | 0.05 |
| 0.08%石墨纳米流体 | 50 | 石墨纳米粒子 | 0.04 |
| 0.08%铜纳米流体 | 50 | 铜纳米粒子 | 0.04 |
| 0.08%氧化铜纳米流体 | 50 | 氧化铜纳米粒子 | 0.04 |
| 0.04%氧化铜纳米流体 | 50 | 氧化铜纳米粒子 | 0.02 |
| 0.06%氧化铜纳米流体 | 50 | 氧化铜纳米粒子 | 0.03 |
| 0.10%氧化铜纳米流体 | 50 | 氧化铜纳米粒子 | 0.05 |
表1 配制各纳米流体所需实验材料用量
Table 1 Experimental materials dosage required for each nanofluid preparation
| 纳米流体体系 | 去离子水体积/ml | 纳米粒子种类 | 纳米粒子质量/g |
|---|---|---|---|
| 0.10%石墨纳米流体 | 50 | 石墨纳米粒子 | 0.05 |
| 0.08%石墨纳米流体 | 50 | 石墨纳米粒子 | 0.04 |
| 0.08%铜纳米流体 | 50 | 铜纳米粒子 | 0.04 |
| 0.08%氧化铜纳米流体 | 50 | 氧化铜纳米粒子 | 0.04 |
| 0.04%氧化铜纳米流体 | 50 | 氧化铜纳米粒子 | 0.02 |
| 0.06%氧化铜纳米流体 | 50 | 氧化铜纳米粒子 | 0.03 |
| 0.10%氧化铜纳米流体 | 50 | 氧化铜纳米粒子 | 0.05 |
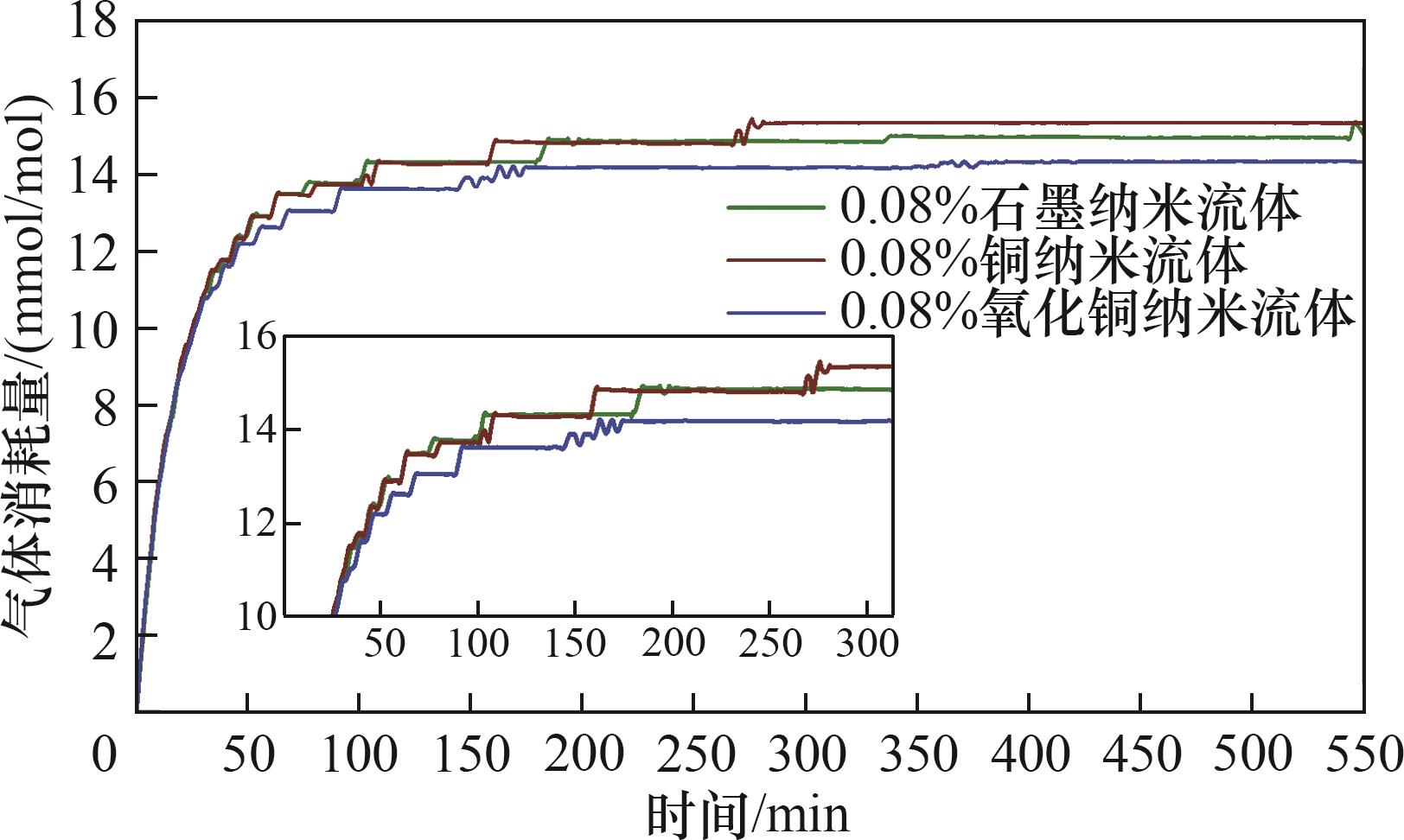
图7 不同种类纳米粒子对水合物生成过程耗气量变化曲线的影响
Fig.7 Effect of different types of nanoparticles on the variation curve of gas consumption in the hydrate generation process
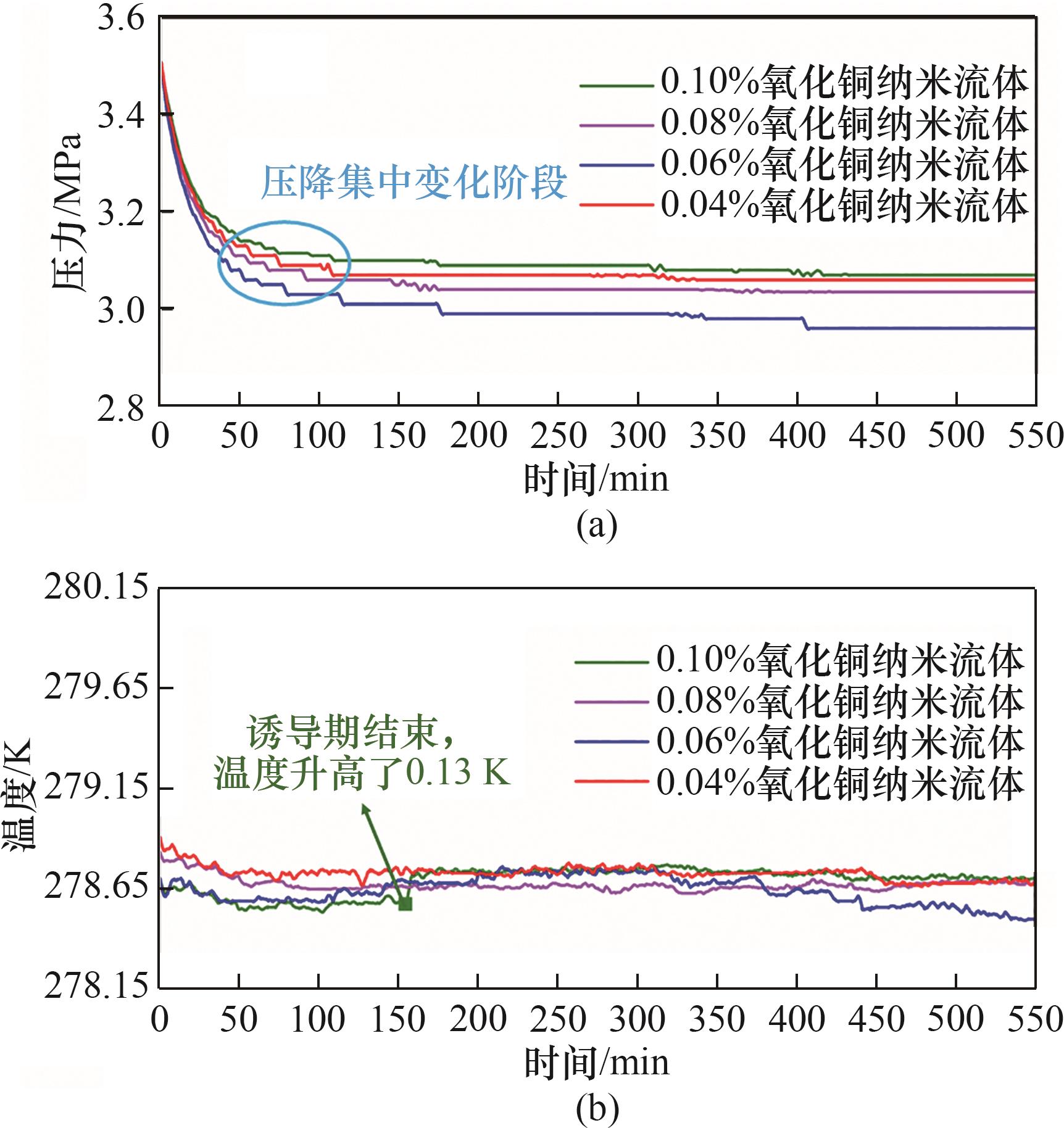
图10 不同质量分数氧化铜纳米流体中水合物生成过程压力、温度变化曲线
Fig.10 Pressure and temperature curve of hydrate formation in copper oxide nanofluids with different mass fractions
| 1 | Boswell R, Yoneda J, Waite W F. India National Gas Hydrate Program Expedition 02 summary of scientific results: evaluation of natural gas-hydrate-bearing pressure cores[J]. Marine and Petroleum Geology, 2019, 108: 143-153. |
| 2 | Song Y C, Yang L, Zhao J F, et al. The status of natural gas hydrate research in China: a review[J]. Renewable and Sustainable Energy Reviews, 2014, 31: 778-791. |
| 3 | Wang J Q, Zhao J F, Zhang Y, et al. Analysis of the effect of particle size on permeability in hydrate-bearing porous media using pore network models combined with CT[J]. Fuel, 2016, 163: 34-40. |
| 4 | Xu H F, Khan M N, Peters C J, et al. Hydrate-based desalination using cyclopentane hydrates at atmospheric pressure[J]. Journal of Chemical & Engineering Data, 2018, 63(4): 1081-1087. |
| 5 | Ponnivalavan B, Abhishek N, He T B, et al. A review of clathrate hydrate based desalination to strengthen energy-water nexus[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(7): 8093-8107. |
| 6 | Liu N, Zhou J L, Gao M, et al. An experimental study on flow and heat transfer characteristics of binary hydrate slurries in a horizontal tube[J]. International Communications in Heat and Mass Transfer, 2019, 102: 34-41. |
| 7 | Sun Q B, Kang Y. Experimental correlation for the formation rate of CO2 hydrate with THF (tetrahydrofuran) for cooling application[J]. Energy, 2015, 91: 712-719. |
| 8 | Veluswamy H P, Kumar R, Linga P. Hydrogen storage in clathrate hydrates: current state of the art and future directions[J]. Applied Energy, 2014, 122: 112-132. |
| 9 | Wang J Q, Zhao J F, Yang M J, et al. Permeability of laboratory-formed porous media containing methane hydrate: observations using X-ray computed tomography and simulations with pore network models[J]. Fuel, 2015, 145: 170-179. |
| 10 | Xia Z M, Li X S, Chen Z Y, et al. Hydrate-based acidic gases capture for clean methane with new synergic additives[J]. Applied Energy, 2017, 207: 584-593. |
| 11 | 樊栓狮. 天然气水合物储存与运输技术[M]. 北京: 化学工业出版社, 2005: 1-30. |
| Fan S S. Storage and Transportation Technology of Natural Gas Hydrate[M]. Beijing: Chemical Industry Press, 2005: 1-30. | |
| 12 | Lee S Y, Kim H C, Lee J D. Morphology study of methane-propane clathrate hydrates on the bubble surface in the presence of SDS or PVCap[J]. Journal of Crystal Growth, 2014, 402: 249-259. |
| 13 | Song Y C, Wang J Q, Liu Y, et al. Analysis of heat transfer influences on gas production from methane hydrates using a combined method[J]. International Journal of Heat and Mass Transfer, 2016, 92: 766-773. |
| 14 | Mori Y H. Recent advances in hydrate-based technologies for natural gas storage-A review[J]. Chinese Journal of Chemical Engineering, 2003, 54(1): 1-17. |
| 15 | 王帅, 杜胜男, 刘胜利, 等. 促进天然气水合物形成的影响因素分析[J]. 当代化工, 2016, 45(2): 367-369, 372. |
| Wang S, Du S N, Liu S L, et al. Analysis of factors of promoting natural gas hydrate formation[J]. Contemporary Chemical Industry, 2016, 45(2): 367-369, 372. | |
| 16 | He Y, Sun M T, Chen C, et al. Surfactant-based promotion to gas hydrate formation for energy storage[J]. Journal of Materials Chemistry A, 2019, 7(38): 21634-21661. |
| 17 | 李玉星, 朱超, 王武昌. 表面活性剂促进CO2水合物生成的实验及动力学模型[J]. 石油化工, 2012, 41(6): 699-703. |
| Li Y X, Zhu C, Wang W C. Promoting effects of surfactants on carbon dioxide hydrate formation and the kinetics[J]. Petrochemical Technology, 2012, 41(6): 699-703. | |
| 18 | Celata G P, D'Annibale F, Mariani A, et al. Heat transfer in water-based SiC and TiO2 nanofluids[J]. Heat Transfer Engineering, 2013, 34(13): 1060-1072. |
| 19 | Li J P, Liang D Q, Guo K H, et al. Formation and dissociation of HFC134a gas hydrate in nano-copper suspension[J]. Energy Conversion and Management, 2006, 47(2): 201-210. |
| 20 | Yu Y S, Xu C G, Li X S. Evaluation of CO2 hydrate formation from mixture of graphite nanoparticle and sodium dodecyl benzene sulfonate[J]. Journal of Industrial and Engineering Chemistry, 2018, 59: 64-69. |
| 21 | Ganji H, Aalaie J, Boroojerdi S H, et al. Effect of polymer nanocomposites on methane hydrate stability and storage capacity [J]. Journal of Petroleum Science and Engineering, 2013, 112: 32-35. |
| 22 | Chari V D, Sharma D V S G K, Prasad P S R, et al. Methane hydrates formation and dissociation in nano silica suspension[J]. Journal of Natural Gas Science and Engineering, 2013, 11: 7-11. |
| 23 | Zhong D L, Wang J L, Lu Y Y, et al. Precombustion CO2 capture using a hybrid process of adsorption and gas hydrate formation[J]. Energy, 2016, 102: 621-629. |
| 24 | Peng D Y, Robinson D B. A new two-constant equation of state[J]. Industrial & Engineering Chemistry Fundamentals, 1976, 15(1): 59-64. |
| 25 | Stryjek R, Vera J H. PRSV2: a cubic equation of state for accurate vapor-liquid equilibria calculations[J]. The Canadian Journal of Chemical Engineering, 1986, 64(5): 820-826. |
| 26 | Stryjek R, Vera J H. PRSV: an improved Peng-Robinson equation of state for pure compounds and mixtures[J]. The Canadian Journal of Chemical Engineering, 1986, 64(2): 323-333. |
| 27 | Mohammadi A, Manteghian M, Haghtalab A, et al. Kinetic study of carbon dioxide hydrate formation in presence of silver nanoparticles and SDS[J]. Chemical Engineering Journal, 2014, 237: 387-395. |
| 28 | McCain W. The Properties of Petroleum Fluids[M]. Tulsa: PennWell Books, 1990. |
| 29 | Klauda J B, Sandler S I. A fugacity model for gas hydrate phase equilibria[J]. Industrial & Engineering Chemistry Research, 2000, 39(9): 3377-3386. |
| 30 | 陈光进, 孙长宇, 马庆兰. 气体水合物科学与技术[M]. 2版. 北京: 化学工业出版社, 2020. |
| Chen G J, Sun C Y, Ma Q L. Gas Hydrate Science and Technology[M]. 2nd ed. Beijing: Chemical Industry Press, 2020. | |
| 31 | 张炜, 李昊阳, 徐纯刚, 等. 气体水合物生成微观机理及分析方法研究进展[J]. 化工学报, 2022, 73(9): 3815-3827. |
| Zhang W, Li H Y, Xu C G, et al. Research progress on the microscopic mechanism and analytical methods of gas hydrate formation[J]. CIESC Journal, 2022, 73(9): 3815-3827. | |
| 32 | Traciak J, Żyła G. Effect of nanoparticles saturation on the surface tension of nanofluids[J]. Journal of Molecular Liquids, 2022, 363: 119937. |
| 33 | Wanic M, Cabaleiro D, Hamze S, et al. Surface tension of ethylene glycol-based nanofluids containing various types of nitrides[J]. Journal of Thermal Analysis and Calorimetry, 2020, 139(2): 799-806. |
| 34 | Zhang L Y, Fu Z B, Liu Y Y, et al. Experimental study on enhancement of falling film absorption process by adding various nanoparticles[J]. International Communications in Heat and Mass Transfer, 2018, 92: 100-106. |
| 35 | Sadeghinezhad E, Mehrali M, Saidur R, et al. A comprehensive review on graphene nanofluids: recent research, development and applications[J]. Energy Conversion and Management, 2016, 111: 466-487. |
| 36 | Buongiorno J, Venerus D C, Prabhat N, et al. A benchmark study on the thermal conductivity of nanofluids[J]. Journal of Applied Physics, 2009, 106(9): 094312. |
| 37 | Li Z, Zhong D L, Lu Y Y, et al. Preferential enclathration of CO2 into tetra-n-butyl phosphonium bromide semiclathrate hydrate in moderate operating conditions: application for CO2 capture from shale gas[J]. Applied energy, 2017, 199: 370-381. |
| [1] | 曾玉娇, 肖炘, 杨刚, 张意博, 郑光明, 李防, 汪凤玲. 基于机理与数据混合驱动的湿法磷酸生产过程代理建模与优化[J]. 化工学报, 2024, 75(3): 936-944. |
| [2] | 王娟, 李秀明, 邵炜涛, 丁续, 霍莹, 付连超, 白云宇, 李迪. 多孔板鼓泡塔流动与传质特性数值模拟[J]. 化工学报, 2024, 75(3): 801-814. |
| [3] | 徐百平, 梁瑞凤, 喻慧文, 吴桂群, 肖书平. 双螺杆挤出机强化三角形转子作用下的腔内分布混合模拟[J]. 化工学报, 2024, 75(3): 858-866. |
| [4] | 李文俊, 赵中阳, 倪震, 周灿, 郑成航, 高翔. 基于气-液传质强化的湿法烟气脱硫CFD模拟研究[J]. 化工学报, 2024, 75(2): 505-519. |
| [5] | 张兆想, 蔡茂坤, 任志英, 贾晓红, 郭飞. 温度及其波动对橡胶密封硫化过程影响的仿真分析[J]. 化工学报, 2024, 75(2): 715-726. |
| [6] | 王学云, 郁肖兵, 彭万旺, 沈岩松. 熔渣气化炉喷嘴燃烧区行为的数值模拟研究[J]. 化工学报, 2024, 75(2): 659-674. |
| [7] | 赵文琪, 邓燕君, 朱春英, 付涛涛, 马友光. 纳米粒子稳定的Pickering乳液及其液滴聚并动力学研究进展[J]. 化工学报, 2024, 75(1): 33-46. |
| [8] | 麻雪怡, 刘克勤, 胡激江, 姚臻. POE溶液聚合反应器内混合与反应过程的CFD研究[J]. 化工学报, 2024, 75(1): 322-337. |
| [9] | 赵若晗, 黄蒙蒙, 朱春英, 付涛涛, 高习群, 马友光. 缩口T型微通道内纳米流体吸收CO2的流动与传质研究[J]. 化工学报, 2024, 75(1): 221-230. |
| [10] | 李亚婷, 王忠东, 董艳鹏, 朱春英, 马友光, 付涛涛. 微通道中毛细流动及其工程应用的研究进展[J]. 化工学报, 2024, 75(1): 159-170. |
| [11] | 崔怡洲, 李成祥, 翟霖晓, 刘束玉, 石孝刚, 高金森, 蓝兴英. 亚毫米气泡和常规尺寸气泡气液两相流流动与传质特性对比[J]. 化工学报, 2024, 75(1): 197-210. |
| [12] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [13] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [14] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [15] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号