CIESC Journal ›› 2023, Vol. 74 ›› Issue (11): 4739-4748.DOI: 10.11949/0438-1157.20230930
• Material science and engineering, nanotechnology • Previous Articles
Xiaolin GAO( ), Changguo CHEN(
), Changguo CHEN( )
)
Received:2023-09-06
Revised:2023-10-24
Online:2024-01-22
Published:2023-11-25
Contact:
Changguo CHEN
通讯作者:
陈昌国
作者简介:高孝麟(1990—),男,博士,624972527@qq.com
CLC Number:
Xiaolin GAO, Changguo CHEN. A study on production of silica from CO2 mineralization by wollastonite promoted via air-driven membrane electrolysis technology[J]. CIESC Journal, 2023, 74(11): 4739-4748.
高孝麟, 陈昌国. 空气驱动的膜电解技术促进硅灰石矿化CO2产白炭黑的研究[J]. 化工学报, 2023, 74(11): 4739-4748.
Add to citation manager EndNote|Ris|BibTeX
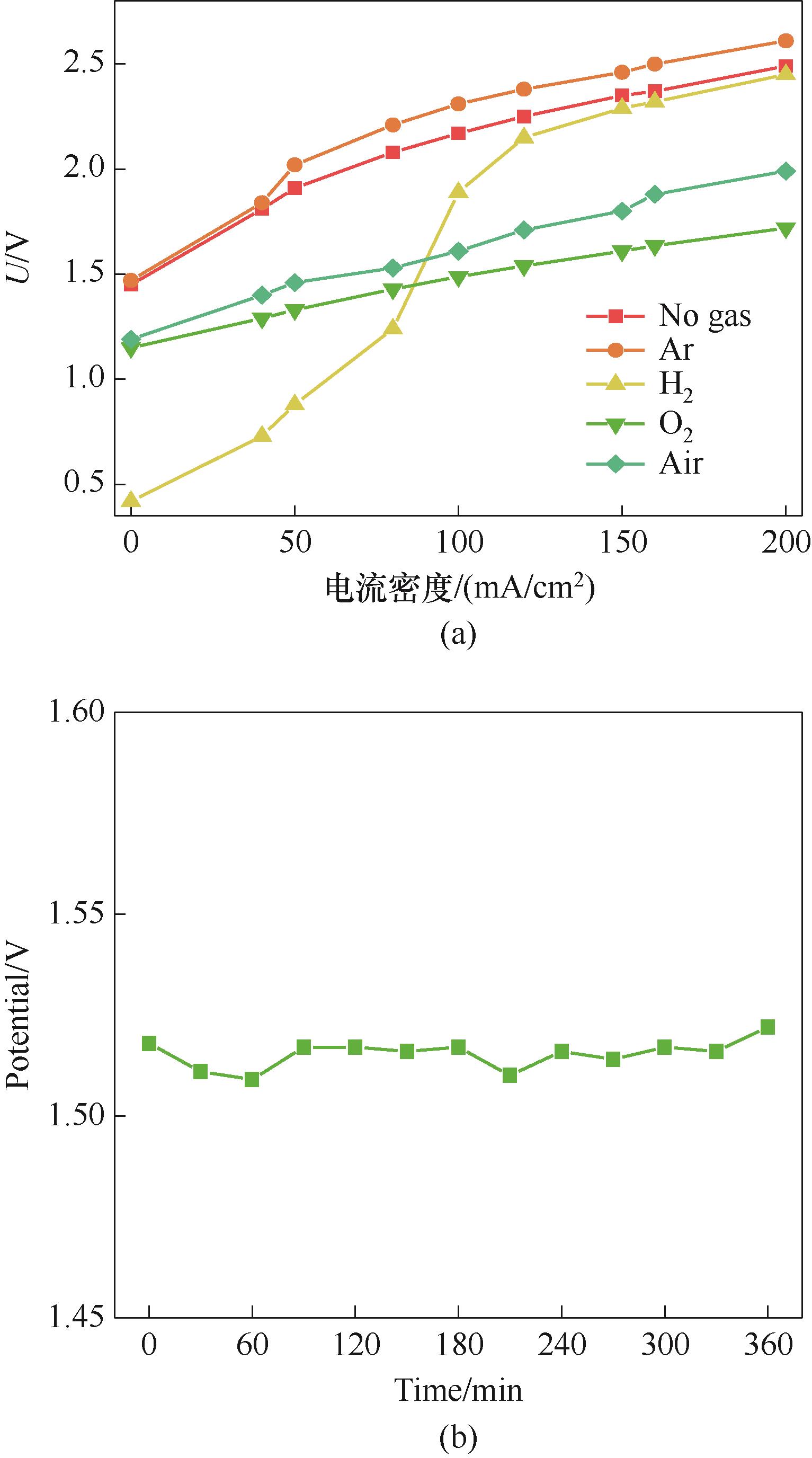
Fig.7 The effect of different gas injected on membrane electrolysis (a) and the voltage change of the air-driven membrane electrolysis stability test (b)
| 电解时间/h | 阴极理论碱浓度/(mol/L) | 阴极实际碱浓度/(mol/L) | 阴极pH | 阴极电解效率/% | 阳极理论酸浓度/(mol/L) | 阳极实际酸浓度/(mol/L) | 阳极pH | 阳极电解效率/% |
|---|---|---|---|---|---|---|---|---|
| 0 | — | -0.0022 | 6.35 | — | — | 0.0022 | 6.35 | — |
| 1 | 0.0309 | 0.02977 | 8.24 | 96.34 | 0.0265 | 0.0263 | 1.86 | 99.25 |
| 2 | 0.0596 | 0.0564 | 8.54 | 94.63 | 0.0552 | 0.0537 | 1.17 | 97.28 |
| 3 | 0.0883 | 0.0804 | 8.69 | 91.05 | 0.0839 | 0.0770 | 1.02 | 93.78 |
| 4 | 0.1170 | 0.1009 | 8.83 | 86.24 | 0.1126 | 0.1002 | 0.95 | 88.99 |
| 5 | 0.1457 | 0.1150 | 8.92 | 78.93 | 0.1414 | 0.1182 | 0.77 | 83.59 |
| 6 | 0.1744 | 0.1218 | 9.01 | 69.84 | 0.1700 | 0.1293 | 0.69 | 76.06 |
Table 1 Changes of pH of anode and cathode and electrolytic efficiency in electrolytic process
| 电解时间/h | 阴极理论碱浓度/(mol/L) | 阴极实际碱浓度/(mol/L) | 阴极pH | 阴极电解效率/% | 阳极理论酸浓度/(mol/L) | 阳极实际酸浓度/(mol/L) | 阳极pH | 阳极电解效率/% |
|---|---|---|---|---|---|---|---|---|
| 0 | — | -0.0022 | 6.35 | — | — | 0.0022 | 6.35 | — |
| 1 | 0.0309 | 0.02977 | 8.24 | 96.34 | 0.0265 | 0.0263 | 1.86 | 99.25 |
| 2 | 0.0596 | 0.0564 | 8.54 | 94.63 | 0.0552 | 0.0537 | 1.17 | 97.28 |
| 3 | 0.0883 | 0.0804 | 8.69 | 91.05 | 0.0839 | 0.0770 | 1.02 | 93.78 |
| 4 | 0.1170 | 0.1009 | 8.83 | 86.24 | 0.1126 | 0.1002 | 0.95 | 88.99 |
| 5 | 0.1457 | 0.1150 | 8.92 | 78.93 | 0.1414 | 0.1182 | 0.77 | 83.59 |
| 6 | 0.1744 | 0.1218 | 9.01 | 69.84 | 0.1700 | 0.1293 | 0.69 | 76.06 |
| 制备过程pH | 比表面积/(m2/g) | 平均孔径/nm | 平均微粒尺寸/nm |
|---|---|---|---|
| 2 | 50 | 9.7 | 119.3 |
| 4 | 88 | 8.3 | 64.7 |
| 6 | 241 | 8.0 | 24.8 |
| 8 | 255 | 5.7 | 23.5 |
Table 2 The specific surface area, pore size and average particle size of SiO2 products obtained under different pH
| 制备过程pH | 比表面积/(m2/g) | 平均孔径/nm | 平均微粒尺寸/nm |
|---|---|---|---|
| 2 | 50 | 9.7 | 119.3 |
| 4 | 88 | 8.3 | 64.7 |
| 6 | 241 | 8.0 | 24.8 |
| 8 | 255 | 5.7 | 23.5 |
| 1 | IEA. CO2 emissions from fuel combustion-highlights 2020[R]. Paris: International Energy Agency, 2020. |
| 2 | IEA. CO2 emissions in 2022[R]. Paris: International Energy Agency, 2023. |
| 3 | IPCC. Climate change 2023: synthesis report[R]. Geneva: Intergovernmental Panel on Climate Change, 2023. |
| 4 | Jayanthakumaran K, Verma R, Liu Y. CO2 emissions, energy consumption, trade and income: a comparative analysis of China and India[J]. Energy Policy, 2012, 42: 450-460. |
| 5 | Parekh A, Chaturvedi G, Dutta A. Sustainability analyses of CO2 sequestration and CO2 utilization as competing options for mitigating CO2 emissions[J]. Sustainable Energy Technologies and Assessments, 2023, 55: 102942. |
| 6 | Zickfeld K, Azevedo D, Mathesius S, et al. Asymmetry in the climate-carbon cycle response to positive and negative CO2 emissions[J]. Nature Climate Change, 2021, 11(7): 613-617. |
| 7 | Liu H J, Were P, Li Q, et al. Worldwide status of CCUS technologies and their development and challenges in China[J]. Geofluids, 2017, 2017: 1-25. |
| 8 | Geerlings H, Zevenhoven R. CO2 mineralization—bridge between storage and utilization of CO2 [J]. Annual Review of Chemical and Biomolecular Engineering, 2013, 4: 103-117. |
| 9 | Faruque Hasan M M, First E L, Boukouvala F, et al. A multi-scale framework for CO2 capture, utilization, and sequestration: CCUS and CCU[J]. Computers & Chemical Engineering, 2015, 81: 2-21. |
| 10 | Ostovari H, Müller L, Mayer F, et al. A climate-optimal supply chain for CO2 capture, utilization, and storage by mineralization[J]. Journal of Cleaner Production, 2022, 360: 131750. |
| 11 | Jiang K, Ashworth P, Zhang S Y, et al. China's carbon capture, utilization and storage (CCUS) policy: a critical review[J]. Renewable and Sustainable Energy Reviews, 2020, 119: 109601. |
| 12 | Xie H P, Yue H R, Zhu J H, et al. Scientific and engineering progress in CO2 mineralization using industrial waste and natural minerals[J]. Engineering, 2015, 1(1): 150-157. |
| 13 | Xie H P, Wang Y F, He Y, et al. Generation of electricity from CO2 mineralization: principle and realization[J]. Science China Technological Sciences, 2014, 57(12): 2335-2343. |
| 14 | Wang C, Yue H R, Li C, et al. Mineralization of CO2 using natural K-feldspar and industrial solid waste to produce soluble potassium[J]. Industrial & Engineering Chemistry Research, 2014, 53(19): 7971-7978. |
| 15 | Zhang R X, Arrigoni A, Panesar D. Could reactive MgO cement be a green solution? The effect of CO2 mineralization and manufacturing route on the potential global warming impact[J]. Cement and Concrete Composites, 2021, 124: 104263. |
| 16 | Wang J J, Watanabe N, Inomoto K, et al. Sustainable process for enhanced CO2 mineralization of calcium silicates using a recyclable chelating agent under alkaline conditions[J]. Journal of Environmental Chemical Engineering, 2022, 10(1): 107055. |
| 17 | Scott A, Oze C, Shah V, et al. Transformation of abundant magnesium silicate minerals for enhanced CO2 sequestration[J]. Communications Earth & Environment, 2021, 2: 25. |
| 18 | Wang F, Dreisinger D. Status of CO2 mineralization and its utilization prospects[J]. Minerals and Mineral Materials, 2022, 1: 4. |
| 19 | Xu H, van Deventer J S J. The geopolymerisation of alumino-silicate minerals[J]. International Journal of Mineral Processing, 2000, 59(3): 247-266. |
| 20 | Yip C K, Lukey G C, Provis J L, et al. Effect of calcium silicate sources on geopolymerisation[J]. Cement and Concrete Research, 2008, 38(4): 554-564. |
| 21 | Flanagan. 2015 minerals yearbook. Asbestos [advance release][R]. Reston: U.S. Geological Survey, 2016. |
| 22 | Zhang S, DePaolo D J. Rates of CO2 mineralization in geological carbon storage[J]. Accounts of Chemical Research, 2017, 50(9): 2075-2084. |
| 23 | Gadikota G, Matter J, Kelemen P, et al. Elucidating the differences in the carbon mineralization behaviors of calcium and magnesium bearing alumino-silicates and magnesium silicates for CO2 storage[J]. Fuel, 2020, 277: 117900. |
| 24 | Ghoorah M, Dlugogorski B Z, Balucan R D, et al. Selection of acid for weak acid processing of wollastonite for mineralisation of CO2 [J]. Fuel, 2014, 122: 277-286. |
| 25 | Xie H P, Wang F H, Wang Y F, et al. CO2 mineralization of natural wollastonite into porous silica and CaCO3 powders promoted via membrane electrolysis[J]. Environmental Earth Sciences, 2018, 77(4): 149. |
| 26 | Rashid M M, Al Mesfer M K, Naseem H, et al. Hydrogen production by water electrolysis: a review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis[J]. International Journal of Engineering and Advanced Technology(IJEAT), 2015, 4(3): 80-93. |
| 27 | Chandran P, Bakshi S, Chatterjee D. Study on the characteristics of hydrogen bubble formation and its transport during electrolysis of water[J]. Chemical Engineering Science, 2015, 138: 99-109. |
| 28 | Christensen J, Albertus P, Sanchez-Carrera R S, et al. A critical review of Li/air batteries[J]. Journal of the Electrochemical Society, 2011, 159(2): R1-R30. |
| 29 | Olabi A G, Sayed E T, Wilberforce T, et al. Metal-air batteries—a review[J]. Energies, 2021, 14(21): 7373. |
| 30 | Pan J, Xu Y Y, Yang H A, et al. Advanced architectures and relatives of air electrodes in Zn-air batteries[J]. Advanced Science, 2018, 5(4): 1700691. |
| 31 | Ahn I K, Joo W, Lee J H, et al. Metal-organic framework-driven porous cobalt disulfide nanoparticles fabricated by gaseous sulfurization as bifunctional electrocatalysts for overall water splitting[J]. Scientific Reports, 2019, 9(1): 19539. |
| 32 | Reier T, Oezaslan M, Strasser P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: a comparative study of nanoparticles and bulk materials[J]. ACS Catalysis, 2012, 2(8): 1765-1772. |
| 33 | Zhang K X, Zou R Q. Advanced transition metal-based OER electrocatalysts: current status, opportunities, and challenges[J]. Small, 2021, 17(37): 2100129. |
| 34 | Su P P, Pei W, Wang X W, et al. Exceptional electrochemical HER performance with enhanced electron transfer between Ru nanoparticles and single atoms dispersed on a carbon substrate[J]. Angewandte Chemie, 2021, 133(29): 16180-16186. |
| 35 | Yu Q M, Zhang Z Y, Qiu S Y, et al. A Ta-TaS2 monolith catalyst with robust and metallic interface for superior hydrogen evolution[J]. Nature Communications, 2021, 12(1): 6051. |
| 36 | Sadeghi E, Peighambardoust N S, Khatamian M, et al. Metal doped layered MgB2 nanoparticles as novel electrocatalysts for water splitting[J]. Scientific Reports, 2021, 11: 3337. |
| 37 | Lazaro A, Brouwers H J H, Quercia G, et al. The properties of amorphous nano-silica synthesized by the dissolution of olivine[J]. Chemical Engineering Journal, 2012, 211/212: 112-121. |
| 38 | 陈天虎. 硅灰石酸溶法制取白炭黑工艺研究[J]. 非金属矿, 1995, 18(3): 45-46, 59. |
| Chen T H. Study on preparation of white carbon black by acid dissolution of wollastonite[J]. Non-Metallic Mines, 1995, 18(3): 45-46, 59. | |
| 39 | Crundwell F K. The mechanism of dissolution of forsterite, olivine and minerals of the orthosilicate group[J]. Hydrometallurgy, 2014, 150: 68-82. |
| 40 | Oelkers E H, Declercq J, Saldi G D, et al. Olivine dissolution rates: a critical review[J]. Chemical Geology, 2018, 500: 1-19. |
| 41 | Diao Y F, Zheng X Y, He B S, et al. Experimental study on capturing CO2 greenhouse gas by ammonia scrubbing[J]. Energy Conversion and Management, 2004, 45(13/14): 2283-2296. |
| 42 | Bai H, Yeh A C. Removal of CO2 greenhouse gas by ammonia scrubbing[J]. Industrial & Engineering Chemistry Research, 1997, 36(6): 2490-2493. |
| 43 | Li X N, Hagaman E, Tsouris C, et al. Removal of carbon dioxide from flue gas by ammonia carbonation in the gas phase[J]. Energy & Fuels, 2003, 17(1): 69-74. |
| 44 | 张昀, 李振中, 李成之, 等. 电站烟气中CO2减排新技术双重效益的研究[J]. 现代电力, 2002, 19(3): 14-15. |
| Zhang Y, Li Z Z, Li C Z, et al. Dual benefits of a new CO2 sequestration technology from fossil fuel power plants[J]. Modern Electric Power, 2002, 19(3): 14-15. |
| [1] | Congqi HUANG, Yimei WU, Jianye CHEN, Shuangquan SHAO. Simulation study of thermal management system of alkaline water electrolysis device for hydrogen production [J]. CIESC Journal, 2023, 74(S1): 320-328. |
| [2] | Yitong LI, Hang GUO, Hao CHEN, Fang YE. Study on operating conditions of proton exchange membrane fuel cells with non-uniform catalyst distributions [J]. CIESC Journal, 2023, 74(9): 3831-3840. |
| [3] | Fei KANG, Weiguang LYU, Feng JU, Zhi SUN. Research on discharge path and evaluation of spent lithium-ion batteries [J]. CIESC Journal, 2023, 74(9): 3903-3911. |
| [4] | Meisi CHEN, Weida CHEN, Xinyao LI, Shangyu LI, Youting WU, Feng ZHANG, Zhibing ZHANG. Advances in silicon-based ionic liquid microparticle enhanced gas capture and conversion [J]. CIESC Journal, 2023, 74(9): 3628-3639. |
| [5] | Yepin CHENG, Daqing HU, Yisha XU, Huayan LIU, Hanfeng LU, Guokai CUI. Application of ionic liquid-based deep eutectic solvents for CO2 conversion [J]. CIESC Journal, 2023, 74(9): 3640-3653. |
| [6] | Jie CHEN, Yongsheng LIN, Kai XIAO, Chen YANG, Ting QIU. Study on catalytic synthesis of sec-butanol by tunable choline-based basic ionic liquids [J]. CIESC Journal, 2023, 74(9): 3716-3730. |
| [7] | Xuejin YANG, Jintao YANG, Ping NING, Fang WANG, Xiaoshuang SONG, Lijuan JIA, Jiayu FENG. Research progress in dry purification technology of highly toxic gas PH3 [J]. CIESC Journal, 2023, 74(9): 3742-3755. |
| [8] | Yali HU, Junyong HU, Suxia MA, Yukun SUN, Xueyi TAN, Jiaxin HUANG, Fengyuan YANG. Development of novel working fluid and study on electrochemical characteristics of reverse electrodialysis heat engine [J]. CIESC Journal, 2023, 74(8): 3513-3521. |
| [9] | Gang YIN, Yihui LI, Fei HE, Wenqi CAO, Min WANG, Feiya YAN, Yu XIANG, Jian LU, Bin LUO, Runting LU. Early warning method of aluminum reduction cell leakage accident based on KPCA and SVM [J]. CIESC Journal, 2023, 74(8): 3419-3428. |
| [10] | Feifei YANG, Shixi ZHAO, Wei ZHOU, Zhonghai NI. Sn doped In2O3 catalyst for selective hydrogenation of CO2 to methanol [J]. CIESC Journal, 2023, 74(8): 3366-3374. |
| [11] | Kaixuan LI, Wei TAN, Manyu ZHANG, Zhihao XU, Xuyu WANG, Hongbing JI. Design of cobalt-nitrogen-carbon/activated carbon rich in zero valent cobalt active site and application of catalytic oxidation of formaldehyde [J]. CIESC Journal, 2023, 74(8): 3342-3352. |
| [12] | Lingding MENG, Ruqing CHONG, Feixue SUN, Zihui MENG, Wenfang LIU. Immobilization of carbonic anhydrase on modified polyethylene membrane and silica [J]. CIESC Journal, 2023, 74(8): 3472-3484. |
| [13] | Xin YANG, Xiao PENG, Kairu XUE, Mengwei SU, Yan WU. Preparation of molecularly imprinted-TiO2 and its properties of photoelectrocatalytic degradation of solubilized PHE [J]. CIESC Journal, 2023, 74(8): 3564-3571. |
| [14] | Mengmeng ZHANG, Dong YAN, Yongfeng SHEN, Wencui LI. Effect of electrolyte types on the storage behaviors of anions and cations for dual-ion batteries [J]. CIESC Journal, 2023, 74(7): 3116-3126. |
| [15] | Yajie YU, Jingru LI, Shufeng ZHOU, Qingbiao LI, Guowu ZHAN. Construction of nanomaterial and integrated catalyst based on biological template: a review [J]. CIESC Journal, 2023, 74(7): 2735-2752. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||