CIESC Journal ›› 2023, Vol. 74 ›› Issue (11): 4419-4432.DOI: 10.11949/0438-1157.20231001
• Reviews and monographs • Previous Articles Next Articles
Zhongliang XIAO1,2( ), Youtao XIANG1, Liubin SONG1(
), Youtao XIANG1, Liubin SONG1( ), Yinjie KUANG1, Tingting ZHAO1, Yubo XIA1, Minzhi XIAO1, Lin JIANG1, Taotao CHEN1, Qian XIAO2
), Yinjie KUANG1, Tingting ZHAO1, Yubo XIA1, Minzhi XIAO1, Lin JIANG1, Taotao CHEN1, Qian XIAO2
Received:2023-09-22
Revised:2023-11-24
Online:2024-01-22
Published:2023-11-25
Contact:
Liubin SONG
肖忠良1,2( ), 向优涛1, 宋刘斌1(
), 向优涛1, 宋刘斌1( ), 匡尹杰1, 赵亭亭1, 夏宇博1, 肖敏之1, 蒋琳1, 陈涛涛1, 肖茜2
), 匡尹杰1, 赵亭亭1, 夏宇博1, 肖敏之1, 蒋琳1, 陈涛涛1, 肖茜2
通讯作者:
宋刘斌
作者简介:肖忠良(1964—),男,博士,教授,xiaozhongliang@163.com
基金资助:CLC Number:
Zhongliang XIAO, Youtao XIANG, Liubin SONG, Yinjie KUANG, Tingting ZHAO, Yubo XIA, Minzhi XIAO, Lin JIANG, Taotao CHEN, Qian XIAO. Progress of mechanochemical recovery of valuable metals from used lithium-ion battery cathode materials[J]. CIESC Journal, 2023, 74(11): 4419-4432.
肖忠良, 向优涛, 宋刘斌, 匡尹杰, 赵亭亭, 夏宇博, 肖敏之, 蒋琳, 陈涛涛, 肖茜. 机械化学法回收废旧锂离子电池正极材料中有价金属的研究进展[J]. 化工学报, 2023, 74(11): 4419-4432.
Add to citation manager EndNote|Ris|BibTeX
| 成分 | 所用材料 | 成本占比/% | 化学特性 | 潜在危害性 |
|---|---|---|---|---|
| 正极材料 | 钴酸锂/锰酸锂/镍酸锂/磷酸铁锂 | 30~35 | 与水、酸、还原剂或氧化剂发生反应 | 重金属污染 |
| 负极材料 | 碳材料/石墨 | 10~15 | 遇明火或高温易爆炸 | 粉尘污染 |
| 电解质 | LiPF6/LiBF4/LiAsF6 | 10~15 | 强腐蚀性,遇水生成HF,氧化生成P2O5等有害物质 | 氟污染、有害气体污染 |
| 电解质溶剂 | 碳酸乙烯酯/碳酸二甲酯 | 10~15 | 水解生成醛和酸,燃烧产生CO、CO2等 | 有机物污染 |
| 隔膜材料 | 聚丙烯/聚乙烯 | 20~30 | 燃烧可产生CO、醛等 | 有机物污染 |
| 黏合剂 | 聚偏氟乙烯/偏氟乙烯 | 10~15 | 受热分解产生HF | 氟污染 |
Table 1 Chemical properties of the main components of lithium-ion batteries and their environmental hazards[9]
| 成分 | 所用材料 | 成本占比/% | 化学特性 | 潜在危害性 |
|---|---|---|---|---|
| 正极材料 | 钴酸锂/锰酸锂/镍酸锂/磷酸铁锂 | 30~35 | 与水、酸、还原剂或氧化剂发生反应 | 重金属污染 |
| 负极材料 | 碳材料/石墨 | 10~15 | 遇明火或高温易爆炸 | 粉尘污染 |
| 电解质 | LiPF6/LiBF4/LiAsF6 | 10~15 | 强腐蚀性,遇水生成HF,氧化生成P2O5等有害物质 | 氟污染、有害气体污染 |
| 电解质溶剂 | 碳酸乙烯酯/碳酸二甲酯 | 10~15 | 水解生成醛和酸,燃烧产生CO、CO2等 | 有机物污染 |
| 隔膜材料 | 聚丙烯/聚乙烯 | 20~30 | 燃烧可产生CO、醛等 | 有机物污染 |
| 黏合剂 | 聚偏氟乙烯/偏氟乙烯 | 10~15 | 受热分解产生HF | 氟污染 |
| 浸出剂 | 正极材料 | 辅助试剂 | 浸出条件 | 浸出率/% | 优点 | 缺点 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 温度/ ℃ | 时间/h | 固液比/ (g/L) | Li | Ni | Co | Mn | |||||
| 硫酸[ | 镍钴锰酸锂 | Na2S2O5 | 60 | 2.5 | 100 | 90 | 90 | 90 | 90 | 操作简单,浸出率高 | 腐蚀性强,浸出液量大 |
| 盐酸[ | 废LIBs | NaClO | 50 | 2 | 20 | 99 | — | 99 | 99 | 浸出率高,氯离子促进金属溶解 | 成本高,溶剂易挥发, 腐蚀性大 |
| 硝酸[ | LiCoO2 | H2O2 | 75 | 1 | 20 | 95 | — | 95 | — | 浓度低 | 产生有毒气体 |
| 磷酸[ | LiCoO2 | 葡萄糖 | 80 | 2 | 2 | 100 | — | 98 | — | 浸出液少,效率高 | 一定的腐蚀性,高温 |
| 甲磺酸+对甲苯磺酸[ | LiFePO4 | H2O2 | 室温 | 1.5 | 40 | 95 | — | — | — | 环保绿色,成本低 | 选择性低 |
| 单宁酸[ | 废LIBs | 醋酸 | 80 | 4 | 20 | 99 | — | 94 | — | 浸出液少,效率高 | 一定的腐蚀性,高温 |
| 柠檬酸[ | 废LIBs | H2O2 | 80 | 1.5 | 20 | 100 | — | 99 | — | 绿色,操作简单 | 无选择性,高温 |
| 柠檬酸+DL-苹果酸[ | 废LIBs | H2O2 | 95 | 0.5 | — | 96 | — | 98 | — | 操作简单,绿色 | 高温,无选择性 |
| 甲磺酸[ | LiCoO2 | H2O2 | 70 | 1 | — | 100 | — | 100 | — | 操作简单,成本低 | 腐蚀性,无选择性 |
| 柠檬酸[ | LiCoO2 | 橘子皮 | 100 | 4 | — | 80.5 | 90.1 | 91.3 | 92.2 | 绿色,成本低 | 高温 |
| 酒石酸[ | LiCoO2 | 抗坏血酸 | 80 | 5 | 2 | 99 | — | 96 | — | 高效,无毒气 | 渗滤液量大,温度高 |
| 硫酸钠[ | LiNi0.5Co0.2Mn0.3O2 | H2O2 | 60 | 0.5 | 20 | >99 | — | >99 | — | 绿色,操作简单 | 高能耗 |
| 氨水硫酸铵[ | LiNi0.5Co0.2Mn0.3O2 | 亚硫酸钠 | 50 | — | 10~50 | 97 | 95 | 89 | — | 操作简单,成本低 | 锰的选择性低 |
| 丙醇酸[ | LiNi1/3Mn1/3Co1/3O2 | H2O2 | 70 | 1/3 | 20 | 95 | 98 | 98 | 98 | 绿色,操作简单 | 一定的腐蚀性,高温 |
| 草酸盐[ | LiNi0.5Co0.2Mn0.3O2 | 柠檬酸 | 90 | 0.5 | 20 | 100 | 100 | 100 | 100 | 操作简单 | 一定的腐蚀性 |
| 乳酸[ | LiNi1/3Mn1/3Co1/3O2 | H2O2 | 70 | 1/3 | 20 | 97.7 | 98.2 | 98.9 | 98.4 | 高效,无毒气 | 高温,成本高 |
| 硫酸+丙醇酸[ | LiNi x Co y Mn z O2 | H2O2 | 70 | 1.35 | 61 | 99.8 | 99.5 | 97.2 | 96.9 | 操作简单,高效 | 一定的腐蚀性,高温 |
Table 2 Comparison of the properties of different leaching agents in the recovery of used LIBs
| 浸出剂 | 正极材料 | 辅助试剂 | 浸出条件 | 浸出率/% | 优点 | 缺点 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 温度/ ℃ | 时间/h | 固液比/ (g/L) | Li | Ni | Co | Mn | |||||
| 硫酸[ | 镍钴锰酸锂 | Na2S2O5 | 60 | 2.5 | 100 | 90 | 90 | 90 | 90 | 操作简单,浸出率高 | 腐蚀性强,浸出液量大 |
| 盐酸[ | 废LIBs | NaClO | 50 | 2 | 20 | 99 | — | 99 | 99 | 浸出率高,氯离子促进金属溶解 | 成本高,溶剂易挥发, 腐蚀性大 |
| 硝酸[ | LiCoO2 | H2O2 | 75 | 1 | 20 | 95 | — | 95 | — | 浓度低 | 产生有毒气体 |
| 磷酸[ | LiCoO2 | 葡萄糖 | 80 | 2 | 2 | 100 | — | 98 | — | 浸出液少,效率高 | 一定的腐蚀性,高温 |
| 甲磺酸+对甲苯磺酸[ | LiFePO4 | H2O2 | 室温 | 1.5 | 40 | 95 | — | — | — | 环保绿色,成本低 | 选择性低 |
| 单宁酸[ | 废LIBs | 醋酸 | 80 | 4 | 20 | 99 | — | 94 | — | 浸出液少,效率高 | 一定的腐蚀性,高温 |
| 柠檬酸[ | 废LIBs | H2O2 | 80 | 1.5 | 20 | 100 | — | 99 | — | 绿色,操作简单 | 无选择性,高温 |
| 柠檬酸+DL-苹果酸[ | 废LIBs | H2O2 | 95 | 0.5 | — | 96 | — | 98 | — | 操作简单,绿色 | 高温,无选择性 |
| 甲磺酸[ | LiCoO2 | H2O2 | 70 | 1 | — | 100 | — | 100 | — | 操作简单,成本低 | 腐蚀性,无选择性 |
| 柠檬酸[ | LiCoO2 | 橘子皮 | 100 | 4 | — | 80.5 | 90.1 | 91.3 | 92.2 | 绿色,成本低 | 高温 |
| 酒石酸[ | LiCoO2 | 抗坏血酸 | 80 | 5 | 2 | 99 | — | 96 | — | 高效,无毒气 | 渗滤液量大,温度高 |
| 硫酸钠[ | LiNi0.5Co0.2Mn0.3O2 | H2O2 | 60 | 0.5 | 20 | >99 | — | >99 | — | 绿色,操作简单 | 高能耗 |
| 氨水硫酸铵[ | LiNi0.5Co0.2Mn0.3O2 | 亚硫酸钠 | 50 | — | 10~50 | 97 | 95 | 89 | — | 操作简单,成本低 | 锰的选择性低 |
| 丙醇酸[ | LiNi1/3Mn1/3Co1/3O2 | H2O2 | 70 | 1/3 | 20 | 95 | 98 | 98 | 98 | 绿色,操作简单 | 一定的腐蚀性,高温 |
| 草酸盐[ | LiNi0.5Co0.2Mn0.3O2 | 柠檬酸 | 90 | 0.5 | 20 | 100 | 100 | 100 | 100 | 操作简单 | 一定的腐蚀性 |
| 乳酸[ | LiNi1/3Mn1/3Co1/3O2 | H2O2 | 70 | 1/3 | 20 | 97.7 | 98.2 | 98.9 | 98.4 | 高效,无毒气 | 高温,成本高 |
| 硫酸+丙醇酸[ | LiNi x Co y Mn z O2 | H2O2 | 70 | 1.35 | 61 | 99.8 | 99.5 | 97.2 | 96.9 | 操作简单,高效 | 一定的腐蚀性,高温 |
| 正极材料 | 共磨剂/条件 | 温度/℃ | 浸出剂/条件 | 沉淀剂 | 产品 | 效率/%(性能) | 文献 |
|---|---|---|---|---|---|---|---|
| LFP | (NH4)2S2O8∶LFP=1.4∶11,5 h, 350 r/min, BMR=30∶1 | — | H2O | — | Li2SO4 | Li=95.3 | [ |
Na3Cit∶LFP=10∶1,5 h, 500 r/min, H2O2=1 ml | — | H2O | — | Li2CO3 | Li=98.9 | [ | |
FeCl3∶LiFePO4=1.2∶1, 5 h,600 r/min | 95 | H2O | NaOH | Li2CO3 | Li=97 | [ | |
LFP∶EDTA-2Na=3∶1, 2 h,S∶L=50 g/L | — | 0.6 mol/L H3PO4, 20 min | — | FePO4·2H2O, Li3PO4 | Li=94.29,Fe=97.67 | [ | |
柠檬酸∶LFP=20∶1, BPR=45,8 h,300 r/min | 95 | H2O | NaOH | Fe(OH)3,Li2CO3 | Li=97.82,Fe=95.62 | [ | |
LiFePO4∶NaCl=1∶2, 500 r/min,6 h | — | H2O | Na2CO3 | NaFePO4,Li2CO3 | — | [ | |
LiFePO4∶草酸=1∶1, 500 r/min,2 h, BMR=20∶1 | — | H2O,30 min | NaOH | FeC2O4·2H2O, Li3PO4 | Li=99,Fe=94 | [ | |
| LCO | LCO∶GS=0.3∶1,400 r/min, 1 h,0.15 mol/L柠檬酸 | — | — | Na2CO3 | CoC2O4·2H2O, Li2CO3 | Li=99.33,Co=98.87 | [ |
| 120 min,500 r/min, NH4Cl 0.03 mol/L,L∶S=15 g/L | — | 1 mol/L H2SO4, 80℃,60 min | — | Li2SO4 | Li=100,Co=99.22 | [ | |
LiCoO2∶EDTA=1∶4,4 h, 600 r/min,BMR=80∶1 | — | H2O | Na2CO3, NaOH | Li2CO3,Co3O4 | Li=99,Co=98 | [ | |
| LiCoO2∶Al=1∶1,2 h | — | H2O | Na2CO3 | Li2CO3 | Co=90,Li=70 | [ | |
| NCM | (NH4)2WO4,300 r/min, NCM-3%(质量分数)W,1 h | 800℃, 4 h | — | — | 钨改性NCM | (145 mA·h/g) | [ |
| Li2CO3,500 r/min,4 h, Li∶TM(Ni,Co,Mn)=1.2∶1 | 800℃,10 h | — | — | 再生NCM | (165 mA·h/g) | [ | |
| Na2S⋅9H2O,600 r/min,15 min | — | H2O,25℃, 30 min | Na2CO3 | Li2CO3, Ni0.5Mn0.3Co0.2(OH)2 | Li=95,Co=100,Ni=100,Mn=100 | [ | |
| LiOH,360 r/min,10 h | 800℃,16 h | — | — | 再生NCM | (176.8 mA·h/g) | [ |
Table 3 MC method for recycling and reusing valuable metals in waste LIBs
| 正极材料 | 共磨剂/条件 | 温度/℃ | 浸出剂/条件 | 沉淀剂 | 产品 | 效率/%(性能) | 文献 |
|---|---|---|---|---|---|---|---|
| LFP | (NH4)2S2O8∶LFP=1.4∶11,5 h, 350 r/min, BMR=30∶1 | — | H2O | — | Li2SO4 | Li=95.3 | [ |
Na3Cit∶LFP=10∶1,5 h, 500 r/min, H2O2=1 ml | — | H2O | — | Li2CO3 | Li=98.9 | [ | |
FeCl3∶LiFePO4=1.2∶1, 5 h,600 r/min | 95 | H2O | NaOH | Li2CO3 | Li=97 | [ | |
LFP∶EDTA-2Na=3∶1, 2 h,S∶L=50 g/L | — | 0.6 mol/L H3PO4, 20 min | — | FePO4·2H2O, Li3PO4 | Li=94.29,Fe=97.67 | [ | |
柠檬酸∶LFP=20∶1, BPR=45,8 h,300 r/min | 95 | H2O | NaOH | Fe(OH)3,Li2CO3 | Li=97.82,Fe=95.62 | [ | |
LiFePO4∶NaCl=1∶2, 500 r/min,6 h | — | H2O | Na2CO3 | NaFePO4,Li2CO3 | — | [ | |
LiFePO4∶草酸=1∶1, 500 r/min,2 h, BMR=20∶1 | — | H2O,30 min | NaOH | FeC2O4·2H2O, Li3PO4 | Li=99,Fe=94 | [ | |
| LCO | LCO∶GS=0.3∶1,400 r/min, 1 h,0.15 mol/L柠檬酸 | — | — | Na2CO3 | CoC2O4·2H2O, Li2CO3 | Li=99.33,Co=98.87 | [ |
| 120 min,500 r/min, NH4Cl 0.03 mol/L,L∶S=15 g/L | — | 1 mol/L H2SO4, 80℃,60 min | — | Li2SO4 | Li=100,Co=99.22 | [ | |
LiCoO2∶EDTA=1∶4,4 h, 600 r/min,BMR=80∶1 | — | H2O | Na2CO3, NaOH | Li2CO3,Co3O4 | Li=99,Co=98 | [ | |
| LiCoO2∶Al=1∶1,2 h | — | H2O | Na2CO3 | Li2CO3 | Co=90,Li=70 | [ | |
| NCM | (NH4)2WO4,300 r/min, NCM-3%(质量分数)W,1 h | 800℃, 4 h | — | — | 钨改性NCM | (145 mA·h/g) | [ |
| Li2CO3,500 r/min,4 h, Li∶TM(Ni,Co,Mn)=1.2∶1 | 800℃,10 h | — | — | 再生NCM | (165 mA·h/g) | [ | |
| Na2S⋅9H2O,600 r/min,15 min | — | H2O,25℃, 30 min | Na2CO3 | Li2CO3, Ni0.5Mn0.3Co0.2(OH)2 | Li=95,Co=100,Ni=100,Mn=100 | [ | |
| LiOH,360 r/min,10 h | 800℃,16 h | — | — | 再生NCM | (176.8 mA·h/g) | [ |
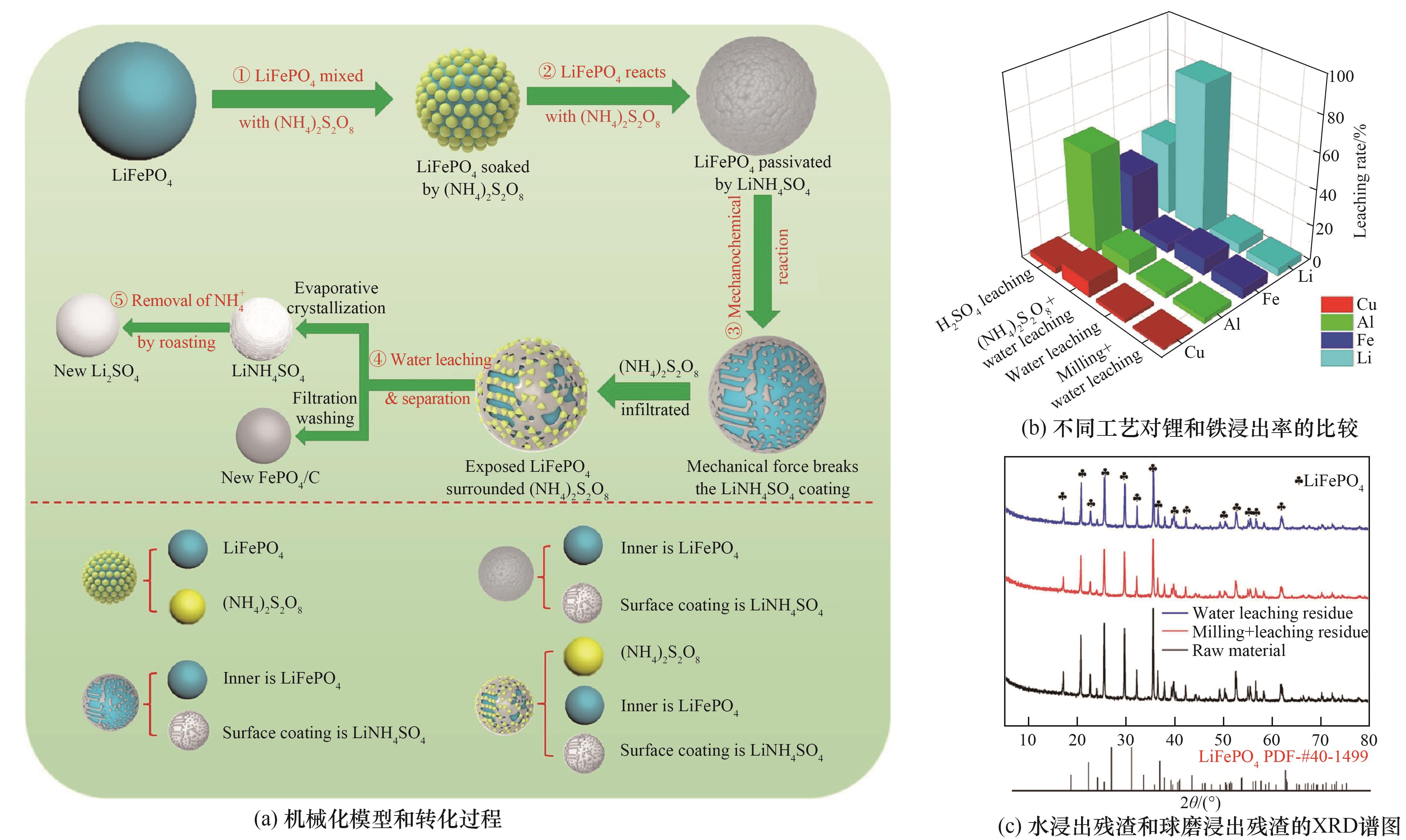
Fig.1 (a) Mechanization model and conversion process; (b) Comparison of lithium and iron leaching rates by different processes; (c) XRD patterns of water leaching residue and ball milling leaching residue[71]
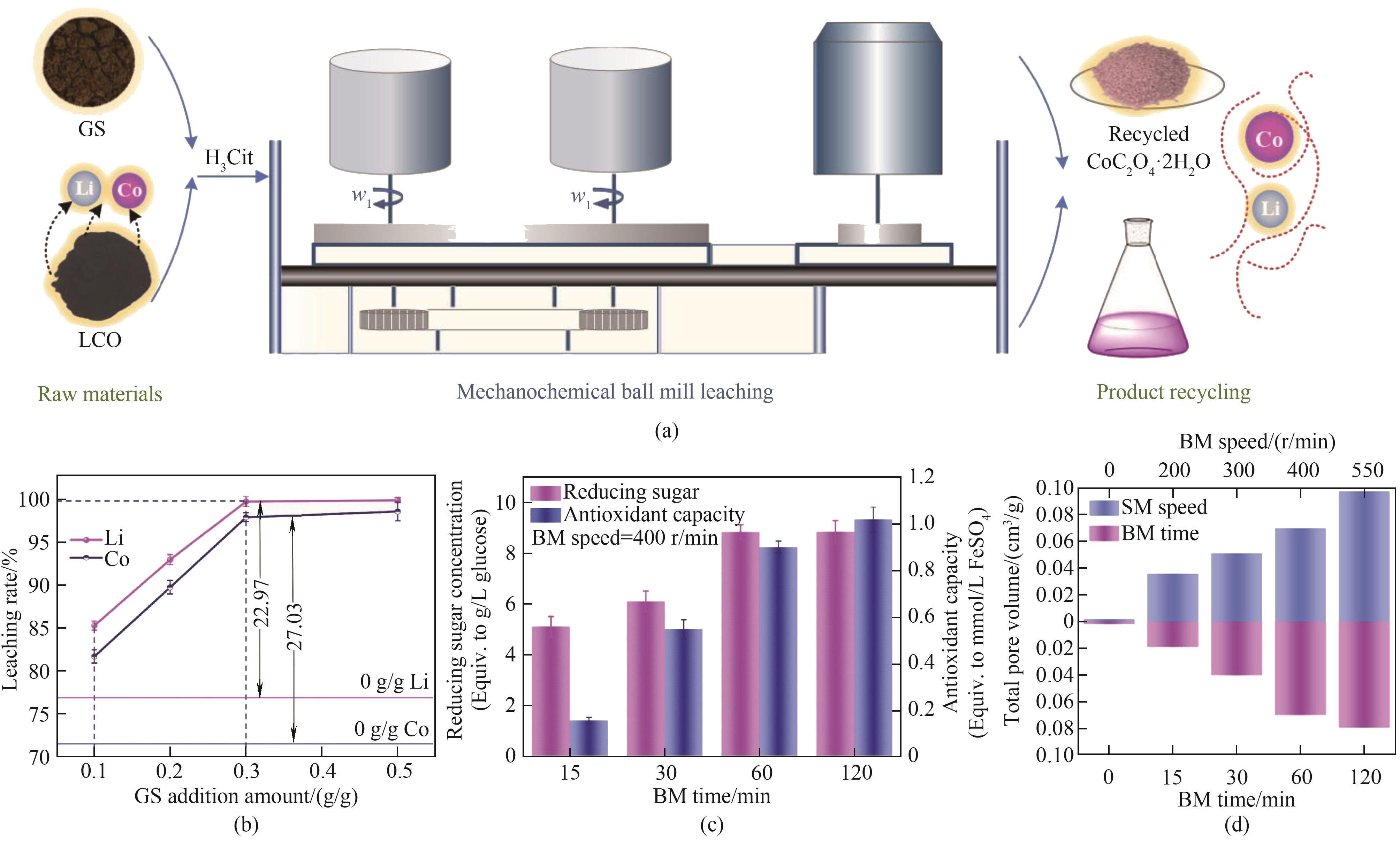
Fig.3 (a) Schematic diagram of the procedure for mechanochemical leaching of valuable metals from LCO; (b) Leaching rates of metals from LCO with different amounts of added GS; (c) Reducing sugar content and antioxidant capacity of GS with different BM times; (d) Total pore volume[77]
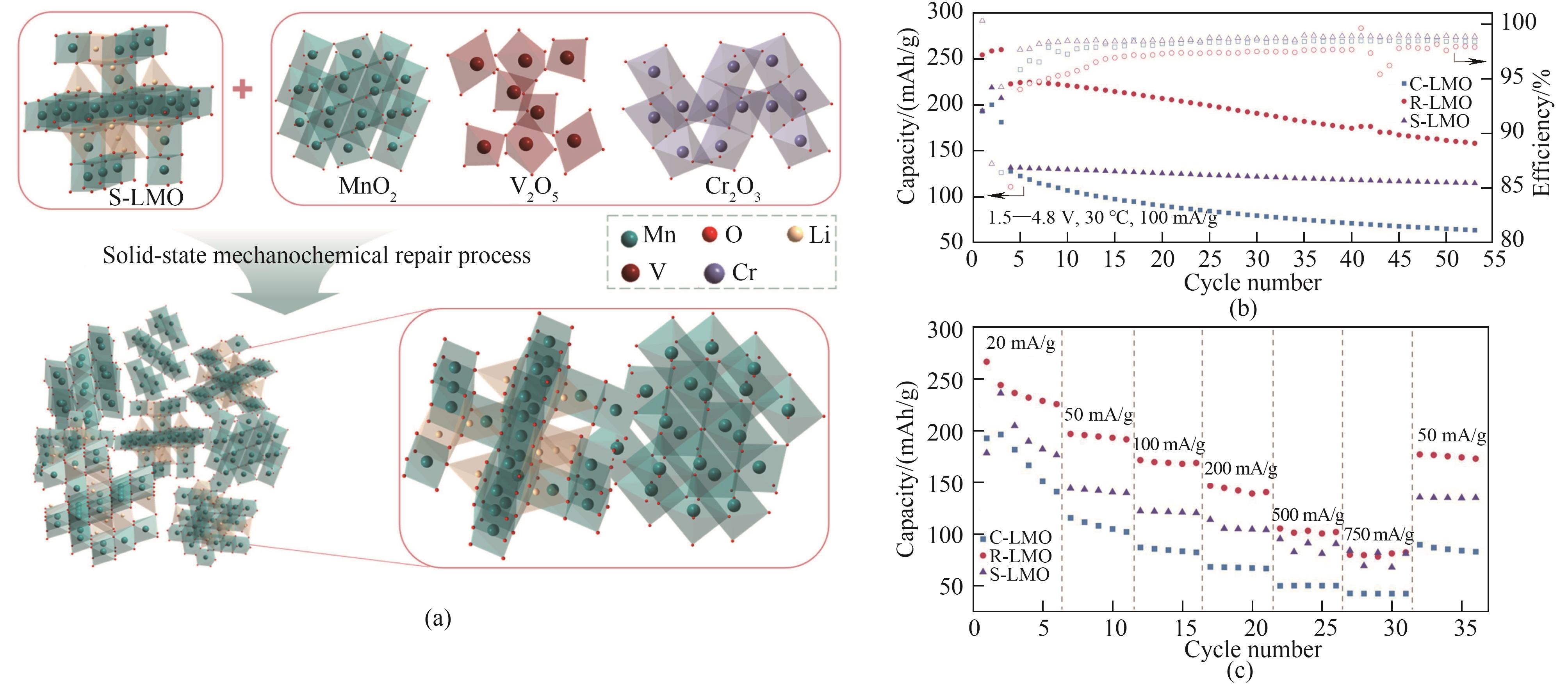
Fig.7 (a) Strategy diagram for repairing LMO cathode materials using mechanochemical technology; (b) cycling performance of repaired R-LMO at 50 mA/g; (c) The rate capability of repaired R-LMO at different current rates[89]
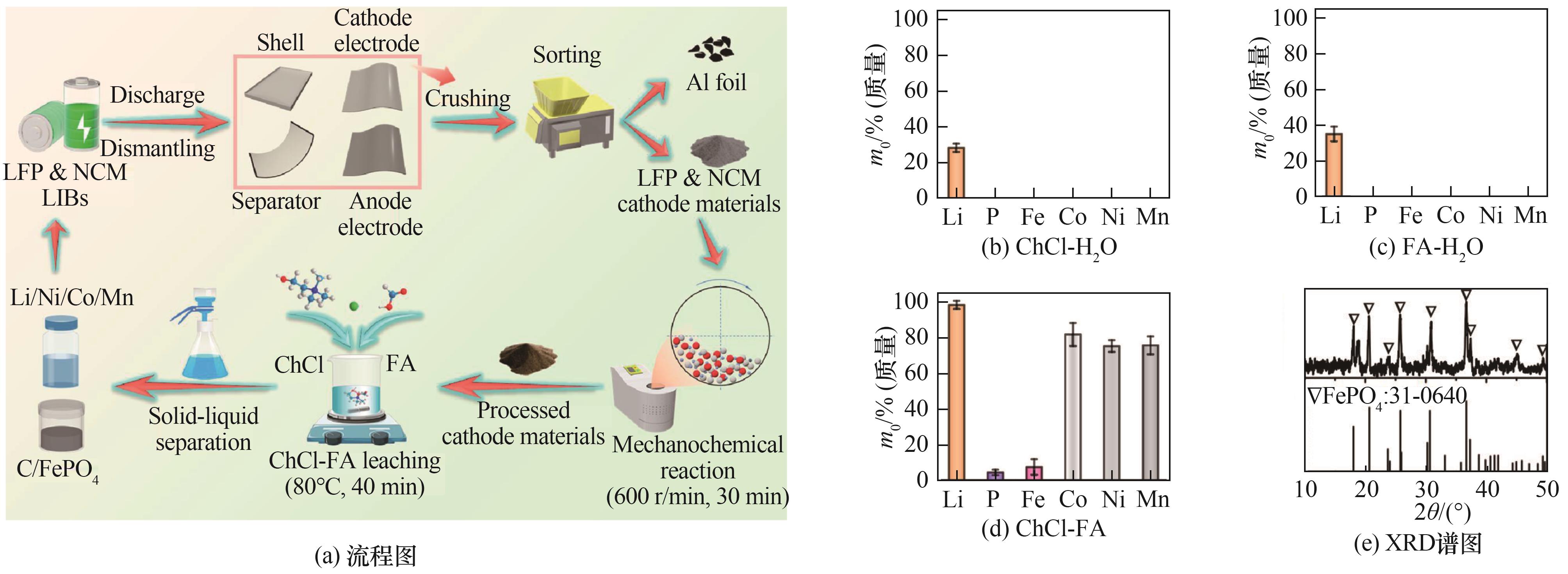
Fig.8 Flow chart of selective extraction of Li, Ni, Co, and Mn from mixed cathode materials of LIBs, as well as XRD patterns of leaching percentage of mixed cathode materials in different solvent systems and residue of mixed cathode materials after extracting critical metals[91]
| 1 | 陈海生, 李泓, 徐玉杰, 等. 2022年中国储能技术研究进展[J]. 储能科学与技术, 2023, 12(5): 1516-1552. |
| Chen H S, Li H, Xu Y J, et al. Research progress on energy storage technologies of China in 2022[J]. Energy Storage Science and Technology, 2023, 12(5): 1516-1552. | |
| 2 | Ren J Z. New energy vehicle in China for sustainable development: analysis of success factors and strategic implications[J]. Transportation Research Part D: Transport and Environment, 2018, 59: 268-288. |
| 3 | Zhang H, Cai G X. Subsidy strategy on new-energy vehicle based on incomplete information: a case in china[J]. Physica A: Statistical Mechanics and Its Applications, 2020, 541: 123370. |
| 4 | Ma X T, Azhari L, Wang Y. Li-ion battery recycling challenges[J]. Chem, 2021, 7(11): 2843-2847. |
| 5 | Wang Y, Yin H Y, An L A. An upcoming global challenge: efficient recycling for end-of-life lithium‐ion batteries[J]. Global Challenges, 2022, 6(12): 2200184. |
| 6 | 徐政和, 刘振达, 王树宾, 等. 湿法回收废旧锂离子电池有价金属的研究进展[J]. 中国矿业大学学报, 2022, 51(3): 454-465. |
| Xu Z H, Liu Z D, Wang S B, et al. Review on hydronmetallurgical recovery of valuable metals from spent lithium-ion batteries[J]. Journal of China University of Mining & Technology, 2022, 51(3): 454-465. | |
| 7 | Zhou L F, Yang D R, Du T, et al. The current process for the recycling of spent lithium ion batteries[J]. Frontiers in Chemistry, 2020, 8: 578044. |
| 8 | 赵丹阳, 张翔, 徐帆, 等. 废旧三元锂离子电池正极材料资源化回收研究进展[J]. 储能科学与技术, 2023, 12(10): 3087-3098. |
| Zhao D Y, Zhang X, Xu F, et al. Progress of resource recovery of retired ternary lithium-ion battery cathode materials[J]. Energy Storage Science and Technology, 2023, 12(10): 3087-3098. | |
| 9 | 王昊, 霍进达, 曲国瑞, 等. 退役锂电池正极材料资源化回收技术研究进展[J]. 化工进展, 2023, 42(5): 2702-2716. |
| Wang H, Huo J D, Qu G R, et al. Research progress of positive electrode material recycling technology for retired lithium battery[J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2702-2716. | |
| 10 | Zhang J K, Azimi G. Recycling of lithium, cobalt, nickel, and manganese from end-of-life lithium-ion battery of an electric vehicle using supercritical carbon dioxide[J]. Resources, Conservation and Recycling, 2022, 187: 106628. |
| 11 | Abdalla A M, Abdullah M F, Dawood M K, et al. Innovative lithium-ion battery recycling: sustainable process for recovery of critical materials from lithium-ion batteries[J]. Journal of Energy Storage, 2023, 67: 107551. |
| 12 | Baum Z J, Bird R E, Yu X A, et al. Lithium-ion battery recycling— overview of techniques and trends[J]. ACS Energy Letters, 2022, 7(2): 712-719. |
| 13 | Fu Y P, He Y Q, Chen H C, et al. Effective leaching and extraction of valuable metals from electrode material of spent lithium-ion batteries using mixed organic acids leachant[J]. Journal of Industrial and Engineering Chemistry, 2019, 79: 154-162. |
| 14 | Huang Y K, Geng Y B, Han G H, et al. A perspective of stepwise utilization of hazardous zinc plant purification residue based on selective alkaline leaching of zinc[J]. Journal of Hazardous Materials, 2020, 389: 122090. |
| 15 | Qiao D H, Wang G S, Gao T M, et al. Potential impact of the end-of-life batteries recycling of electric vehicles on lithium demand in China: 2010—2050[J]. Science of the Total Environment, 2021, 764: 142835. |
| 16 | Zhang G W, He Y Q, Wang H F, et al. Removal of organics by pyrolysis for enhancing liberation and flotation behavior of electrode materials derived from spent lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(5): 2205-2214. |
| 17 | Francine D C, Mentore V, Laura C. Valorization of resources from end-of-life lithium-ion batteries: a review[J]. Critical Reviews in Environmental Science and Technology, 2022, 52(12): 2060-2103. |
| 18 | Makuza B, Tian Q H, Guo X Y, et al. Pyrometallurgical options for recycling spent lithium-ion batteries: a comprehensive review[J]. Journal of Power Sources, 2021, 491: 229622. |
| 19 | Chen M Y, Ma X T, Chen B, et al. Recycling end-of-life electric vehicle lithium-ion batteries[J]. Joule, 2019, 3(11): 2622-2646. |
| 20 | Jie Y F, Yang S H, Li Y, et al. Oxidizing roasting behavior and leaching performance for the recovery of spent LiFePO4 batteries[J]. Minerals, 2020, 10(11): 949. |
| 21 | Ren G X, Xiao S W, Xie M Q, et al. Recovery of valuable metals from spent lithium ion batteries by smelting reduction process based on FeO-SiO2-Al2O3 slag system[J]. Transactions of Nonferrous Metals Society of China, 2017, 27(2): 450-456. |
| 22 | Dang H, Chang Z D, Wu X, et al. Na2SO4-NaCl binary eutectic salt roasting to enhance extraction of lithium from pyrometallurgical slag of spent lithium-ion batteries[J]. Chinese Journal of Chemical Engineering, 2022, 41: 294-300. |
| 23 | Fahimi A, Alessandri I, Cornelio A, et al. A microwave-enhanced method able to substitute traditional pyrometallurgy for the future of metals supply from spent lithium-ion batteries[J]. Resources, Conservation and Recycling, 2023, 194: 106989. |
| 24 | 肖忠良, 尹碧露, 宋刘斌, 等. 废旧锂离子电池回收工艺研究进展及其安全风险分析[J]. 化工学报, 2023, 74(4): 1446-1456. |
| Xiao Z L, Yin B L, Song L B, et al. Research progress of waste lithium-ion battery recycling process and its safety risk analysis[J]. CIESC Journal, 2023, 74(4): 1446-1456. | |
| 25 | Barik S P, Prabaharan G, Kumar L. Leaching and separation of Co and Mn from electrode materials of spent lithium-ion batteries using hydrochloric acid: laboratory and pilot scale study[J]. Journal of Cleaner Production, 2017, 147: 37-43. |
| 26 | Porvali A, Aaltonen M, Ojanen S, et al. Mechanical and hydrometallurgical processes in HCl media for the recycling of valuable metals from Li-ion battery waste[J]. Resources, Conservation and Recycling, 2019, 142: 257-266. |
| 27 | Xing L, Bao J R, Zhou S Y, et al. Ultra-fast leaching of critical metals from spent lithium-ion batteries cathode materials achieved by the synergy-coordination mechanism[J]. Chemical Engineering Journal, 2021, 420: 129593. |
| 28 | Chen D D, Rao S, Wang D X, et al. Synergistic leaching of valuable metals from spent Li-ion batteries using sulfuric acid-L-ascorbic acid system[J]. Chemical Engineering Journal, 2020, 388: 124321. |
| 29 | Li J S, Yang X Y, Yin Z L. Recovery of manganese from sulfuric acid leaching liquor of spent lithium-ion batteries and synthesis of lithium ion-sieve[J]. Journal of Environmental Chemical Engineering, 2018, 6(5): 6407-6413. |
| 30 | Chen H, Gu S, Guo Y X, et al. Leaching of cathode materials from spent lithium-ion batteries by using a mixture of ascorbic acid and HNO3 [J]. Hydrometallurgy, 2021, 205: 105746. |
| 31 | Peng C, Liu F P, Wang Z L, et al. Selective extraction of lithium (Li) and preparation of battery grade lithium carbonate (Li2CO3) from spent Li-ion batteries in nitrate system[J]. Journal of Power Sources, 2019, 415: 179-188. |
| 32 | Lee C K, Rhee K I. Reductive leaching of cathodic active materials from lithium ion battery wastes[J]. Hydrometallurgy, 2003, 68(1/2/3): 5-10. |
| 33 | Sun L, Qiu K Q. Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries[J]. Journal of Hazardous Materials, 2011, 194: 378-384. |
| 34 | Vieceli N, Nogueira C A, Guimarães C, et al. Hydrometallurgical recycling of lithium-ion batteries by reductive leaching with sodium metabisulphite[J]. Waste Management, 2018, 71: 350-361. |
| 35 | Lee C K, Rhee K I. Preparation of LiCoO2 from spent lithium-ion batteries[J]. Journal of Power Sources, 2002, 109(1): 17-21. |
| 36 | Meng Q, Zhang Y J, Dong P. Use of glucose as reductant to recover Co from spent lithium ions batteries[J]. Waste Management, 2017, 64: 214-218. |
| 37 | Yadav P, Jie C J, Tan S, et al. Recycling of cathode from spent lithium iron phosphate batteries[J]. Journal of Hazardous Materials, 2020, 399: 123068. |
| 38 | Prasetyo E, Muryanta W A, Anggraini A G, et al. Tannic acid as a novel and green leaching reagent for cobalt and lithium recycling from spent lithium-ion batteries[J]. Journal of Material Cycles and Waste Management, 2022, 24(3): 927-938. |
| 39 | Santana I L, Moreira T F M, Lelis M F F, et al. Photocatalytic properties of Co3O4/LiCoO2 recycled from spent lithium-ion batteries using citric acid as leaching agent[J]. Materials Chemistry and Physics, 2017, 190: 38-44. |
| 40 | Musariri B, Akdogan G, Dorfling C, et al. Evaluating organic acids as alternative leaching reagents for metal recovery from lithium ion batteries[J]. Minerals Engineering, 2019, 137: 108-117. |
| 41 | Wang B, Lin X Y, Tang Y Y, et al. Recycling LiCoO2 with methanesulfonic acid for regeneration of lithium-ion battery electrode materials[J]. Journal of Power Sources, 2019, 436: 226828. |
| 42 | Wu Z R, Soh T, Chan J J, et al. Repurposing of fruit peel waste as a green reductant for recycling of spent lithium-ion batteries[J]. Environmental Science & Technology, 2020, 54(15): 9681-9692. |
| 43 | Nayaka G P, Pai K V, Santhosh G, et al. Dissolution of cathode active material of spent Li-ion batteries using tartaric acid and ascorbic acid mixture to recover Co[J]. Hydrometallurgy, 2016, 161: 54-57. |
| 44 | Fang J H, Ding Z P, Ling Y, et al. Green recycling and regeneration of LiNi0.5Co0.2Mn0.3O2 from spent lithium-ion batteries assisted by sodium sulfate electrolysis[J]. Chemical Engineering Journal, 2022, 440: 135880. |
| 45 | Zheng X H, Gao W F, Zhang X H, et al. Spent lithium-ion battery recycling—reductive ammonia leaching of metals from cathode scrap by sodium sulphite[J]. Waste Management, 2017, 60: 680-688. |
| 46 | Fan E S, Yang J B, Huang Y X, et al. Leaching mechanisms of recycling valuable metals from spent lithium-ion batteries by a malonic acid-based leaching system[J]. ACS Applied Energy Materials, 2020, 3(9): 8532-8542. |
| 47 | Gao R C, Sun C H, Xu L J, et al. Recycling LiNi0.5Co0.2Mn0.3O2 material from spent lithium-ion batteries by oxalate co-precipitation[J]. Vacuum, 2020, 173: 109181. |
| 48 | Li L, Fan E S, Guan Y B, et al. Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(6): 5224-5233. |
| 49 | Fan M, Chang X, Guo Y J, et al. Increased residual lithium compounds guided design for green recycling of spent lithium-ion cathodes[J]. Energy & Environmental Science, 2021, 14(3): 1461-1468. |
| 50 | Kaupp G. Mechanochemistry: the varied applications of mechanical bond-breaking[J]. CrystEngComm, 2009, 11(3): 388-403. |
| 51 | Dong D, Zhang Y S, Shan M Y, et al. Application of mechanochemical technology for removal/solidification pollutant and preparation/recycling energy storage materials[J]. Journal of Cleaner Production, 2022, 348: 131351. |
| 52 | Amusat S O, Kebede T G, Dube S, et al. Ball-milling synthesis of biochar and biochar-based nanocomposites and prospects for removal of emerging contaminants: a review[J]. Journal of Water Process Engineering, 2021, 41: 101993. |
| 53 | Kumar M, Xiong X N, Wan Z H, et al. Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials[J]. Bioresource Technology, 2020, 312: 123613. |
| 54 | Rajaonarivony K R, Mayer-Laigle C, Piriou B, et al. Comparative comminution efficiencies of rotary, stirred and vibrating ball-mills for the production of ultrafine biomass powders[J]. Energy, 2021, 227: 120508. |
| 55 | Adamczyk-Habrajska M, Szafraniak-Wiza I, Goryczka T, et al. Impedance studies of K0.5Na0.5NbO3 ceramics prepared from mechanochemically activated powders[J]. Materials Chemistry and Physics, 2020, 242: 122451. |
| 56 | Deng S S, Feng N N, Kang S G, et al. Mechanochemical formation of chlorinated phenoxy radicals and their roles in the remediation of hexachlorobenzene contaminated soil[J]. Journal of Hazardous Materials, 2018, 352: 172-181. |
| 57 | Madanayake S N, Manipura A, Thakuria R, et al. Opportunities and challenges in mechanochemical cocrystallization toward scaled-up pharmaceutical manufacturing[J]. Organic Process Research & Development, 2023, 27(3): 409-422. |
| 58 | Solares-Briones M, Coyote-Dotor G, Páez-Franco J C, et al. Mechanochemistry: a green approach in the preparation of pharmaceutical cocrystals[J]. Pharmaceutics, 2021, 13(6): 790. |
| 59 | 耿晨旭, 孙玉绣, 黄宏亮, 等. 机械化学法合成小尺寸MOF填料助力高性能CO2分离[J]. 化工学报, 2021, 72(9): 4750-4758. |
| Geng C X, Sun Y X, Huang H L, et al. Mechanochemically synthesized small sized MOF fillers assisted for highly efficient CO2 separation[J]. CIESC Journal, 2021, 72(9): 4750-4758. | |
| 60 | 李玉洁, 苗晋朋, 孙雪娇, 等. 机械化学法合成金属有机骨架材料HKUST-1及其吸附苯性能[J]. 化工学报, 2015, 66(2): 793-799. |
| Li Y J, Miao J P, Sun X J, et al. Mechano-chemical synthesis of HKUST-1 with high capacity of benzene adsorption[J]. CIESC Journal, 2015, 66(2): 793-799 | |
| 61 | 贺理珀, 孙淑英, 于建国. 退役锂离子电池中有价金属回收研究进展[J]. 化工学报, 2018, 69(1): 327-340. |
| He L P, Sun S Y, Yu J G. Review on processes and technologies for recovery of valuable metals from spent lithium-ion batteries[J]. CIESC Journal, 2018, 69(1): 327-340. | |
| 62 | Yang Y X, Zheng X H, Cao H B, et al. A closed-loop process for selective metal recovery from spent lithium iron phosphate batteries through mechanochemical activation[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(11): 9972-9980. |
| 63 | 王晨, 高宏, 应媛芳, 等. 机械化学法活化磷矿的机理研究[J]. 硅酸盐通报, 2018, 37(12): 4007-4011. |
| Wang C, Gao H, Ying Y F, et al. Study on the mechanism of mechanochemical activited phosphate[J]. Bulletin of the Chinese Ceramic Society, 2018, 37(12): 4007-4011. | |
| 64 | Su F Y, Zhou X Y, Liu X J, et al. High-efficiency preferential extraction of lithium from spent lithium-ion battery cathode powder via synergistic treatment of mechanochemical activation and oxidation roasting[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(43): 15685-15698. |
| 65 | Wang M M, Tan Q Y, Li J H. Unveiling the role and mechanism of mechanochemical activation on lithium cobalt oxide powders from spent lithium-ion batteries[J]. Environmental Science & Technology, 2018, 52(22): 13136-13143. |
| 66 | Arshad F, Li L, Amin K, et al. A comprehensive review of advancement in recycling anode and electrolyte from spent lithium ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(36): 13527-13554. |
| 67 | Qu G R, Li B, Wei Y G. A novel approach for the recovery and cyclic utilization of valuable metals by co-smelting spent lithium-ion batteries with copper slag[J]. Chemical Engineering Journal, 2023, 451: 138897. |
| 68 | Yang C, Zhang J L, Liang G Q, et al. An advanced strategy of “metallurgy before sorting” for recycling spent entire ternary lithium-ion batteries[J]. Journal of Cleaner Production, 2022, 361: 132268. |
| 69 | Shih Y J, Chien S K, Jhang S R, et al. Chemical leaching, precipitation and solvent extraction for sequential separation of valuable metals in cathode material of spent lithium ion batteries[J]. Journal of the Taiwan Institute of Chemical Engineers, 2019, 100: 151-159. |
| 70 | Dolotko O, Hlova I Z, Mudryk Y, et al. Mechanochemical recovery of Co and Li from LCO cathode of lithium-ion battery[J]. Journal of Alloys and Compounds, 2020, 824: 153876. |
| 71 | Liu G Q, Liu Z J, Gu J, et al. A facile new process for the efficient conversion of spent LiFePO4 batteries via (NH4)2S2O8-assisted mechanochemical activation coupled with water leaching[J]. Chemical Engineering Journal, 2023, 471: 144265. |
| 72 | Zhang Q Y, Fan E S, Lin J, et al. Acid-free mechanochemical process to enhance the selective recycling of spent LiFePO4 batteries[J]. Journal of Hazardous Materials, 2023, 443: 130160. |
| 73 | Wu L X, Zhang F S, Zhang Z Y,et al. An environmentally friendly process for selective recovery of lithium and simultaneous synthesis of LiFe5O8 from spent LiFePO4 battery by mechanochemical[J]. Journal of Cleaner Production, 2023, 396: 136504. |
| 74 | Li L, Bian Y F, Zhang X X, et al. A green and effective room-temperature recycling process of LiFePO4 cathode materials for lithium-ion batteries[J]. Waste Management, 2019, 85: 437-444. |
| 75 | Liu K, Tan Q Y, Liu L L, et al. Acid-free and selective extraction of lithium from spent lithium iron phosphate batteries via a mechanochemically induced isomorphic substitution[J]. Environmental Science & Technology, 2019, 53(16): 9781-9788. |
| 76 | Xu B, Dong P, Duan J G, et al. Regenerating the used LiFePO4 to high performance cathode via mechanochemical activation assisted V5+ doping[J]. Ceramics International, 2019, 45(9): 11792-11801. |
| 77 | Zhang S Y, Zhang C L, Zhang X H, et al. A mechanochemical method for one-step leaching of metals from spent LIBs[J]. Waste Management, 2023, 161: 245-253. |
| 78 | Zhang S Y, Zhang C L, Zhang X H, et al. Recovery of Li and Co from spent Li-ion batteries by mechanochemical integration with NH4Cl [J]. ACS Sustainable Chemistry & Engineering, 2022, 10(17): 5611-5620. |
| 79 | Wang M M, Zhang C C, Zhang F S. An environmental benign process for cobalt and lithium recovery from spent lithium-ion batteries by mechanochemical approach[J]. Waste Management, 2016, 51: 239-244. |
| 80 | Luo B, Jiang B, Peng P, et al. Improving the electrochemical performance of LiNi1/3Co1/3Mn1/3O2 cathode material via tungsten modification[J]. Electrochimica Acta, 2019, 297: 398-405. |
| 81 | Meng X Q, Hao J, Cao H B, et al. Recycling of LiNi1/3Co1/3Mn1/3O2 cathode materials from spent lithium-ion batteries using mechanochemical activation and solid-state sintering[J]. Waste Management, 2019, 84: 54-63. |
| 82 | Yang Y X, Yang H L, Cao H B, et al. Direct preparation of efficient catalyst for oxygen evolution reaction and high-purity Li2CO3 from spent LiNi0.5Mn0.3Co0.2O2 batteries[J]. Journal of Cleaner Production, 2019, 236: 117576. |
| 83 | Jin S, Liang J Q, Mu D Y, et al. Structural evolution of layered oxide cathodes for spent Li-ion batteries: degradation mechanism and repair strategy[J]. SusMat, 2023, 3(3): 362-378. |
| 84 | Gao G L, Luo X M, Liu N, et al. Exploration of sequential mechanochemical activation and complexation leaching for enhanced recovery of valuable metals from spent lithium-ion batteries[J]. Ionics, 2023, 29(9): 3585-3596. |
| 85 | Cai L, Lin J A, Fan E S, et al. Eco-friendly organic acid-assisted mechanochemical process for metal extraction from spent lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(32): 10649-10657. |
| 86 | Dolotko O, Gehrke N, Malliaridou T, et al. Universal and efficient extraction of lithium for lithium-ion battery recycling using mechanochemistry[J]. Communications Chemistry, 2023, 6: 49. |
| 87 | Xie J Y, Huang K Y, Nie Z L, et al. An effective process for the recovery of valuable metals from cathode material of lithium-ion batteries by mechanochemical reduction[J]. Resources, Conservation and Recycling, 2021, 168: 105261. |
| 88 | Liang Z L, Peng G W, Hu J P, et al. Mechanochemically assisted persulfate activation for the facile recovery of metals from spent lithium ion batteries[J]. Waste Management, 2022, 150: 290-300. |
| 89 | Lin J, Chen X, Fan E S, et al. A green repair pathway for spent spinel cathode material: coupled mechanochemistry and solid-phase reactions[J]. eScience, 2023, 3(3): 100110. |
| 90 | He S C, Zhou A, Jiang T, et al. Mechanochemical enhanced lithium selective extraction from spent cathode material via two-step molten salt treatments[J]. Journal of Power Sources, 2023, 580:233406. |
| 91 | Wang M M, Liu K, Xu Z B, et al. Selective extraction of critical metals from spent lithium-ion batteries[J]. Environmental Science & Technology, 2023, 57(9): 3940-3950. |
| 92 | Jiang Y Z, Chen X P, Yan S X, et al. Mechanochemistry-induced recycling of spent lithium-ion batteries for synergistic treatment of mixed cathode powders[J]. Green Chemistry, 2022, 24(15): 5987-5997. |
| [1] | Chao KANG, Jinpeng QIAO, Shengchao YANG, Chao PENG, Yuanpeng FU, Bin LIU, Jianrong LIU, Aleksandrova TATIANA, Chenlong DUAN. Research progress on activation extraction of valuable metals in coal gangue [J]. CIESC Journal, 2023, 74(7): 2783-2799. |
| [2] | Jing LI, Conghao SHEN, Daliang GUO, Jing LI, Lizheng SHA, Xin TONG. Research progress in the application of lignin-based carbon fiber composite materials in energy storage components [J]. CIESC Journal, 2023, 74(6): 2322-2334. |
| [3] | Zhongliang XIAO, Bilu YIN, Liubin SONG, Yinjie KUANG, Tingting ZHAO, Cheng LIU, Rongyao YUAN. Research progress of waste lithium-ion battery recycling process and its safety risk analysis [J]. CIESC Journal, 2023, 74(4): 1446-1456. |
| [4] | Pengpeng WANG, Yanggang JIA, Xia SHAO, Jie CHENG, Aiqin MAO, Jie TAN, Daolai FANG. Preparation and lithium storage performance of K+-doped spinel (Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)3O4 high-entropy oxide anode materials [J]. CIESC Journal, 2022, 73(12): 5625-5637. |
| [5] | Liubin SONG, Yixuan WANG, Yinjie KUANG, Yubo XIA, Zhongliang XIAO. Development and prospect of pivotal materials and technologies in sodium-ion batteries [J]. CIESC Journal, 2022, 73(11): 4814-4825. |
| [6] | Yiwei ZHOU, Zhuo CHEN, Jianhong XU. Progress and prospect of recycling spent lithium battery cathode materials by hydrometallurgy [J]. CIESC Journal, 2022, 73(1): 85-96. |
| [7] | Tao HU, Xiong ZHANG, Yabin AN, Chen LI, Yanwei MA. Research progress of carbon cathode materials for Li-ion capacitors [J]. CIESC Journal, 2020, 71(6): 2530-2546. |
| [8] | Jie WANG, Yuan LI, Hailei ZHAO. Synthesis and lithium storage performance of three-dimensional Co3O4 micro-flowers assembled with nanoparticles [J]. CIESC Journal, 2020, 71(4): 1844-1850. |
| [9] | Zhibo ZHANG, Kunyao PENG, Maoning GENG, Xinyue ZHAO, Si LIU, Changbao ZHU. Recent progress on cathode materials for potassium-ion batteries [J]. CIESC Journal, 2020, 71(10): 4429-4444. |
| [10] | Liubin SONG, Anxian LI, Zhongliang XIAO, Zhenzhen CHI, Zhong CAO. Application research status of first-principles in lithium-ion battery electrode materials [J]. CIESC Journal, 2019, 70(6): 2051-2059. |
| [11] | WANG Jian, GUO Hang, YE Fang, MA Chongfang. Numerical simulation of effect of phase change material on temperature distribution in power battery pack of vehicle [J]. CIESC Journal, 2018, 69(4): 1611-1619. |
| [12] | GAO Xiang, GUO Yuan, WEI Difeng, LUO Yingwu, SU Rongxin. Recent progress on binders for silicon-based anodes in lithium-ion batteries [J]. CIESC Journal, 2018, 69(11): 4605-4613. |
| [13] | HE Lipo, SUN Shuying, YU Jianguo. Review on processes and technologies for recovery of valuable metals from spent lithium-ion batteries [J]. CIESC Journal, 2018, 69(1): 327-340. |
| [14] | WANG Jian, GUO Hang, YE Fang, MA Chongfang. Numerical simulation of effect of heat pipe cooling device on temperature distribution in lithium-ion battery pack of vehicle [J]. CIESC Journal, 2016, 67(S2): 340-347. |
| [15] | LI Yanwei, LI Shiyu, XIE Zhiping, YAO Jinhuan, JIANG Jiqiong, ZHANG Lingzhi. Surface morphology and sodium storage performance of V2O5 thin film electrode prepared by CTAB assisted electrodeposition [J]. CIESC Journal, 2016, 67(11): 4771-4778. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||