化工学报 ›› 2020, Vol. 71 ›› Issue (2): 755-765.DOI: 10.11949/0438-1157.20190680
收稿日期:2019-06-18
修回日期:2019-08-08
出版日期:2020-02-05
发布日期:2020-02-05
通讯作者:
张光义
作者简介:温宏炎(1994—),男,硕士研究生,
Hongyan WEN1,2( ),Yuming ZHANG1,Dexin JI1,2,Guangyi ZHANG2(
),Yuming ZHANG1,Dexin JI1,2,Guangyi ZHANG2( )
)
Received:2019-06-18
Revised:2019-08-08
Online:2020-02-05
Published:2020-02-05
Contact:
Guangyi ZHANG
摘要:
采用热重分析法研究了不同升温速率下油泥焦、褐煤及其混合物燃烧特性,并利用Kissinger-Akahira-Sunose(KAS)、Flynn-Wall-Ozawa(FWO)和Friedma(FR)等方法计算其燃烧动力学参数。结果表明,油泥焦燃烧主要是固定碳燃烧过程,而褐煤燃烧是挥发分和少量固定碳连续燃烧的过程。褐煤比油泥焦具有更好的燃烧特性,平均活化能更低。油泥焦和褐煤共燃过程中存在明显的协同促进作用,当混合燃料中褐煤占比为75%时协同促进效应达到最强。通过比较KAS、FWO和FR的结果发现,FR法能够更好地体现反应变化的趋势,而KAS法和FWO法的结果具有较高的准确性。通过比较油泥焦和褐煤共燃动力学参数的理论计算值与实验计算值发现,利用热重分析预测混合燃料的燃烧性质具有较高的可靠性,对油泥焦与褐煤共燃技术的应用具有重要的指导作用。
中图分类号:
温宏炎, 张玉明, 纪德馨, 张光义. 油泥焦与褐煤共燃特性及动力学[J]. 化工学报, 2020, 71(2): 755-765.
Hongyan WEN, Yuming ZHANG, Dexin JI, Guangyi ZHANG. Co-combustion of oil sludge char and brown coal: characteristics and kinetics[J]. CIESC Journal, 2020, 71(2): 755-765.
| Sample | Proximate analysis/%(mass) | Ultimate analysis/%(mass) | HHVs/ (MJ/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Var | Mar | Aar | FCar① | Nd | Cd | Hd | Sd | Od① | ||
| SC | 4.91 | 1.01 | 74.40 | 19.68 | 0.45 | 15.96 | 0.51 | 0.47 | 6.95 | 5.43 |
| 25BC75SC | 13.87 | 7.38 | 58.13 | 20.62 | 0.68 | 26.04 | 1.17 | 0.54 | 8.81 | 9.07 |
| 50BC50SC | 19.75 | 13.63 | 42.94 | 23.68 | 1.02 | 34.13 | 1.74 | 0.61 | 12.78 | 13.25 |
| 75BC25SC | 28.16 | 19.75 | 27.16 | 25.93 | 1.33 | 45.21 | 2.20 | 0.73 | 17.10 | 16.98 |
| BC | 33.73 | 26.09 | 11.07 | 29.11 | 1.78 | 56.29 | 3.56 | 0.95 | 22.44 | 21.46 |
表1 燃料样品的工业分析和元素分析
Table 1 Proximate and ultimate of various blended samples
| Sample | Proximate analysis/%(mass) | Ultimate analysis/%(mass) | HHVs/ (MJ/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Var | Mar | Aar | FCar① | Nd | Cd | Hd | Sd | Od① | ||
| SC | 4.91 | 1.01 | 74.40 | 19.68 | 0.45 | 15.96 | 0.51 | 0.47 | 6.95 | 5.43 |
| 25BC75SC | 13.87 | 7.38 | 58.13 | 20.62 | 0.68 | 26.04 | 1.17 | 0.54 | 8.81 | 9.07 |
| 50BC50SC | 19.75 | 13.63 | 42.94 | 23.68 | 1.02 | 34.13 | 1.74 | 0.61 | 12.78 | 13.25 |
| 75BC25SC | 28.16 | 19.75 | 27.16 | 25.93 | 1.33 | 45.21 | 2.20 | 0.73 | 17.10 | 16.98 |
| BC | 33.73 | 26.09 | 11.07 | 29.11 | 1.78 | 56.29 | 3.56 | 0.95 | 22.44 | 21.46 |
| Sample | Component content/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Na2O | MgO | Al2O3 | SiO2 | SO3 | K2O | CaO | Fe2O3 | Others | |
| SC | 2.45 | 3.06 | 15.50 | 57.31 | 3.93 | 2.59 | 7.53 | 5.89 | 1.74 |
| BC | — | 3.94 | 8.56 | 12.63 | 31.65 | 0.54 | 34.46 | 6.52 | 1.70 |
表2 油泥焦灰渣和褐煤灰渣的XRF分析
Table 2 XRF analysis of oil sludge char ash and coal ash
| Sample | Component content/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Na2O | MgO | Al2O3 | SiO2 | SO3 | K2O | CaO | Fe2O3 | Others | |
| SC | 2.45 | 3.06 | 15.50 | 57.31 | 3.93 | 2.59 | 7.53 | 5.89 | 1.74 |
| BC | — | 3.94 | 8.56 | 12.63 | 31.65 | 0.54 | 34.46 | 6.52 | 1.70 |
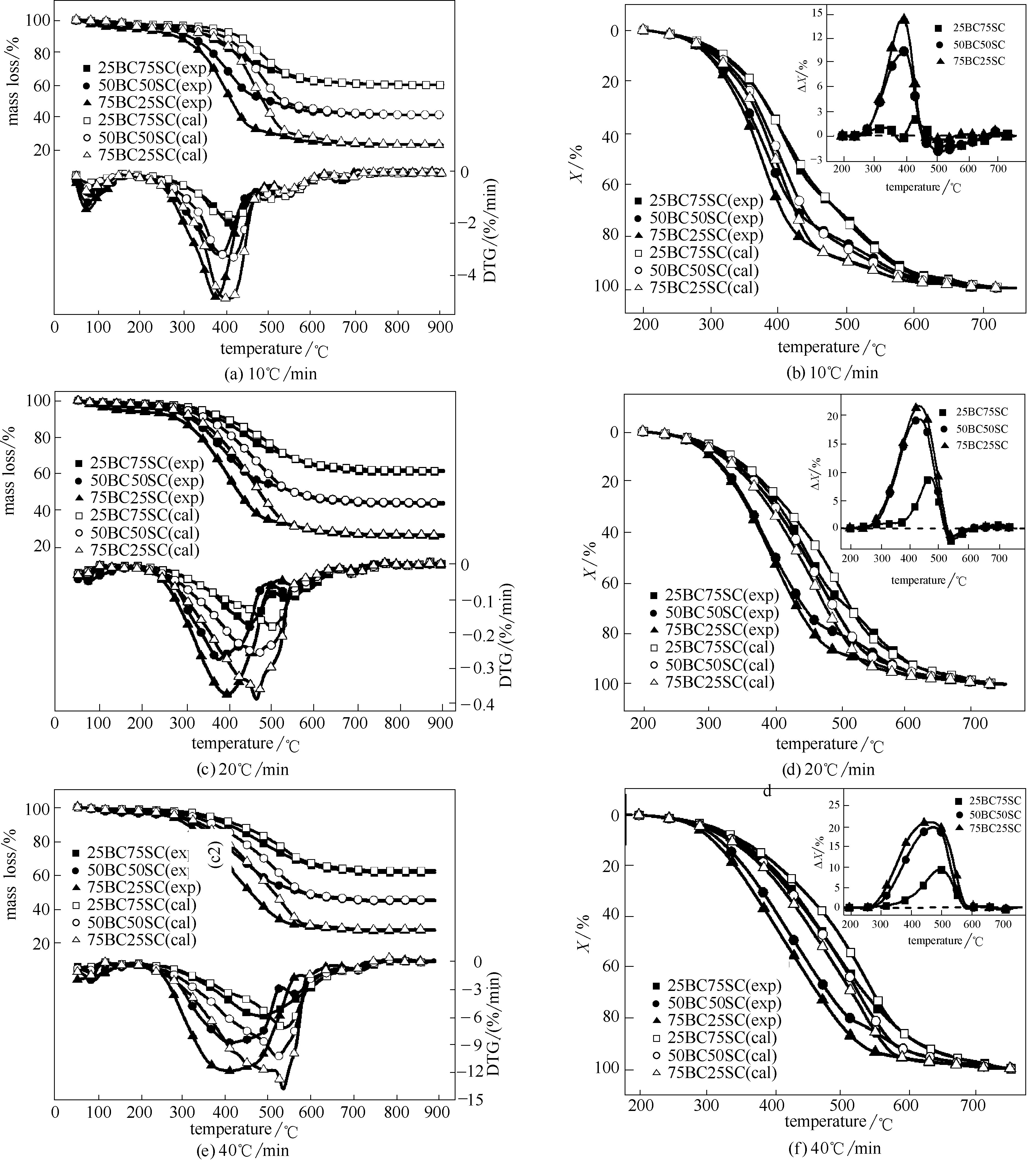
图2 不同升温速率下混合样品的TG、DTG、转化率和?X曲线的实验计算值与理论计算值
Fig.2 Experimental and theoretical TG, DTG, conversion and ?X curves of various blended samples at different heating rates
| Sample | β/(℃/min) | Ti/℃ | Tmax/℃ | Th/℃ | Kr×106/(min-1·℃-2) | Gb×106/(min-1·℃-2) | S×108/(min-2·℃-3) |
|---|---|---|---|---|---|---|---|
| SC | 10 | 426. | 497 | 713 | 6.43 | 5.51 | 0.22 |
| 20 | 441 | 515 | 727 | 12.03 | 10.34 | 0.73 | |
| 40 | 458 | 534 | 753 | 19.26 | 16.63 | 1.71 | |
| 25BC75SC | 10 | 308 | 412 | 675 | 20.45 | 15.29 | 1.48 |
| 20 | 333 | 447 | 694 | 31.02 | 23.11 | 4.07 | |
| 40 | 350 | 469 | 712 | 52.41 | 39.11 | 13.84 | |
| 50BC50SC | 10 | 284 | 378 | 654 | 40.92 | 30.74 | 4.26 |
| 20 | 291 | 380 | 672 | 71.21 | 52.56 | 14.31 | |
| 40 | 295 | 409 | 690 | 109.63 | 79.07 | 42.74 | |
| 75BC25SC | 10 | 279 | 372 | 614 | 67.70 | 50.78 | 9.81 |
| 20 | 289 | 385 | 632 | 96.38 | 72.35 | 27.00 | |
| 40 | 291 | 396 | 644 | 162.49 | 119.41 | 95.63 | |
| BC | 10 | 306 | 397 | 613 | 64.41 | 49.63 | 11.64 |
| 20 | 316 | 427 | 621 | 87.82 | 64.99 | 29.56 | |
| 40 | 331 | 470 | 648 | 155.62 | 109.59 | 100.14 |
表3 不同升温速率下混合样品的燃烧特性参数
Table 3 Combustion characteristic parameters of various blended samples at different heating rates
| Sample | β/(℃/min) | Ti/℃ | Tmax/℃ | Th/℃ | Kr×106/(min-1·℃-2) | Gb×106/(min-1·℃-2) | S×108/(min-2·℃-3) |
|---|---|---|---|---|---|---|---|
| SC | 10 | 426. | 497 | 713 | 6.43 | 5.51 | 0.22 |
| 20 | 441 | 515 | 727 | 12.03 | 10.34 | 0.73 | |
| 40 | 458 | 534 | 753 | 19.26 | 16.63 | 1.71 | |
| 25BC75SC | 10 | 308 | 412 | 675 | 20.45 | 15.29 | 1.48 |
| 20 | 333 | 447 | 694 | 31.02 | 23.11 | 4.07 | |
| 40 | 350 | 469 | 712 | 52.41 | 39.11 | 13.84 | |
| 50BC50SC | 10 | 284 | 378 | 654 | 40.92 | 30.74 | 4.26 |
| 20 | 291 | 380 | 672 | 71.21 | 52.56 | 14.31 | |
| 40 | 295 | 409 | 690 | 109.63 | 79.07 | 42.74 | |
| 75BC25SC | 10 | 279 | 372 | 614 | 67.70 | 50.78 | 9.81 |
| 20 | 289 | 385 | 632 | 96.38 | 72.35 | 27.00 | |
| 40 | 291 | 396 | 644 | 162.49 | 119.41 | 95.63 | |
| BC | 10 | 306 | 397 | 613 | 64.41 | 49.63 | 11.64 |
| 20 | 316 | 427 | 621 | 87.82 | 64.99 | 29.56 | |
| 40 | 331 | 470 | 648 | 155.62 | 109.59 | 100.14 |
| Sample | X | KAS | FWO | FR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E/(kJ/mol) | k0/min-1 | R2 | E/(kJ/mol) | k0/min-1 | R2 | E/(kJ/mol) | k0/min-1 | R2 | ||
| SC | 0.1 | 152.7 | 1.71×107 | 0.796 | 156.3 | 3.79×1013 | 0.822 | 178.3 | 5.44×1011 | 0.899 |
| 0.2 | 208.7 | 8.12×1010 | 0.981 | 210.1 | 1.09×1017 | 0.983 | 234.9 | 2.57×1015 | 0.978 | |
| 0.3 | 197.2 | 7.52×109 | 0.998 | 199.5 | 1.19×1016 | 0.998 | 204.3 | 1.19×1013 | 0.986 | |
| 0.4 | 209.9 | 3.78×1010 | 1.000 | 211.9 | 5.54×1016 | 1.000 | 187.4 | 6.11×1011 | 0.983 | |
| 0.5 | 209.4 | 2.36×1010 | 0.999 | 211.7 | 3.62×1016 | 0.999 | 189.2 | 5.15×1011 | 0.979 | |
| 0.6 | 202.6 | 5.06×109 | 0.997 | 205.5 | 8.64×1015 | 0.997 | 153.3 | 1.48×109 | 0.981 | |
| 0.7 | 192.6 | 6.74×108 | 0.987 | 196.4 | 1.33×1015 | 0.988 | 161.1 | 3.05×109 | 0.966 | |
| 0.8 | 181.3 | 7.41×107 | 0.980 | 186.0 | 1.74×1014 | 0.982 | 154.3 | 6.66×108 | 0.950 | |
| 0.9 | 190.0 | 9.15×107 | 0.948 | 194.9 | 2.16×1014 | 0.955 | 169.3 | 1.63×109 | 0.850 | |
| Ave | 193.8 | — | — | 196.9 | — | — | 181.3 | — | — | |
| BC | 0.1 | 152.4 | 3.64×109 | 0.998 | 154.2 | 5.68×1015 | 0.998 | 138.2 | 7.93×1010 | 0.917 |
| 0.2 | 127.4 | 6.60×106 | 1.000 | 131.0 | 1.65×1013 | 1.000 | 114.5 | 2.52×108 | 0.920 | |
| 0.3 | 107.8 | 8.10×104 | 0.997 | 112.8 | 3.03×1011 | 0.998 | 81.2 | 3.40×105 | 0.949 | |
| 0.4 | 89.0 | 1.65×103 | 0.993 | 95.3 | 9.44×109 | 0.996 | 69.4 | 3.29×104 | 0.940 | |
| 0.5 | 76.8 | 1.40×102 | 0.980 | 84.0 | 1.12×109 | 0.986 | 62.8 | 9.29×103 | 0.939 | |
| 0.6 | 68.6 | 2.73×10 | 0.994 | 76.5 | 2.83×108 | 0.996 | 61.8 | 7.04×103 | 0.934 | |
| 0.7 | 62.5 | 8.34 | 0.991 | 71.0 | 1.08×108 | 0.990 | 69.8 | 2.35×104 | 0.965 | |
| 0.8 | 57.3 | 3.20 | 0.983 | 66.4 | 5.10×107 | 0.982 | 92.4 | 7.62×105 | 0.976 | |
| 0.9 | 61.0 | 5.98 | 0.904 | 70.3 | 9.01×107 | 0.932 | 117.3 | 3.26×107 | 0.969 | |
| Ave | 89.2 | — | — | 95.7 | — | — | 89.7 | — | — | |
表4 油泥焦和褐煤的动力学参数
Table 4 Kinetic parameters of oil sludge char and coal
| Sample | X | KAS | FWO | FR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E/(kJ/mol) | k0/min-1 | R2 | E/(kJ/mol) | k0/min-1 | R2 | E/(kJ/mol) | k0/min-1 | R2 | ||
| SC | 0.1 | 152.7 | 1.71×107 | 0.796 | 156.3 | 3.79×1013 | 0.822 | 178.3 | 5.44×1011 | 0.899 |
| 0.2 | 208.7 | 8.12×1010 | 0.981 | 210.1 | 1.09×1017 | 0.983 | 234.9 | 2.57×1015 | 0.978 | |
| 0.3 | 197.2 | 7.52×109 | 0.998 | 199.5 | 1.19×1016 | 0.998 | 204.3 | 1.19×1013 | 0.986 | |
| 0.4 | 209.9 | 3.78×1010 | 1.000 | 211.9 | 5.54×1016 | 1.000 | 187.4 | 6.11×1011 | 0.983 | |
| 0.5 | 209.4 | 2.36×1010 | 0.999 | 211.7 | 3.62×1016 | 0.999 | 189.2 | 5.15×1011 | 0.979 | |
| 0.6 | 202.6 | 5.06×109 | 0.997 | 205.5 | 8.64×1015 | 0.997 | 153.3 | 1.48×109 | 0.981 | |
| 0.7 | 192.6 | 6.74×108 | 0.987 | 196.4 | 1.33×1015 | 0.988 | 161.1 | 3.05×109 | 0.966 | |
| 0.8 | 181.3 | 7.41×107 | 0.980 | 186.0 | 1.74×1014 | 0.982 | 154.3 | 6.66×108 | 0.950 | |
| 0.9 | 190.0 | 9.15×107 | 0.948 | 194.9 | 2.16×1014 | 0.955 | 169.3 | 1.63×109 | 0.850 | |
| Ave | 193.8 | — | — | 196.9 | — | — | 181.3 | — | — | |
| BC | 0.1 | 152.4 | 3.64×109 | 0.998 | 154.2 | 5.68×1015 | 0.998 | 138.2 | 7.93×1010 | 0.917 |
| 0.2 | 127.4 | 6.60×106 | 1.000 | 131.0 | 1.65×1013 | 1.000 | 114.5 | 2.52×108 | 0.920 | |
| 0.3 | 107.8 | 8.10×104 | 0.997 | 112.8 | 3.03×1011 | 0.998 | 81.2 | 3.40×105 | 0.949 | |
| 0.4 | 89.0 | 1.65×103 | 0.993 | 95.3 | 9.44×109 | 0.996 | 69.4 | 3.29×104 | 0.940 | |
| 0.5 | 76.8 | 1.40×102 | 0.980 | 84.0 | 1.12×109 | 0.986 | 62.8 | 9.29×103 | 0.939 | |
| 0.6 | 68.6 | 2.73×10 | 0.994 | 76.5 | 2.83×108 | 0.996 | 61.8 | 7.04×103 | 0.934 | |
| 0.7 | 62.5 | 8.34 | 0.991 | 71.0 | 1.08×108 | 0.990 | 69.8 | 2.35×104 | 0.965 | |
| 0.8 | 57.3 | 3.20 | 0.983 | 66.4 | 5.10×107 | 0.982 | 92.4 | 7.62×105 | 0.976 | |
| 0.9 | 61.0 | 5.98 | 0.904 | 70.3 | 9.01×107 | 0.932 | 117.3 | 3.26×107 | 0.969 | |
| Ave | 89.2 | — | — | 95.7 | — | — | 89.7 | — | — | |
| Stage | X | KAS | FWO | FR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E/(kJ/mol) | k0/min-1 | R2 | E/(kJ/mol) | k0/min-1 | R2 | E/(kJ/mol) | k0/min-1 | R2 | ||
| volatile (exp) | 0.1 | 127.2 | 2.48×107 | 0.998 | 130.1 | 5.37×1013 | 0.998 | 125.5 | 7.59×109 | 0.882 |
| 0.2 | 122.4 | 3.11×106 | 0.997 | 126.1 | 8.24×1012 | 0.998 | 120.8 | 1.04×109 | 0.987 | |
| 0.3 | 120.6 | 1.17×106 | 0.992 | 124.9 | 3.46×1012 | 0.993 | 115.8 | 2.36×108 | 0.989 | |
| 0.4 | 113.5 | 2.05×105 | 0.993 | 118.5 | 7.25×1011 | 0.994 | 100.8 | 1.21×107 | 0.989 | |
| 0.5 | 106.3 | 4.16×104 | 0.994 | 111.9 | 1.76×1011 | 0.995 | 88.9 | 1.34×106 | 0.986 | |
| 0.6 | 97.6 | 7.37×103 | 0.991 | 103.9 | 3.80×1010 | 0.993 | 81.9 | 3.90×105 | 0.993 | |
| 0.7 | 92.8 | 2.80×103 | 0.995 | 99.5 | 1.65×1010 | 0.996 | 81.5 | 3.73×105 | 0.981 | |
| 0.8 | 90.7 | 1.82×103 | 0.998 | 97.8 | 1.17×1010 | 0.998 | 92.8 | 2.49×106 | 0.945 | |
| 0.9 | 96.2 | 4.51×103 | 0.990 | 103.3 | 2.71×1010 | 0.975 | 120.5 | 2.40×108 | 0.920 | |
| Ave | 107.5 | — | — | 112.9 | — | — | 103.2 | — | — | |
| fixed carbon (exp) | 0.1 | 135.4 | 6.05×104 | 0.962 | 141.0 | 2.04×1011 | 0.968 | 186.4 | 5.66×1011 | 0.915 |
| 0.2 | 151.7 | 1.23×106 | 0.941 | 156.6 | 3.42×1012 | 0.950 | 194.3 | 1.44×102 | 0.926 | |
| 0.3 | 163.4 | 8.80×106 | 0.930 | 168.0 | 2.17×1013 | 0.939 | 196.2 | 1.47×1012 | 0.936 | |
| 0.4 | 172.7 | 3.72×107 | 0.936 | 177.0 | 8.48×1013 | 0.945 | 196.2 | 1.13×1012 | 0.957 | |
| 0.5 | 181.4 | 1.29×108 | 0.938 | 185.4 | 2.75×1014 | 0.946 | 194.9 | 7.15×1011 | 0.954 | |
| 0.6 | 187.6 | 2.85×108 | 0.931 | 191.5 | 5.85×1014 | 0.940 | 191.8 | 3.45×1011 | 0.928 | |
| 0.7 | 185.3 | 1.67×108 | 0.929 | 189.5 | 3.62×1014 | 0.939 | 180.9 | 5.62×1010 | 0.937 | |
| 0.8 | 185.4 | 1.39×108 | 0.948 | 189.9 | 3.11×1014 | 0.955 | 180.2 | 4.02×1010 | 0.975 | |
| 0.9 | 179.1 | 4.17×107 | 0.967 | 184.2 | 1.05×1014 | 0.971 | 178.6 | 2.45×1010 | 0.971 | |
| Ave | 171.3 | — | — | 175.9 | — | — | 188.8 | — | — | |
| volatile (cal) | 0.1 | 204.6 | 4.37×1014 | 0.938 | 203.6 | 3.73×1020 | 0.943 | 193.6 | 1.35×1016 | 0.936 |
| 0.2 | 176.9 | 2.56×1011 | 0.950 | 177.9 | 3.27×1017 | 0.955 | 169.7 | 2.12×1013 | 0.956 | |
| 0.3 | 158.1 | 2.43×109 | 0.976 | 160.4 | 4.19×1015 | 0.979 | 138.4 | 2.41×1010 | 0.998 | |
| 0.4 | 135.7 | 1.96×107 | 0.999 | 139.4 | 4.79×1013 | 0.999 | 109.3 | 7.74×107 | 0.981 | |
| 0.5 | 119.1 | 6.24×105 | 0.997 | 123.9 | 2.06×1012 | 0.998 | 97.7 | 7.80×106 | 0.955 | |
| 0.6 | 108.7 | 7.16×104 | 0.991 | 114.3 | 2.94×1011 | 0.993 | 92.7 | 2.87×106 | 0.963 | |
| 0.7 | 101.6 | 1.68×104 | 0.985 | 107.8 | 8.16×1010 | 0.987 | 90.7 | 1.94×106 | 0.954 | |
| 0.8 | 97.2 | 6.75×103 | 0.976 | 103.8 | 3.72×1010 | 0.981 | 90.1 | 1.93×106 | 0.933 | |
| 0.9 | 94.5 | 4.17×103 | 0.965 | 101.5 | 2.52×1010 | 0.972 | 95.0 | 6.03×106 | 0.930 | |
| Ave | 132.9 | — | — | 137.0 | — | — | 119.7 | — | — | |
| fixed carbon (cal) | 0.1 | 120.1 | 8.21×103 | 0.999 | 126.2 | 3.35×1010 | 1.000 | 98.9 | 2.12×106 | 0.890 |
| 0.2 | 121.7 | 1.89×104 | 0.999 | 127.9 | 7.64×1010 | 0.999 | 104.7 | 5.04×106 | 0.910 | |
| 0.3 | 133.2 | 1.53×105 | 0.970 | 139.0 | 5.36×1011 | 0.975 | 113.8 | 1.97×107 | 0.993 | |
| 0.4 | 150.2 | 2.42×106 | 0.995 | 155.2 | 6.87×1012 | 0.996 | 132.1 | 2.87×108 | 0.990 | |
| 0.5 | 161.9 | 1.56×107 | 1.000 | 166.5 | 3.90×1013 | 1.000 | 148.8 | 3.29×109 | 0.997 | |
| 0.6 | 170.9 | 6.19×107 | 1.000 | 175.2 | 1.43×1014 | 1.000 | 158.7 | 1.34×1010 | 0.986 | |
| 0.7 | 210.6 | 2.29×1010 | 0.999 | 213.1 | 3.63×1016 | 0.999 | 217.1 | 5.91×1013 | 0.979 | |
| 0.8 | 240.5 | 1.78×1012 | 0.999 | 241.7 | 2.23×1018 | 0.999 | 386.9 | 1.79×1024 | 0.956 | |
| 0.9 | 262.4 | 3.97×1013 | 0.979 | 262.6 | 4.31×1019 | 0.982 | 509.5 | 4.58×1031 | 0.904 | |
| Ave | 174.6 | — | — | 178.6 | — | — | 207.8 | — | — | |
表5 75BC25SC样本燃烧活化能实验计算值与理论计算值对比
Table 5 Comparison of experimental and theoretic calculated value of 75BC25SC
| Stage | X | KAS | FWO | FR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E/(kJ/mol) | k0/min-1 | R2 | E/(kJ/mol) | k0/min-1 | R2 | E/(kJ/mol) | k0/min-1 | R2 | ||
| volatile (exp) | 0.1 | 127.2 | 2.48×107 | 0.998 | 130.1 | 5.37×1013 | 0.998 | 125.5 | 7.59×109 | 0.882 |
| 0.2 | 122.4 | 3.11×106 | 0.997 | 126.1 | 8.24×1012 | 0.998 | 120.8 | 1.04×109 | 0.987 | |
| 0.3 | 120.6 | 1.17×106 | 0.992 | 124.9 | 3.46×1012 | 0.993 | 115.8 | 2.36×108 | 0.989 | |
| 0.4 | 113.5 | 2.05×105 | 0.993 | 118.5 | 7.25×1011 | 0.994 | 100.8 | 1.21×107 | 0.989 | |
| 0.5 | 106.3 | 4.16×104 | 0.994 | 111.9 | 1.76×1011 | 0.995 | 88.9 | 1.34×106 | 0.986 | |
| 0.6 | 97.6 | 7.37×103 | 0.991 | 103.9 | 3.80×1010 | 0.993 | 81.9 | 3.90×105 | 0.993 | |
| 0.7 | 92.8 | 2.80×103 | 0.995 | 99.5 | 1.65×1010 | 0.996 | 81.5 | 3.73×105 | 0.981 | |
| 0.8 | 90.7 | 1.82×103 | 0.998 | 97.8 | 1.17×1010 | 0.998 | 92.8 | 2.49×106 | 0.945 | |
| 0.9 | 96.2 | 4.51×103 | 0.990 | 103.3 | 2.71×1010 | 0.975 | 120.5 | 2.40×108 | 0.920 | |
| Ave | 107.5 | — | — | 112.9 | — | — | 103.2 | — | — | |
| fixed carbon (exp) | 0.1 | 135.4 | 6.05×104 | 0.962 | 141.0 | 2.04×1011 | 0.968 | 186.4 | 5.66×1011 | 0.915 |
| 0.2 | 151.7 | 1.23×106 | 0.941 | 156.6 | 3.42×1012 | 0.950 | 194.3 | 1.44×102 | 0.926 | |
| 0.3 | 163.4 | 8.80×106 | 0.930 | 168.0 | 2.17×1013 | 0.939 | 196.2 | 1.47×1012 | 0.936 | |
| 0.4 | 172.7 | 3.72×107 | 0.936 | 177.0 | 8.48×1013 | 0.945 | 196.2 | 1.13×1012 | 0.957 | |
| 0.5 | 181.4 | 1.29×108 | 0.938 | 185.4 | 2.75×1014 | 0.946 | 194.9 | 7.15×1011 | 0.954 | |
| 0.6 | 187.6 | 2.85×108 | 0.931 | 191.5 | 5.85×1014 | 0.940 | 191.8 | 3.45×1011 | 0.928 | |
| 0.7 | 185.3 | 1.67×108 | 0.929 | 189.5 | 3.62×1014 | 0.939 | 180.9 | 5.62×1010 | 0.937 | |
| 0.8 | 185.4 | 1.39×108 | 0.948 | 189.9 | 3.11×1014 | 0.955 | 180.2 | 4.02×1010 | 0.975 | |
| 0.9 | 179.1 | 4.17×107 | 0.967 | 184.2 | 1.05×1014 | 0.971 | 178.6 | 2.45×1010 | 0.971 | |
| Ave | 171.3 | — | — | 175.9 | — | — | 188.8 | — | — | |
| volatile (cal) | 0.1 | 204.6 | 4.37×1014 | 0.938 | 203.6 | 3.73×1020 | 0.943 | 193.6 | 1.35×1016 | 0.936 |
| 0.2 | 176.9 | 2.56×1011 | 0.950 | 177.9 | 3.27×1017 | 0.955 | 169.7 | 2.12×1013 | 0.956 | |
| 0.3 | 158.1 | 2.43×109 | 0.976 | 160.4 | 4.19×1015 | 0.979 | 138.4 | 2.41×1010 | 0.998 | |
| 0.4 | 135.7 | 1.96×107 | 0.999 | 139.4 | 4.79×1013 | 0.999 | 109.3 | 7.74×107 | 0.981 | |
| 0.5 | 119.1 | 6.24×105 | 0.997 | 123.9 | 2.06×1012 | 0.998 | 97.7 | 7.80×106 | 0.955 | |
| 0.6 | 108.7 | 7.16×104 | 0.991 | 114.3 | 2.94×1011 | 0.993 | 92.7 | 2.87×106 | 0.963 | |
| 0.7 | 101.6 | 1.68×104 | 0.985 | 107.8 | 8.16×1010 | 0.987 | 90.7 | 1.94×106 | 0.954 | |
| 0.8 | 97.2 | 6.75×103 | 0.976 | 103.8 | 3.72×1010 | 0.981 | 90.1 | 1.93×106 | 0.933 | |
| 0.9 | 94.5 | 4.17×103 | 0.965 | 101.5 | 2.52×1010 | 0.972 | 95.0 | 6.03×106 | 0.930 | |
| Ave | 132.9 | — | — | 137.0 | — | — | 119.7 | — | — | |
| fixed carbon (cal) | 0.1 | 120.1 | 8.21×103 | 0.999 | 126.2 | 3.35×1010 | 1.000 | 98.9 | 2.12×106 | 0.890 |
| 0.2 | 121.7 | 1.89×104 | 0.999 | 127.9 | 7.64×1010 | 0.999 | 104.7 | 5.04×106 | 0.910 | |
| 0.3 | 133.2 | 1.53×105 | 0.970 | 139.0 | 5.36×1011 | 0.975 | 113.8 | 1.97×107 | 0.993 | |
| 0.4 | 150.2 | 2.42×106 | 0.995 | 155.2 | 6.87×1012 | 0.996 | 132.1 | 2.87×108 | 0.990 | |
| 0.5 | 161.9 | 1.56×107 | 1.000 | 166.5 | 3.90×1013 | 1.000 | 148.8 | 3.29×109 | 0.997 | |
| 0.6 | 170.9 | 6.19×107 | 1.000 | 175.2 | 1.43×1014 | 1.000 | 158.7 | 1.34×1010 | 0.986 | |
| 0.7 | 210.6 | 2.29×1010 | 0.999 | 213.1 | 3.63×1016 | 0.999 | 217.1 | 5.91×1013 | 0.979 | |
| 0.8 | 240.5 | 1.78×1012 | 0.999 | 241.7 | 2.23×1018 | 0.999 | 386.9 | 1.79×1024 | 0.956 | |
| 0.9 | 262.4 | 3.97×1013 | 0.979 | 262.6 | 4.31×1019 | 0.982 | 509.5 | 4.58×1031 | 0.904 | |
| Ave | 174.6 | — | — | 178.6 | — | — | 207.8 | — | — | |
| 1 | Shen L, Zhang D K. An experimental study of oil recovery from sewage sludge by low-temperature pyrolysis in a fluidised-bed [J]. Fuel, 2003, 82(4): 465-472. |
| 2 | Gong Z Q, Du A, Wang Z B, et al. Experimental study on pyrolysis characteristics of oil sludge with a tube furnace reactor [J]. Energy & Fuels, 2017, 31(8): 8102-8108. |
| 3 | Du Y, Jiang X, Lv G, et al. Thermal behavior and kinetics of bio-ferment residue/coal blends during co-pyrolysis[J]. Energy Conversion and Management, 2014, 88: 459-463. |
| 4 | Sahu S G, Sarkar P, Chakraborty N, et al. Thermogravimetric assessment of combustion characteristics of blends of a coal with different biomass chars[J]. Fuel Processing Technology, 2010, 91(3): 369-378. |
| 5 | Yang J L, Chen H X, Zhao W T, et al. Combustion kinetics and emission characteristics of peat by using TG-FTIR technique[J]. Journal of Thermal Analysis and Calorimetry, 2016, 124(1): 519-528. |
| 6 | Xu T, Ning X J, Wang G W, et al. Combustion characteristics and kinetic analysis of co-combustion between bag dust and pulverized coal[J]. International Journal of Minerals Metallurgy and Materials, 2018, 25(12): 1412-1422. |
| 7 | Tong W, Liu Q C, Ran G J, et al. Experiment and expectation: co-combustion behavior of anthracite and biomass char[J]. Bioresource Technology, 2019, 280(5): 421-420. |
| 8 | Sarkar P, Sahu S G, Mukherjee A, et al. Co-combustion studies for potential application of sawdust or its low temperature char as co-fuel with coal[J]. Applied Thermal Engineering, 2014, 63(3): 616-623. |
| 9 | 张锦萍, 王长安, 贾晓威, 等. 半焦-烟煤混燃特性及动力学分析[J]. 化工学报, 2018, 69(8): 3611-3618. |
| Zhang J P, Wang C A, Jia X W, et al. Co-combustion characteristics and kinetic analysis of semi-coke and bituminous coal[J]. CIESC Journal, 2018, 69(8): 3611-3618. | |
| 10 | Gong Z Q, Wang Z T, Wang Z B, et al. Study on the migration characteristics of nitrogen and sulfur during co-combustion of oil sludge char and microalgae residue[J]. Fuel, 2019, 238(15): 1-9. |
| 11 | Javier P, Carlos H, Carmen B, et al. Investigation on co-firing of coal mine waste residues in pulverized coal combustion systems[J]. Energy, 2017, 140: 58-68. |
| 12 | Ke M L, Wen J L, Wei H C, et al. Thermogravimetric analysis and kinetics of co-pyrolysis of raw/torrefied wood and coal blends[J]. Applied Energy, 2013, 105: 57-65. |
| 13 | Van D B, Anderson S L. Breakdown and combustion of JP-10 fuel catalyzed by nanoparticulate CeO2 and Fe2O3 [J].Energy & Fuels, 2006, 20: 1886-1894. |
| 14 | Chen Y, Mori S, Pan W. Studying the mechanism of ignition of coal particles by TG-DTA[J]. Thermochimica Acta, 1996, 275: 149-158. |
| 15 | Wang G, Zhang J, Chang W, et al. Structural features and gasification reactivity of biomass chars pyrolyzed in different atmospheres at high temperature[J]. Energy, 2018, 147: 25-35. |
| 16 | 白刚, 周西华, 宋东平, 等.不同变质程度煤燃烧特性及动力学参数研究[J].中国安全科学学报, 2017, 27(9): 63-68. |
| Bai G, Zhou X H, Song D P, et al. Research on coal’s combustion characteristics and kinetics parameters as a function of its metamorphic degree[J]. China Safety Science Journal, 2017, 27(9): 63-68. | |
| 17 | Folgueras M B, Díaz R M, Xiberta J, et al. Thermogravimetric analysis of the co-combustion of coal and sewage sludge[J]. Fuel, 2003, 82(15/16/17): 2051-2055. |
| 18 | Wang H Y, Zhang J L, Wang G W, et al. Characteristics and kinetic analysis of co-combustion of brown coal and anthracite[J]. Journal of Thermal Analysis and Calorimetry, 2016, 126(2): 447-454. |
| 19 | Colomba D B. Combustion and gasification rates of lignocellulosic chars[J]. Progress in Energy and Combustion Science, 2009, 35 (2): 121-140. |
| 20 | Huang L, Liu J, He Y, et al. Thermodynamics and kinetics parameters of co-combustion between sewage sludge and water hyacinth in CO2/O2 atmosphere as biomass to solid biofuel[J]. Bioresource Technology, 2016, 218: 631-642. |
| 21 | Mathieu M, Sébastien P, Enrica M, et al. Kinetic study and modelling of char combustion in TGA in isothermal conditions[J]. Fuel, 2017, 203: 522-536. |
| 22 | 王擎, 王海刚, 孙佰仲, 等. 油页岩及其半焦混烧特性的热重试验研究和动力学分析[J]. 化工学报, 2007, 58(11): 2882-2888. |
| Wang Q, Wang H G, Sun B Z, et al. Thermo-gravimetric study and kinetic analysis of blended combustion characteristics of oil shale and semi-coke[J]. Journal of Chemical Industry and Engineering(China), 2007, 58(11): 2882-2888. | |
| 23 | Xu F F, Wang B, Yang D, et al. Thermal degradation of typical plastics under high heating rate conditions by TG-FTIR: pyrolysis behaviors and kinetic analysis[J]. Energy Conversion and Management, 2018, 171(1): 1106-1115. |
| 24 | Coats A W, Redfern J P. Kinetic parameters from thermogravimetric data [J]. Nature, 1964, 201: 68-69. |
| 25 | Doyle C D. Estimating isothermal life from thermogravimetric data [J]. Journal of Applied Polymer Science, 1962, 6: 639-642. |
| 26 | Kissinger H E. Reaction kinetics in differential thermal analysis [J]. Analytical Chemistry, 1957, 29: 1702-1706. |
| 27 | Joseph H F, Leo A W. A quick, direct method for the determination of activation energy from thermogravimetric data[J]. Polymer Letters, 1966, 4: 323-328. |
| 28 | Miura K. A new and simple method to estimate f(E) and k0(E) in the distributed activation-energy model from three sets of experimental-data[J]. Energy & Fuels, 1995, 9(2): 302-307. |
| 29 | Friedman H L. New methods for evaluating kinetic parameters from thermal analysis data[J]. Polymer Letters, 1969, 7: 41-46. |
| 30 | Andrés A C. Anka B, Nico Z,et al. How to determine consistent biomass pyrolysis kinetics in a parallel reaction scheme[J]. Fuel, 2014, 123: 230-240. |
| 31 | Janković B, Mentus S, Jelić D. A kinetic study of nonisothermal decomposition process of anhydrous nickel nitrate under air atmosphere[J]. Physica B Condensed Matter, 2009, 404(16): 2263-2269. |
| 32 | Cai J, Wu W, Liu R, et al. An overview of distributed activation energy model and its application in the pyrolysis of lignocellulosic biomass[J]. Renewable and Sustainable Energy Reviews, 2014, 36: 236-246. |
| 33 | 郑瑛, 池保华, 王保文, 等. 几种等转化率法在动力学研究中的应用与比较[J]. 煤炭转化, 2006, 29(4): 34-37. |
| Zheng Y, Chi B H, Wang B W, et al. Application and comp arison of equal conversion rate methods in the decomposition kinetics research[J]. Coal Conversion, 2006, 29(4): 34-37. | |
| 34 | 孙庆雷, 李文, 陈皓侃, 等. DEAM和Coats-Redfern积分法研究煤半焦燃烧动力学的比较[J]. 化工学报, 2003, 54(11): 1598-1602. |
| Sun Q L, Li W, Chen H K, et al. Comparison between DAEM and Coats-Redfern method for combustion kinetics of coal char[J]. Journal of Chemical Industry and Engineering(China), 2003, 54(11): 1598-1602. |
| [1] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [2] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [3] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [4] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [5] | 肖明堃, 杨光, 黄永华, 吴静怡. 浸没孔液氧气泡动力学数值研究[J]. 化工学报, 2023, 74(S1): 87-95. |
| [6] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [7] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [8] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [9] | 杨越, 张丹, 郑巨淦, 涂茂萍, 杨庆忠. NaCl水溶液喷射闪蒸-掺混蒸发的实验研究[J]. 化工学报, 2023, 74(8): 3279-3291. |
| [10] | 曾如宾, 沈中杰, 梁钦锋, 许建良, 代正华, 刘海峰. 基于分子动力学模拟的Fe2O3纳米颗粒烧结机制研究[J]. 化工学报, 2023, 74(8): 3353-3365. |
| [11] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [12] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [13] | 何宣志, 何永清, 闻桂叶, 焦凤. 磁液液滴颈部自相似破裂行为[J]. 化工学报, 2023, 74(7): 2889-2897. |
| [14] | 李艳辉, 丁邵明, 白周央, 张一楠, 于智红, 邢利梅, 高鹏飞, 王永贞. 非常规服役超临界锅炉的微纳尺度腐蚀动力学模型建立及应用[J]. 化工学报, 2023, 74(6): 2436-2446. |
| [15] | 周继鹏, 何文军, 李涛. 异形催化剂上乙烯催化氧化失活动力学反应工程计算[J]. 化工学报, 2023, 74(6): 2416-2426. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号