化工学报 ›› 2020, Vol. 71 ›› Issue (11): 4981-4989.DOI: 10.11949/0438-1157.20200248
收稿日期:2020-03-11
修回日期:2020-05-18
出版日期:2020-11-05
发布日期:2020-11-05
通讯作者:
纪红兵
作者简介:陈亚举(1989—),男,博士,讲师,基金资助:
Yaju CHEN1( ),Zhongxiu LIANG2,Xiantai ZHOU2,Hongbing JI1,2(
),Zhongxiu LIANG2,Xiantai ZHOU2,Hongbing JI1,2( )
)
Received:2020-03-11
Revised:2020-05-18
Online:2020-11-05
Published:2020-11-05
Contact:
Hongbing JI
摘要:
通过仿生催化,将苯乙烯、氧气(O2)和二氧化碳(CO2)直接合成环状碳酸酯在现代化学中极具学术研究意义和工业应用价值。采用钴卟啉-四丁基溴化铵为双组分催化剂,以2-氧代环戊烷羧酸甲酯为助剂,在O2和CO2条件下,直接将苯乙烯转化为碳酸苯乙烯酯。系统考察了催化剂用量等因素对催化性能的影响。在最佳反应条件下,苯乙烯的转化率高达99%,环状碳酸酯的收率可达35%。利用在线紫外与在线红外探讨了该串联反应可能的机理。结果表明,钴中心与2-氧代环戊烷羧酸甲酯的环内氧原子配位后活化氧气形成过氧活性物种,进而形成高价钴-氧中间体,其通过传递氧原子给苯乙烯而生成环氧苯乙烷。而后,环氧苯乙烷在四丁基溴化铵的催化作用下开环,并通过CO2插入反应和分子内闭环反应最终生成环状碳酸酯。
中图分类号:
陈亚举,梁中秀,周贤太,纪红兵. 钴卟啉-四丁基溴化铵串联催化苯乙烯在氧气和二氧化碳中合成碳酸苯乙烯酯[J]. 化工学报, 2020, 71(11): 4981-4989.
Yaju CHEN,Zhongxiu LIANG,Xiantai ZHOU,Hongbing JI. CoTPP-TBAB catalyzed tandem transformation of styrene carbonate from styrene in the presence of O2 and CO2[J]. CIESC Journal, 2020, 71(11): 4981-4989.
| Entry | CoTPP/mmol | TBAB/mmol | MCC/mmol | Reaction time /h | Conv. ②/% | Yield ② /% | ||
|---|---|---|---|---|---|---|---|---|
| SC | SO | BA | ||||||
| 1 | 0.005 | — | 5.0 | 12 | 99 | 1 | 7 | 13 |
| 2 | — | 0.1 | 5.0 | 12 | 46 | 5 | 1 | 3 |
| 3 | 0.005 | 0.1 | 5.0 | 12 | 99 | 20 | 2 | 13 |
| 4 | 0.005 | 0.3 | 5.0 | 12 | 99 | 20 | 2 | 10 |
| 5 | 0.005 | 0.5 | 5.0 | 12 | 99 | 21 | 2 | 10 |
| 6 | 0.005 | 0.5 | 5.0 | 26 | 99 | 21 | 2 | 7 |
| 7 | 0.005 | 0.5 | 1.0 | 12 | 47 | 14 | 1 | 12 |
| 8 | 0.005 | 0.5 | 2.0 | 12 | 99 | 35 | 2 | 12 |
| 9 | 0.005 | 0.5 | 3.0 | 12 | 99 | 34 | 3 | 14 |
表1 苯乙烯、O2和CO2直接合成碳酸苯乙烯酯的反应结果
Table 1 Results of the direct synthesis of styrene carbonate from styrene, dioxygen and carbon dioxide①
| Entry | CoTPP/mmol | TBAB/mmol | MCC/mmol | Reaction time /h | Conv. ②/% | Yield ② /% | ||
|---|---|---|---|---|---|---|---|---|
| SC | SO | BA | ||||||
| 1 | 0.005 | — | 5.0 | 12 | 99 | 1 | 7 | 13 |
| 2 | — | 0.1 | 5.0 | 12 | 46 | 5 | 1 | 3 |
| 3 | 0.005 | 0.1 | 5.0 | 12 | 99 | 20 | 2 | 13 |
| 4 | 0.005 | 0.3 | 5.0 | 12 | 99 | 20 | 2 | 10 |
| 5 | 0.005 | 0.5 | 5.0 | 12 | 99 | 21 | 2 | 10 |
| 6 | 0.005 | 0.5 | 5.0 | 26 | 99 | 21 | 2 | 7 |
| 7 | 0.005 | 0.5 | 1.0 | 12 | 47 | 14 | 1 | 12 |
| 8 | 0.005 | 0.5 | 2.0 | 12 | 99 | 35 | 2 | 12 |
| 9 | 0.005 | 0.5 | 3.0 | 12 | 99 | 34 | 3 | 14 |
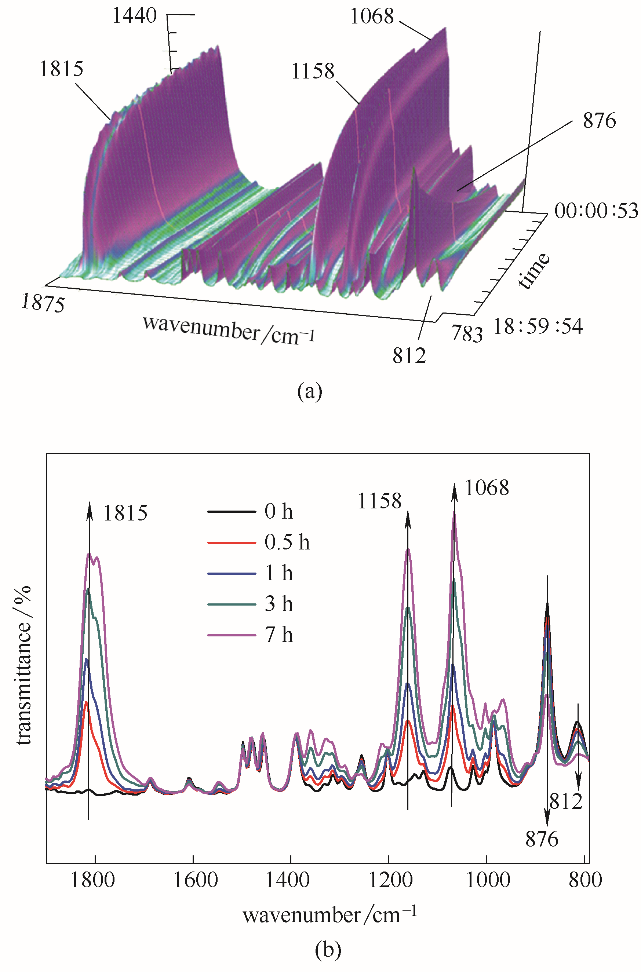
图11 TBAB催化环氧苯乙烷和CO2环加成反应的在线红外光谱三维图(a);环氧化物和环状碳酸酯中官能团红外特征吸收峰在不同时间的变化(b)
Fig.11 Three-dimensional stack plot of in-situ IR spectra collected every 1 min during the coupling reaction of SO and CO2 over TBAB(a); changes of the epoxide ring and SC vibrational peaks at different time(b)
| 1 | Rahman F A, Aziz M M A, Saidur R, et al. Pollution to solution: capture and sequestration of carbon dioxide (CO2) and its utilization as a renewable energy source for a sustainable future[J]. Renew. Sust. Energy Rev., 2017, 71: 112-126. |
| 2 | Appel A M, Bercaw J E, Bocarsly A B, et al. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation[J]. Chem. Rev., 2013, 113(8): 6621-6658. |
| 3 | Tapia J F D, Lee J Y, Ooi R E H, et al. A review of optimization and decision-making models for the planning of CO2 capture, utilization and storage (CCUS) systems[J]. Sustainable Production Consumption, 2018, 13: 1-15. |
| 4 | Dowell N M, Fennell P S, Shah N, et al. The role of CO2 capture and utilization in mitigating climate change[J]. Nat. Clim. Change, 2017, 7(4): 243-249. |
| 5 | He M Y, Sun Y H, Han B X. Green carbon science: scientific basis for integrating carbon resource processing, utilization, and recycling[J]. Angew. Chem. Int. Ed., 2013, 52(37): 9620-9633. |
| 6 | Artz J, Muller T E, Thenert K, et al. Sustainable conversion of carbon dioxide: an integrated review of catalysis and life cycle assessment[J]. Chem. Rev., 2018, 118(2): 434-504. |
| 7 | Sakakura T, Choi J C, Yasuda H. Transformation of carbon dioxide[J]. Chem. Rev., 2007, 107(6): 2365-2387. |
| 8 | Aresta M, Dibenedetto A, Angelini A. Catalysis for the valorization of exhaust carbon: from CO2 to chemicals, materials, and fuels. Technological use of CO2[J]. Chem. Rev., 2014, 114(3): 1709-1742. |
| 9 | 张文珍, 张宁, 郭春晓, 等. 二氧化碳参与的环化反应最新研究进展[J]. 有机化学, 2017, 37(6): 1309-1321. |
| Zhang W Z, Zhang N, Guo C X, et al. Recent progress in the cyclization reactions using carbon dioxide[J]. Chin. J. Org. Chem., 2017, 37(6): 1309-1321. | |
| 10 | Song Q W, Zhou Z H, He L N. Efficient, selective and sustainable catalysis of carbon dioxide[J]. Green Chem., 2017, 19(16): 3707-3728. |
| 11 | Sakakura T, Kohno K. The synthesis of organic carbonates from carbon dioxide[J]. Chem. Commun., 2009, (11): 1312-1330. |
| 12 | Shaikh R R, Pornpraprom S, D􀆳Elia V. Catalytic strategies for the cycloaddition of pure, diluted and waste CO2 to epoxides under ambient conditions[J]. ACS Catal.,2018, 8(1): 419-450. |
| 13 | Schäffner B, Friederike S, Verevkin S P, et al. Organic carbonates as solvents in synthesis and catalysis[J]. Chem. Rev., 2010, 110(8): 4554–4581. |
| 14 | Shaikh A G, Sivaram S. Organic carbonates[J]. Chem. Rev., 1996, 96(3): 951-976. |
| 15 | North M, Pasquale R, Young C. Synthesis of cyclic carbonates from epoxides and CO2[J]. Green Chem., 2010, 12(9): 1514-1539. |
| 16 | Liang J, Huang Y B, Cao R. Metal-organic frameworks and porous organic polymers for sustainable fixation of carbon dioxide into cyclic carbonates[J]. Coord. Chem. Rev., 2019, 378: 32-65. |
| 17 | 罗荣昌, 周贤太, 杨智, 等. 均相体系中酸碱协同催化二氧化碳与环氧化物的环加成反应[J]. 化工学报, 2016, 67(1): 258-276. |
| Luo R C, Zhou X T, Yang Z, et al. Acid-base synergistic effect promoted cycloaddition reaction from CO2 with epoxide in homogenous catalysis systems[J]. CIESC Journal, 2016, 67(1): 258-276. | |
| 18 | Liu J, Yang G Q, Liu Y, et al. Metal-free imidazolium hydrogen carbonate ionic liquids as bifunctional catalysts for the one-pot synthesis of cyclic carbonates from olefins and CO2[J]. Green Chem., 2019, 21(14): 3834-3838. |
| 19 | Sun J, Fujita S, Bhanage B M, et al. One-pot synthesis of styrene carbonate from styrene in tetrabutylammonium bromide[J]. Catal. Today, 2004, 93-95: 383-388. |
| 20 | Sun J, Fujita S, Bhanage B M, et al. Direct oxidative carboxylation of styrene to styrene carbonate in the presence of ionic liquids[J]. Catal. Commun., 2004, 5(2): 83-87. |
| 21 | Sun J M, Fujita S, Zhao F Y, et al. A direct synthesis of styrene carbonate from styrene with the Au/SiO2ZnBr2/Bu4NBr catalyst system[J]. J. Catal., 2005, 230(2): 398-405. |
| 22 | 孙建敏, 王路, 王亚丽, 等. 溴化锌-卤化正四丁基铵高效催化合成苯乙烯环状碳酸酯[J]. 高等学校化学学报, 2007, 28(3): 502-505. |
| Sun J M, Wang L, Wang Y L, et al. Synthesis of styrene carbonate catalyzed by efficiently by zinc bromide and tetra-n-butylammonium halides[J]. Chem. J. Chin. Univ., 2007, 28(3): 502-505. | |
| 23 | Wang Y L, Sun J H, Xiang D, et al. A facile, direct synthesis of styrene carbonate from styrene and CO2 catalyzed by Au/Fe(OH)3-ZnBr2/Bu4NBr system[J]. Catal. Lett., 2009, 129(3/4): 437-443. |
| 24 | Xiang D, Liu X F, Sun J S, et al. A novel route for synthesis of styrene carbonate using styrene and CO2 as substrates over basic resin R201 supported Au catalyst[J]. Catal. Today, 2009, 148(3/4): 383-388. |
| 25 | Han Q X, Qi B, Ren W M, et al. Polyoxometalate-based homochiral metal-organic frameworks for tandem asymmetric transformation of cyclic carbonates from olefins[J]. Nat.Commun., 2015, 6: 10007-10014. |
| 26 | Ono F, Qiao K, Tomida D, et al. Direct preparation of styrene carbonates from styrene using an ionic-liquid-based one-pot multistep synthetic process[J]. Appl. Catal. A, 2007, 333(1): 107-113. |
| 27 | Eghbali N, Li C J. Conversion of carbon dioxide and olefins into cyclic carbonates in water[J]. Green Chem., 2007, 9(3): 213-215. |
| 28 | Zou B, Hu C W. Synthesis of cyclic carbonates from alkenyl and alkynyl substrates[J]. Chin. J. Chem., 2017, 35(5): 541-550. |
| 29 | Mukherjee A. Biomimetics, Learning from Nature[M]. Vukovar: IntechOpen, 2010. |
| 30 | Que L J, Tolman W B. Biologically inspired oxidation catalysis[J]. Nature, 2008, 455: 333-340. |
| 31 | Pereira M M, Dias L D, Calvete M J F. Metalloporphyrins: bioinspired oxidation catalysts[J]. ACS Catal., 2018, 8(11): 10784-10808. |
| 32 | 周贤太, 纪红兵. 金属卟啉仿生催化氧化合成有机化工产品[J]. 精细化工, 2013, 30(4): 425-432. |
| Zhou X T, Ji H B. synthesis of organic chemical products by metalloporphyrins-based biomimetic catalytic oxidation[J]. Fine Chem., 2013, 30(4): 425-432. | |
| 33 | Zhou X T, Ji H B. Biomimetic kinetics and mechanism of cyclohexene epoxidation catalyzed by metalloporphyrins[J]. Chem. Eng. J., 2010, 156(2): 411-417. |
| 34 | Zhou X T, Tang Q H, Ji H B. Remarkable enhancement of aerobic epoxidation reactivity for olefins catalyzed by μ-oxo-bisiron(III) porphyrins under ambient conditions[J]. Tetrahedron Lett., 2009, 50(47): 6601-6605. |
| 35 | Schroder K, Join B, Amali A J, et al. A biomimetic iron catalyst for the epoxidation of olefins with molecular oxygen at room temperature[J]. Angew. Chem. Int. Ed., 2011, 50(6): 1425-1429. |
| 36 | Rocha C C, Onfroy T, Launay F. Towards a combined use of Mn(Salen) and quaternary ammonium salts as catalysts for the direct conversion of styrene to styrene carbonate in the presence of dioxygen and carbon dioxide[J]. Comptes Rendus Chimie, 2015, 18(3): 270-276. |
| 37 | Niño M E, Giraldo S A, Páez-Mozo E A. Olefin oxidation with dioxygen catalyzed by porphyrins and phthalocyanines intercalated in α-zirconium phosphat[J]. J. Mol. Catal. A-Chem., 2001, 175(1/2): 139-151. |
| 38 | Luo R C, Zhou X T, Zhang W Y, et al. New bi-functional zinc catalysts based on robust and easy-to-handle N-chelating ligands for the synthesis of cyclic carbonates from epoxides and CO2 under mild conditions[J]. Green Chem., 2014, 16(9): 4179-4189. |
| 39 | D'Elia V, Ghani A A, Monassier A, et al. Dynamics of the NbCl5-catalyzed cycloaddition of propylene oxide and CO2: assessing the dual role of the nucleophilic Co-catalysts[J]. Chem. Eur. J., 2014, 20(37): 11870-11882. |
| 40 | Punniyamurthy T, Bhatia B, Reddy M M, et al. A versatile cobalt(Ⅱ)-Schiff base catalyzed oxidation of organic substrates with dioxygen: scope and mechanism[J]. Tetrahedron, 1997, 53(22): 7649-7670. |
| 41 | Yang Z Z, Zhao Y N, He L N. CO2 chemistry: task-specific ionic liquids for CO2 capture/activation and subsequent conversion[J]. RSC Adv., 2011, 1(4): 545-567. |
| 42 | Chen Y J, Luo R C, Bao J H, et al. Function-oriented ionic polymers having high-density active sites for sustainable carbon dioxide conversion[J]. J. Mater. Chem. A, 2018, 6(19): 9172-9182. |
| 43 | Chen Y J, Luo R C, Xu Q H, et al. Charged metalloporphyrin polymers for cooperative synthesis of cyclic carbonates from CO2 under ambient conditions[J]. ChemSusChem, 2017, 10(11): 2534-2541. |
| [1] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [2] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [3] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [4] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [5] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [6] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [7] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [8] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [9] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [10] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [11] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [12] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [13] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [14] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [15] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号