化工学报 ›› 2020, Vol. 71 ›› Issue (1): 68-80.DOI: 10.11949/0438-1157.20191225
收稿日期:2019-10-23
修回日期:2019-10-29
出版日期:2020-01-05
发布日期:2020-01-05
通讯作者:
唐睿康
作者简介:潘海华(1975—),男,博士,副教授,基金资助:
Haihua PAN1,3( ),Ruikang TANG2,3(
),Ruikang TANG2,3( )
)
Received:2019-10-23
Revised:2019-10-29
Online:2020-01-05
Published:2020-01-05
Contact:
Ruikang TANG
摘要:
牙齿、骨骼、贝壳等生物矿物具有多级有序的结构和优异的力学性能,是生物矿化过程调控下的矿化结晶产物。生物矿化中的矿物与生物有机基质之间的界面分子识别和结晶调控策略为深入理解化学工程中的“信息传递和转化”范式提供了良好的学习素材。以生物矿化典型无机矿物磷酸钙和碳酸钙体系为例,从生物矿物-溶液界面结构、生物分子与矿物晶面的分子识别、矿物结晶调控三个层面综述了生物矿化的化学调控原理,并从信息传递和转化的化学工程范式出发,分析了生物矿化中分子工程和结晶调控策略。绿色高效的生物矿化过程调控策略有望应用于未来化学工程以解决目前面临的需求倍增和资源短缺的全球性问题。
中图分类号:
潘海华, 唐睿康. 生物矿化及仿生矿化中的信息传递和转化[J]. 化工学报, 2020, 71(1): 68-80.
Haihua PAN, Ruikang TANG. Information transfer and transformation in bio/biomimetic-mineralization[J]. CIESC Journal, 2020, 71(1): 68-80.
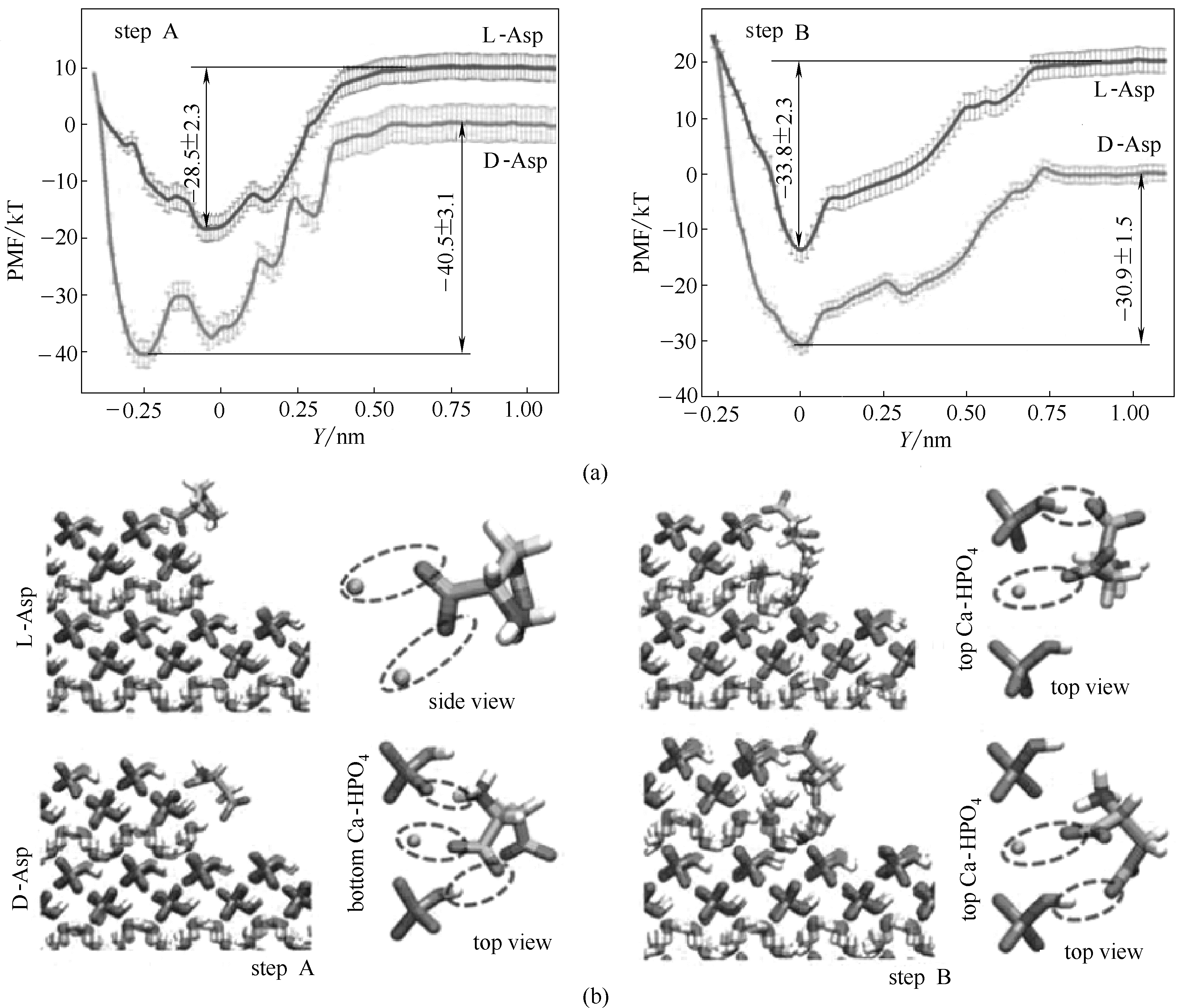
图6 D-和L-Asp在DCPD台阶([101] step A 和 step B)的吸附自由能曲线(a);Asp在台阶上的稳定吸附构型(b) [12]
Fig.6 Free energy profiles for adsorption of D- and L-Asp on [101] step A and step B (a); Snapshots of stable configurations in adsorbed state at free energy minimum(b)[12]
| 1 | Poliakoff M, Licence P. Sustainable technology: green chemistry[J]. Nature, 2007, 450(7171): 810-812. |
| 2 | Lowenstam H, Weiner S. On Biomineralization[M]. USA: Oxford University Press, 1989: 1-49. |
| 3 | 金涌, 程易, 颜彬航. 化学反应工程的前世、今生与未来[J].化工学报, 2013, 64(1): 34-43. |
| Jin Y, Cheng Y, Yan B H. Past, present and future of chemical reaction engineering[J]. CIESC Journal, 2013, 64(1): 34-43. | |
| 4 | 胡英, 刘洪来. 分子工程和化学工程[J]. 化学进展, 1995, 7(3): 235-249. |
| Hu Y, Liu H L. Molecular engineering and chemical engineering[J]. Prog. Chem., 1995, 7(3): 235-249. | |
| 5 | Park C, Fenter P, Zhang Z, et al. Structure of the fluorapatite (100)-water interface by high-resolution X-ray reflectivity[J]. Am. Mineral., 2004, 89(11/12): 1647-1654. |
| 6 | Pareek A, Torrelles X, Angermund K, et al. Structure of interfacial water on fluorapatite (100) surface[J]. Langmuir, 2008, 24(6): 2459-2464. |
| 7 | Wilson E E, Awonusi A, Morris M D, et al. Highly ordered interstitial water observed in bone by nuclear magnetic resonance[J]. J. Bone Miner. Res., 2005, 20(4): 625-634. |
| 8 | Pan H, Tao J, Wu T, et al. Molecular simulation of water behaviors on crystal faces of hydroxyapatite[J]. Front. Chem. China, 2007, 2(2): 156-163. |
| 9 | Arsic J, Kaminski D, Poodt P, et al. Liquid ordering at the Brushite-{010}-water interface[J]. Phys. Rev. B, 2004, 69(24): 1-4. |
| 10 | Chu Y S, Lister T E, Cullen W G, et al. Commensurate water monolayer at the RuO2 (110) /water interface[J]. Phys. Rev. Lett., 2001, 86(15): 3364-3367. |
| 11 | Arsic J, Kaminski D M, Radenovic N, et al. Thickness-dependent ordering of water layers at the NaCl(100) surface[J]. J. Chem. Phys., 2004, 120(20): 9720-9724. |
| 12 | Jiang W, Pan H, Zhang Z, et al. Switchable chiral selection of aspartic acids by dynamic states of brushite[J]. J. Am. Chem. Soc., 2017, 139(25): 8562-8569. |
| 13 | Heberling F, Trainor T P, Lützenkirchen J, et al. Structure and reactivity of the calcite-water interface[J]. J. Colloid Interface Sci., 2011, 354(2): 843-857. |
| 14 | Imada H, Kimura K, Onishi H. Water and 2-propanol structured on calcite (104) probed by frequency-modulation atomic force microscopy[J]. Langmuir, 2013, 29(34): 10744-10751. |
| 15 | Fenter P, Kerisit S, Raiteri P, et al. Is the calcite-water interface understood? Direct comparisons of molecular dynamics simulations with specular X-ray reflectivity data[J]. J. Phys. Chem. C, 2013, 117(10): 5028-5042. |
| 16 | Wang Y, Von Euw S, Fernandes F M, et al. Water-mediated structuring of bone apatite[J]. Nat. Mater., 2013, 12(12): 1144-1153. |
| 17 | Drouet C, Aufray M, Rollin-Martinet S, et al. Nanocrystalline apatites: the fundamental role of water[J]. Am. Mineral., 2018, 103(4): 550-564. |
| 18 | Abrams C, Bussi G. Enhanced sampling in molecular dynamics using metadynamics, replica-exchange, and temperature-acceleration[J]. Entropy, 2014, 16(1): 163-199. |
| 19 | Tracey J, Miyazawa K, Spijker P, et al. Understanding 2D atomic resolution imaging of the calcite surface in water by frequency modulation atomic force microscopy[J]. Nanotechnology, 2016, 27(41): 415709. |
| 20 | Zhang Z, Wu T, Wang Q, et al. Impact of interfacial high-density water layer on accurate estimation of adsorption free energy by Jarzynski s equality[J]. J. Chem. Phys., 2014, 140(3): 034706. |
| 21 | Dove P M, Czank C A. Crystal chemical controls on the dissolution kinetics of the isostructural sulfates: celestite, anglesite, and barite[J]. Geochim. Cosmochim. Acta, 1995, 59(10): 1907-1915. |
| 22 | Piana S, Jones F, Gale J D. Assisted desolvation as a key kinetic step for crystal growth[J]. J. Am. Chem. Soc., 2006, 128(41): 13568-13574. |
| 23 | Pan H, Tao J, Xu X, Tang R. Adsorption processes of Gly and Glu amino acids on hydroxyapatite surfaces at the atomic level[J]. Langmuir, 2007, 23(17): 8972-8981. |
| 24 | Zhang Z, Pan H, Tang R. Molecular dynamics simulations of the adsorption of amino acids on the hydroxyapatite {100} -water interface[J]. Front. Mater. Sci. China, 2008, 2(3): 239-245. |
| 25 | Hu Y Y, Rawal A, Schmidt-Rohr K. Strongly bound citrate stabilizes the apatite nanocrystals in bone[J]. Proc. Natl. Acad. Sci. U. S. A., 2010, 107(52): 22425-22429. |
| 26 | Jiang W, Pan H, Cai Y, et al. Atomic force microscopy reveals hydroxyapatite-citrate interfacial structure at the atomic level[J]. Langmuir, 2008, 24(21): 12446-12451. |
| 27 | Jiang W, Chu X, Wang B, et al. Biomimetically triggered inorganic crystal transformation by biomolecules: a new understanding of biomineralization[J]. J. Phys. Chem. B, 2009, 113(31): 10838-10844. |
| 28 | Gajjeraman S, Narayanan K, Hao J, et al. Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite[J]. J. Biol. Chem., 2007, 282(2): 1193-1204. |
| 29 | Weiner S. Biomineralization: a structural perspective[J]. J. Struct. Biol., 2008, 163(3): 229-234. |
| 30 | Beniash E, Simmer J P, Margolis H C. The effect of recombinant mouse amelogenins on the formation and organization of hydroxyapatite crystals in vitro[J]. J. Struct. Biol., 2005, 149(2): 182-190. |
| 31 | Le Norcy E, Kwak S Y, Wiedemann-Bidlack F B, et al. Potential role of the amelogenin N-terminus in the regulation of calcium phosphate formation in vitro[J]. Cells Tissues Organs, 2011, 194(2/3/4): 188-193. |
| 32 | Tao J, Shin Y, Jayasinha R, et al. The energetic basis for hydroxyapatite mineralization by amelogenin variants provides insights into the origin of amelogenesis imperfecta[J]. Proc. Natl. Acad. Sci. U. S. A., 2019, 116(28): 13867-13872. |
| 33 | Fang P A, Conway J F, Margolis H C, et al. Hierarchical self-assembly of amelogenin and the regulation of biomineralization at the nanoscale[J]. Proc. Natl. Acad. Sci. U. S. A., 2011, 108(34): 14097-14102. |
| 34 | Du C, Falini G, Fermani S, et al. Supramolecular assembly of amelogenin nanospheres into birefringent microribbons[J]. Science, 2005, 307(5714): 1450-1454. |
| 35 | Shaw W J, Campbell A A, Paine M L, et al. The COOH terminus of the amelogenin, LRAP, is oriented next to the hydroxyapatite surface[J]. J. Biol. Chem., 2004, 279(39): 40263-40266. |
| 36 | Moradian-Oldak J, Bouropoulos N, Wang L, et al. Analysis of self-assembly and apatite binding properties of amelogenin proteins lacking the hydrophilic C-terminal[J]. Matrix Biol., 2002, 21(2): 197-205. |
| 37 | Chen X, Wang Q, Shen J, et al. Adsorption of leucine-rich amelogenin protein on hydroxyapatite (001) surface through —COO— claws[J]. J. Phys. Chem. C, 2007, 111(3): 1284-1290. |
| 38 | Salazar V S, Gamer L W, Rosen V. BMP signalling in skeletal development, disease and repair[J]. Nat. Rev. Endocrinol., 2016, 12(4): 203-221. |
| 39 | Dong X, Wang Q, Wu T, Pan H. Understanding adsorption-desorption dynamics of BMP-2 on hydroxyapatite (001) surface[J]. Biophys. J., 2007, 93(3): 750-759. |
| 40 | Shen J W, Wu T, Wang Q, et al. Molecular simulation of protein adsorption and desorption on hydroxyapatite surfaces[J]. Biomaterials, 2008, 29(5): 513-532. |
| 41 | Mann S, Hannington J P, Williams R J P. Phospholipid vesicles as a model system for biomineralization[J]. Nature, 1986, 324(6097): 565-567. |
| 42 | Fricke M, Volkmer D. Crystallization of calcium carbonate beneath insoluble monolayers: suitable models of mineral-matrix interactions in biomineralization? [J]. Top. Curr. Chem., 2007, 270: 1-41. |
| 43 | Aizenberg J, Black A J, Whitesides G M. Oriented growth of calcite controlled by self-assembled monolayers of functionalized alkanethiols supported on gold and silver[J]. J. Am. Chem. Soc., 1999, 121(18): 4500-4509. |
| 44 | Travaille A M, Kaptijn L, Verwer P, et al. Highly oriented self-assembled monolayers as templates for epitaxial calcite growth[J]. J. Am. Chem. Soc., 2003, 125(38): 11571-11577. |
| 45 | Hartgerink J D, Beniash E, Stupp S I. Self-assembly and mineralization of peptide-amphiphile nanofibers[J]. Science, 2001, 294(5547): 1684-1688. |
| 46 | Spoerke E D, Anthony S G, Stupp S I. Enzyme directed templating of artificial bone mineral[J]. Adv. Mater., 2009, 21(4): 425-430. |
| 47 | Kim Y Y, Ganesan K, Yang P, et al. An artificial biomineral formed by incorporation of copolymer micelles in calcite crystals[J]. Nat. Mater., 2011, 10(11): 890-896. |
| 48 | Cho K R, Kim Y Y, Yang P, et al. Direct observation of mineral-organic composite formation reveals occlusion mechanism[J]. Nat. Commun., 2016, 7: 10187. |
| 49 | Loo R W, Goh M C. Potassium ion mediated collagen microfibril assembly on mica[J]. Langmuir, 2008, 24(23): 13276-13278. |
| 50 | Kang S, Li H, Huynh T, et al. Molecular mechanism of surface-assisted epitaxial self-assembly of amyloid-like peptides[J]. ACS Nano, 2012, 6(10): 9276-9282. |
| 51 | Chen J, Zhu E, Liu J, et al. Building two-dimensional materials one row at a time: avoiding the nucleation barrier[J]. Science, 2018, 362(6419): 1135-1139. |
| 52 | Orme C, Noy A, Wierzbicki A, et al. Formation of chiral morphologies through selective binding of amino acids to calcite surface steps[J]. Nature, 2001, 411(6839): 775-779. |
| 53 | Wu Y J, Tsai T W T, Chan J C C. Asymmetric crystal morphology of apatite induced by the chirality of dicarboxylate additives[J]. Cryst. Growth Des., 2012, 12(2): 547-549. |
| 54 | Jiang W, Pacella M S, Athanasiadou D, et al. Chiral acidic amino acids induce chiral hierarchical structure in calcium carbonate[J]. Nat. Commun., 2017, 8(1): 15066. |
| 55 | De Yoreo J J, Gilbert P U P A, Sommerdijk N A J M, et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments[J]. Science, 2015, 349(6247): aaa6760. |
| 56 | De Yoreo J J, Vekilov P G. Principles of crystal nucleation and growth[J]. Rev. Mineral. Geochemistry, 2003, 54(1): 57-93. |
| 57 | Hu Q, Nielsen M H, Freeman C L, et al. The thermodynamics of calcite nucleation at organic interfaces: classical vs. non-classical pathways[J]. Faraday Discuss., 2012, 159: 509-523. |
| 58 | Wang B, Liu P, Liu Z, et al. Biomimetic construction of cellular shell by adjusting the interfacial energy[J]. Biotechnol. Bioeng., 2014, 111(2): 386-395. |
| 59 | Jiang S, Chen Y, Pan H, et al. Faster nucleation at lower pH: amorphous phase mediated nucleation kinetics[J]. Phys. Chem. Chem. Phys., 2013, 15(30): 12530-12533. |
| 60 | Jiang S, Pan H, Chen Y, et al. Amorphous calcium phosphate phase-mediated crystal nucleation kinetics and pathway[J]. Faraday Discuss., 2015, 179: 451-461. |
| 61 | Wang Y N, Jiang S, Pan H, et al. Less is more: silicate in the crystallization of hydroxyapatite in simulated body fluids[J]. CrystEngComm, 2016, 18(3): 379-383. |
| 62 | Chen Y, Gu W, Pan H, et al. Stabilizing amorphous calcium phosphate phase by citrate adsorption[J]. CrystEngComm, 2014, 16(10): 1864-1867. |
| 63 | Jiang S, Jin W, Wang Y N, et al. Effect of the aggregation state of amorphous calcium phosphate on hydroxyapatite nucleation kinetics[J]. RSC Adv., 2017, 7(41): 25497-25503. |
| 64 | Ding H, Pan H, Xu X, et al. Toward a detailed understanding of magnesium ions on hydroxyapatite crystallization inhibition[J]. Cryst. Growth Des., 2014, 14(2): 763-769. |
| 65 | Jin W, Liu Z, Wu Y, et al. Synergic effect of Sr2+ and Mg2+ on the stabilization of amorphous calcium phosphate[J]. Cryst. Growth Des., 2018, 18: 6054-6060. |
| 66 | Lupulescu A I, Rimer J D. In situ imaging of silicalite-1 surface growth reveals the mechanism of crystallization[J]. Science, 2014, 344(6185): 729-732. |
| 67 | Teng H H, Dove P M, Orme C A, et al. Thermodynamics of calcite growth: baseline for understanding biomineral formation[J]. Science, 1998, 282(5389): 724-727. |
| 68 | Piana S, Jones F, Gale J D. Aspartic acid as a crystal growth catalyst[J]. CrystEngComm, 2007, 9(12): 1187-1191. |
| 69 | Jiang W, Pan H, Tao J, et al. Dual roles of borax in kinetics of calcium sulfate dihydrate formation[J]. Langmuir, 2007, 23(9): 5070-5076. |
| 70 | Baumgartner J, Dey A, Bomans P H H, et al. Nucleation and growth of magnetite from solution[J]. Nat. Mater., 2013, 12(4): 310-314. |
| 71 | Liu Z, Pan H, Zhu G, et al. Realignment of nanocrystal aggregates into single crystals as a result of inherent surface stress[J]. Angew. Chem. Int. Ed., 2016, 55(41): 12836-12840. |
| 72 | Banfield J F, Welch S A, Zhang H, et al. Aggregation-based crystal growth and microstructure development in natural iron oxyhydroxide biomineralization products[J]. Science, 2000, 289(5480): 751-754. |
| 73 | Li D, Nielsen M H, Lee J R I, et al. Direction-specific interactions control crystal growth by oriented attachment[J]. Science, 2012, 336(6084): 1014-1018. |
| 74 | Tao J, Pan H, Zeng Y, et al. Roles of amorphous calcium phosphate and biological additives in the assembly of hydroxyapatite nanoparticles[J]. J. Phys. Chem. B, 2007, 111(47): 13410-13418. |
| 75 | Zhu G, Yao S, Zhai H, et al. Evolution from classical to non-classical aggregation-based crystal growth of calcite by organic additive control[J]. Langmuir, 2016, 32(35): 8999-9004. |
| 76 | Wang L, Guan X, Yin H, et al. Mimicking the self-organized microstructure of tooth enamel[J]. J. Phys. Chem. C, 2008, 112(15): 5892-5899. |
| 77 | Yu H P, Zhu Y J, Lu B Q. Dental enamel-mimetic large-sized multi-scale ordered architecture built by a well controlled bottom-up strategy[J]. Chem. Eng. J., 2019, 360: 1633-1645. |
| 78 | Fan Y, Sun Z, Moradian-Oldak J. Controlled remineralization of enamel in the presence of amelogenin and fluoride[J]. Biomaterials, 2009, 30(4): 478-483. |
| 79 | Ruan Q, Zhang Y, Yang X, et al. An amelogenin-chitosan matrix promotes assembly of an enamel-like layer with a dense interface[J]. Acta Biomater., 2013, 9(7): 7289-7297. |
| 80 | Li L, Mao C, Wang J, et al. Bio-inspired enamel repair via glu-directed assembly of apatite nanoparticles: an approach to biomaterials with optimal characteristics[J]. Adv. Mater., 2011, 23(40): 4695-4701. |
| 81 | Shao C, Jin B, Mu Z, et al. Repair of tooth enamel by a biomimetic mineralization frontier ensuring epitaxial growth[J]. Sci. Adv., 2019, 5: eaaw9569. |
| 82 | Olszta M J, Cheng X, Jee S S, et al. Bone structure and formation: a new perspective[J]. Mater. Sci. Eng. R, 2007, 58(3/4/5): 77-116. |
| 83 | Niu L, Jee S E, Jiao K, et al. Collagen intrafibrillar mineralization as a result of the balance between osmotic equilibrium and electroneutrality[J]. Nat. Mater., 2016, 16(3): 370-378. |
| 84 | Shao C, Zhao R, Jiang S, et al. Citrate improves collagen mineralization via interface wetting: a physicochemical understanding of biomineralization control[J]. Adv. Mater., 2018, 30(8): 1704876. |
| 85 | Xiao C, Li M, Wang B, et al. Total morphosynthesis of biomimetic prismatic-type CaCO3 thin films[J]. Nat. Commun., 2017, 8(1): 1398. |
| 86 | Mao L B, Gao H L, Yao H B, et al. Synthetic nacre by predesigned matrix-directed mineralization[J]. Science, 2016, 354(6308): 107-110. |
| [1] | 周晓庆, 李春煜, 杨光, 蔡爱峰, 吴静怡. 液滴撞击不同曲率过冷波纹面结冰动力学行为及机理研究[J]. 化工学报, 2023, 74(S1): 141-153. |
| [2] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [3] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [4] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [5] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [6] | 赵佳佳, 田世祥, 李鹏, 谢洪高. SiO2-H2O纳米流体强化煤尘润湿性的微观机理研究[J]. 化工学报, 2023, 74(9): 3931-3945. |
| [7] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [8] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [9] | 傅予, 刘兴翀, 王瀚雨, 李海敏, 倪亚飞, 邹文静, 雷月, 彭永姗. F3EACl修饰层对钙钛矿太阳能电池性能提升的研究[J]. 化工学报, 2023, 74(8): 3554-3563. |
| [10] | 胡兴枝, 张皓焱, 庄境坤, 范雨晴, 张开银, 向军. 嵌有超小CeO2纳米粒子的碳纳米纤维的制备及其吸波性能[J]. 化工学报, 2023, 74(8): 3584-3596. |
| [11] | 林典, 江国梅, 徐秀彬, 赵波, 刘冬梅, 吴旭. 硅基类液防原油黏附涂层的研制及其减阻性能研究[J]. 化工学报, 2023, 74(8): 3438-3445. |
| [12] | 张贲, 王松柏, 魏子亚, 郝婷婷, 马学虎, 温荣福. 超亲水多孔金属结构驱动的毛细液膜冷凝及传热强化[J]. 化工学报, 2023, 74(7): 2824-2835. |
| [13] | 董明, 徐进良, 刘广林. 超临界水非均质特性分子动力学研究[J]. 化工学报, 2023, 74(7): 2836-2847. |
| [14] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [15] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号