化工学报 ›› 2020, Vol. 71 ›› Issue (10): 4327-4349.DOI: 10.11949/0438-1157.20200693
收稿日期:2020-06-02
修回日期:2020-07-17
出版日期:2020-10-05
发布日期:2020-10-05
通讯作者:
赵宇飞
作者简介:来天艺 (1996—),女,硕士研究生,基金资助:
Tianyi LAI( ),Jikang WANG,Tian LI,Sha BAI,Xiaojie HAO,Yufei ZHAO(
),Jikang WANG,Tian LI,Sha BAI,Xiaojie HAO,Yufei ZHAO( ),Xue DUAN
),Xue DUAN
Received:2020-06-02
Revised:2020-07-17
Online:2020-10-05
Published:2020-10-05
Contact:
Yufei ZHAO
摘要:
加氢/氧化催化是现代化学工业中最广泛的催化过程,传统加氢/氧化催化需要高温高压、消耗大量氢气/氧气(或双氧水等),且存在高成本、高能耗、低选择性等问题。因此,如何在温和条件下绿色高效地实现加氢还原/氧化反应,是目前催化领域的研究热点和难点之一。光电催化过程因其能量来源广泛、清洁环保,且结合了光催化和电催化的优势,已成为当前研究热点,而光电分解水产生H2/O2过程涉及高反应活性的中间物种(活性氢*H、活性氧*O)的产生。利用光电解水产生的中间*H/*O物种,并使其直接参与加氢/氧化催化过程,实现一步光电解水制活性氢/氧耦合加氢/氧化过程,有望极大提高反应的效率。通过对催化剂结构进行调控,使得耦合过程可在温和的反应条件下进行,同时可避免因使用氢气/氧气等导致的安全和耗能等问题。本文综述了光电分解水过程,传统化工的加氢/氧化过程以及光电分解水与加氢/氧化耦合反应等方面的发展,介绍了水滑石基纳米材料在光电解水耦合加氢/氧化过程中的结构和性能优势,并对未来研究方向进行了展望,以期为高附加值有机化学品的高选择性低成本制备提供思路。
中图分类号:
来天艺,王纪康,李天,白莎,郝晓杰,赵宇飞,段雪. 光电解水产活性氢/氧耦合加氢/氧化过程用水滑石基纳米材料[J]. 化工学报, 2020, 71(10): 4327-4349.
Tianyi LAI,Jikang WANG,Tian LI,Sha BAI,Xiaojie HAO,Yufei ZHAO,Xue DUAN. Photoelectrochemical water splitting into active hydrogen/oxygen species coupling with hydrogenation/oxidation process using layered double hydroxides-based nanocatalysts[J]. CIESC Journal, 2020, 71(10): 4327-4349.

图1 LDHs材料结构示意图(a)[17]; 常见LDHs材料pH = 7时的价导带位置图(b)[22]
Fig.1 Schematic illustration of LDHs(a)[17]; Conduction band and valence band potentials of some common LDH materials versus NHE at pH 7 (b)[22]

图2 ZnTi-LDH高分散结构示意图(a); Ti基LDH光催化分解水产氢性能对比(b); 甲醇溶剂中·O2-ESR检测图谱(c)[29];Ti3+自掺杂的NiTi-LDH能带结构示意图及可见光照条件下O2生成过程示意图(d); 不同厚度NiTi-LDH荧光光谱(e); 以10-2 mol·L-1 AgNO3 为牺牲剂不同厚度NiTi-LDH的分解水产O2性能示意图(f)[30]
Fig.2 A polyhedral representation of the ZnTi-LDH structure (a); H2 evolution productivity of MTi-LDH (b); ESR spectra recorded for DMPO-·O2- in methanol dispersion (c)[29]; Proposed structural model of energy states for the Ti3+ self-doped NiTi-LDH and schematic illustration of the O2 evolution process over the NiTi-LDH nanosheet under visible-light irradiation (d); Fluorescence spectra (e); O2 evolution from aqueous solution using 10-2 mol·L-1 AgNO3 as the sacrificial acceptor under visible-light using NiTi-LDH with different thickness (f)[30]

图3 Cu2O@ZnCr-LDH制备过程及核壳结构示意图(a); Cu2O@ZnCr-LDH光催化水分解气体产率随时间变化(b); Cu2O@ZnCr-LDH体系中光生电子分离传输方式示意图(c)[33]; Cu2O@ZnCr-LDH产气性能对比(d); Cu2O@ZnCr-LDH CDB-PAS表征中S及W参数意义(e)[34]
Fig.3 Schematic illustration for the preparation of Cu2O@ZnCr-LDH hollow coreshell photocatalyst(a); Rate of gas generation as function of irradiation time (b); Schematic illustration for the photoexcited electron separation/transport in the Cu2O@ZnCr-LDH system (c)[33]; Gas generation rate (d); Schematic definition for S- and W-parameters in CDB-PAS measurements of Cu2O@ZnCr-LDH (e)[34]
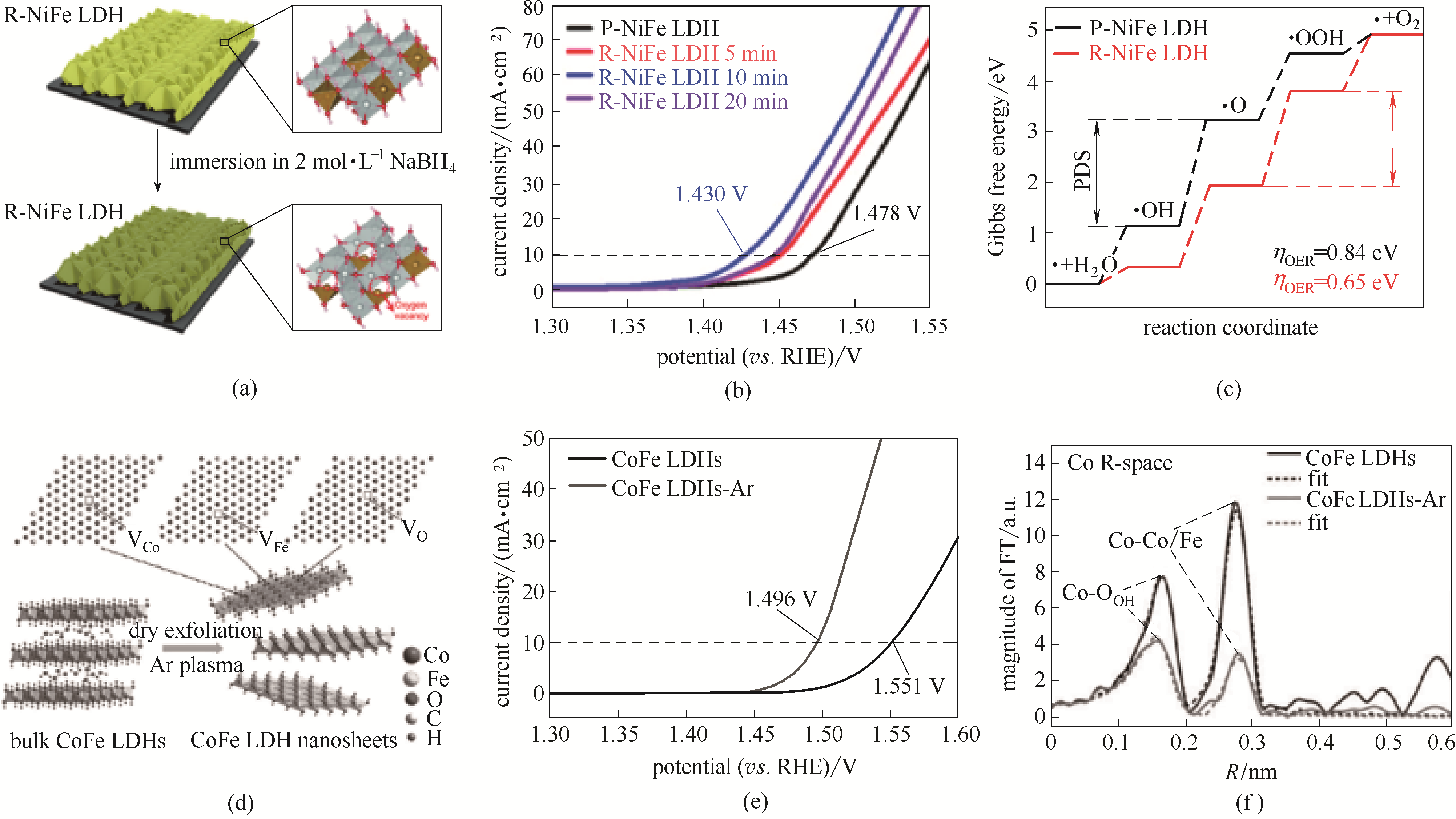
图4 NiFe-LDH 纳米棒电极中引入O缺陷过程示意图(a); NaBH4溶液浸泡处理NiFe-LDH LSV曲线(b); NaBH4溶液浸泡处理NiFe-LDH DFT计算结果(c)[39]; Plasma过程对CoFe-LDH进行干法剥离示意图(d); 块体CoFe-LDH及超薄CoFe-LDH LSV性能(e); Plasma过程处理后的CoFe-LDH Co元素XAFS R空间测试结果(f)[9]
Fig.4 Schematic illustration of introducing oxygen vacancy defects to NiFe-LDH nanoarray electrode (a); Linear sweep voltammetry polarization curves of as-prepared NiFe-LDH after NaBH4 treatment (b); Free energy plots calculated results of OER process NiFe-LDHs treated by NaBH4 (c)[39]; CoFe-LDH nanosheets by Ar plasma exfoliation (d); LSV curves of bulk CoFe-LDHs and ultrathin CoFe-LDH nanosheets (e); Magnitude of the k3-weighted Fourier transforms of the Fe edge XANES spectra for bulk CoFe-LDH and ultrathin CoFe-LDH (f)[9]

图5 CoNiP@LDH层状阵列制备过程示意图(a); 水分解体系实物图(b); 不同材料进行电极装配所得LSV表征结论(c)[44];*H在不同催化材料中Gibbs自由能计算结果(d)[48]
Fig.5 Schematic illustration for the synthesis of CoNiP@NiFe-LDH hierarchical arrays (a); Photographs of water-splitting system (b); LSV results of two-electrode cell assembled by various materials (c)[44]; The *H Gibbs free energy of different catalysts (d)[48]

图6 TiO2/ZnFe-LDH-PE 复合催化剂合成过程示意图(a); 复合催化剂光阳极J-V曲线(b); 催化过程中材料水氧化过程示意图(c)[50]; ZnO@CoNi-LDH核壳结构纳米阵列制备示意图(d); 电沉积过程总时间对光电转换效率影响(e); ZnO@CoNi-LDH光催化水氧化催化过程机理示意图(f)[53]
Fig.6 Schematic illustration for the fabrication of TiO2/ZnFe-LDH-PE NAs (a); J-V curves (b); Schematic illustration for the PEC water oxidation process over the TiO2/ZnFe-LDH photoanode (c)[50]; Schematic illustration of the fabrication of ZnO@ CoNi-LDH core-shell NWs array(d); IPCE for ZnO@LDH electrode with various LDH deposition time (e); Schematic illustration of the photoelectrochemical water oxidation process by the as-obtained ZnO@ CoNi-LDH core-shell NWs array (f)[53]

图7 C3N4和MMO@C3N4的能带结构和电荷转移示意图(a); MMO@C3N4、Ni@C3N4、Fe@C3N4和MMO/C3N4-Mix光合成H2O2的浓度-时间曲线(b)[60]; TiO2-ZnTiO3、ZnTi-MMO和P25光合成H2O2的浓度-时间曲线(c); H2O2在TiO2-ZnTiO3、ZnTi-MMO、P25上的分解曲线(d)[61]
Fig.7 Scheme of energy levels and charge transfer pathways of C3N4 and MMO@C3N4(a); The light-driven H2O2 generation in O2-equilibrated conditions over MMO@C3N4, Ni@C3N4, Fe@C3N4, and MMO/C3N4-Mix (b)[60]; The light-driven H2O2 generation (c); H2O2 decomposition over TiO2-ZnTiO3, ZnTi-MMO and P25 (d)[61]

图8 四种不同Cu/Fe比例下生成醇类物及长链醇类物转化率(a); Fe1、Cu4及CuxFey CO-TPD表征结果(b)[69]; Ni-NiO结构的电子自旋共振-结果(c)[70]; DFT计算Fe3O4、4O/Fe和4O/Fe3Zn光催化剂催化过程CO2生成,C2H4吸附及氢化能量势垒(d); ZnCoAl-LDH为前体H2 300~700℃还原制备Co基催化剂过程示意图(e)[71]
Fig.8 Alcohols STY at different pressures over catalysts with four Cu/Fe ratios (1/1, 2/1, 4/1, and 6/1)(a); CO-TPD profiles of Fe1, Cu4, and CuxFey samples (b)[69]; ESR spectrum over Ni-NiO structure (c)[70]; The potential energy profiles for CO2 formation, C2H4 adsorption, and hydrogenation under excited states on Fe3O4, 4O/Fe, and 4O/Fe3Zn (d); Fabrication of Co-x catalysts by H2 reduction of ZnCoAl-LDH nanosheets at 300—700℃ (e)[71]

图9 MgFe-LDH可控制备花样形貌微球结构催化剂过程示意图(a); N2吸脱附测定MgFe-LDH纳米微球结构比表面积与孔径分布(内置图)(b); MgFe-LDH微纳米球结构修饰电极伏安特性曲线(c)[76]; Au负载Al基LDH焙烧制备过程示意图(d)[77]; Au/HT催化剂通过DP法催化醇类化合物脱氢生成羰基化合物效率(e)[78]
Fig.9 Schematic illustration of the morphological evolution process of the as-obtained flower-like hierarchical LDH microspheres(a); N2-sorption isotherms and pore size distribution (inset) of MgFe-LDH microspheres with different inner architecture (b); Cyclic voltammograms at the MgFe-LDH microspheres modified electrodes (c)[76]; Design schematic of the Au-NCs/LDH catalyst (d)[77]; Time course for the dehydrogenation of benzyl alcohol over Au/HT catalyst prepared by the DP method (e)[78]

图11 单层NiAl-LDH在不同波段光照CO2还原产CH4、CO及H2选择性对比(a); 缺陷单层NiAl-LDH层板结构示意图,VM代表材料中金属缺陷 (M=Ni, Al), VOH 代表材料中OH缺陷(b); 光敏化剂Ru(bpy)3Cl2 单线态及三线激发态能级及单层NiAl-LDH中CBM、缺陷态、VBM相对于NHE能级关系(c)[86]; 以CoS纳米片作为光阴极 S空位还原氢化芳香硝基化合物生成功能性芳香氨基化合物过程示意图(d)[87]; 以Pd-P为阴极,以H2O (D2O)作为H源或D源对炔烃进行部分氢化过程示意图(e)[88]
Fig.11 Selectivity of CH4, CO, and H2 in CO2PR on monolayer NiAl-LDH under different wavelength (a); Illustration for the species of defects on monolayer NiAl-LDH. VM represents metal defect (M=Ni, Al), VOH represents the hydroxyl defect (b); The energy levels for the singlet and triplet excited states of photosensitizer Ru(bpy)3Cl2, together with the band edge placements for the CBM, defect state, and valence band minimum (VBM) of monolayer NiAl-LDH (VNi&OH) versus the normal hydrogen electrode (NHE) (c)[86]; Illustration of sulfur vacancy-promoted selective synthesis of functionalized aminoarenes via transfer hydrogenation of nitroarenes with H2O as the hydrogen source over a cobalt sulfide nanosheet cathode (d)[87]; Illustration of selective transfer semihydrogenation of alkynes with H2O (D2O) as the H (D) source over a Pd-P cathode (e)[88]
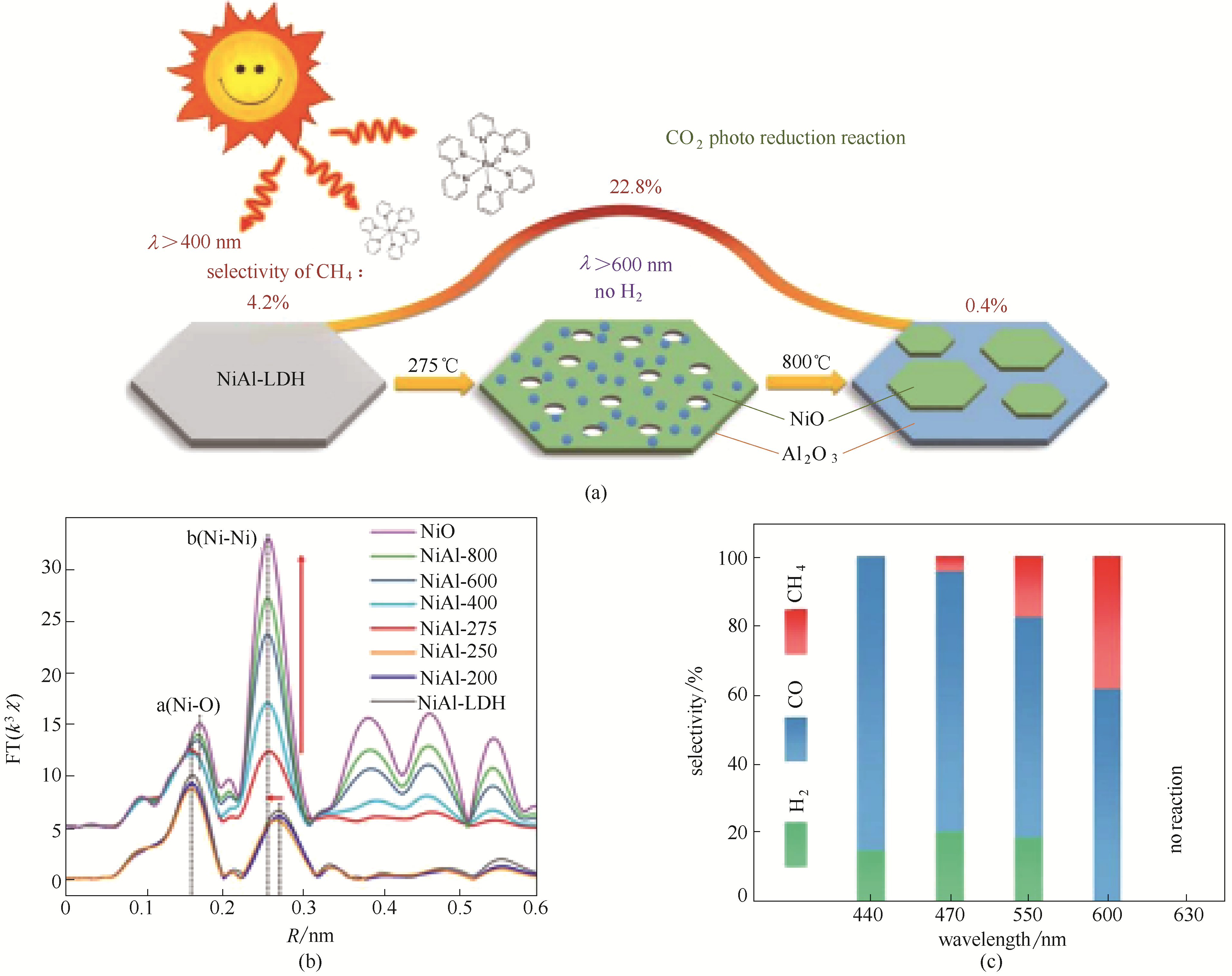
图12 NiAl-LDH经焙烧拓扑转变形成高缺陷浓度NiO结构催化CO2还原示意图(a); NiO, NiAl-x, NiAl-LDH 的EXAFS R空间图(b); NiAl-275在不同波长的单色光下催化CO2还原选择性对比(c)[89]
Fig.12 Schematic illustration of defect-containing NiO derived from the topological transformation of NiAl-LDH(a); The corresponding k3-weighted FT spectra of NiO, NiAl-x, and NiAl-LDH (b); Selectivity of NiAl-275 under different monochromatic light (c)[89]
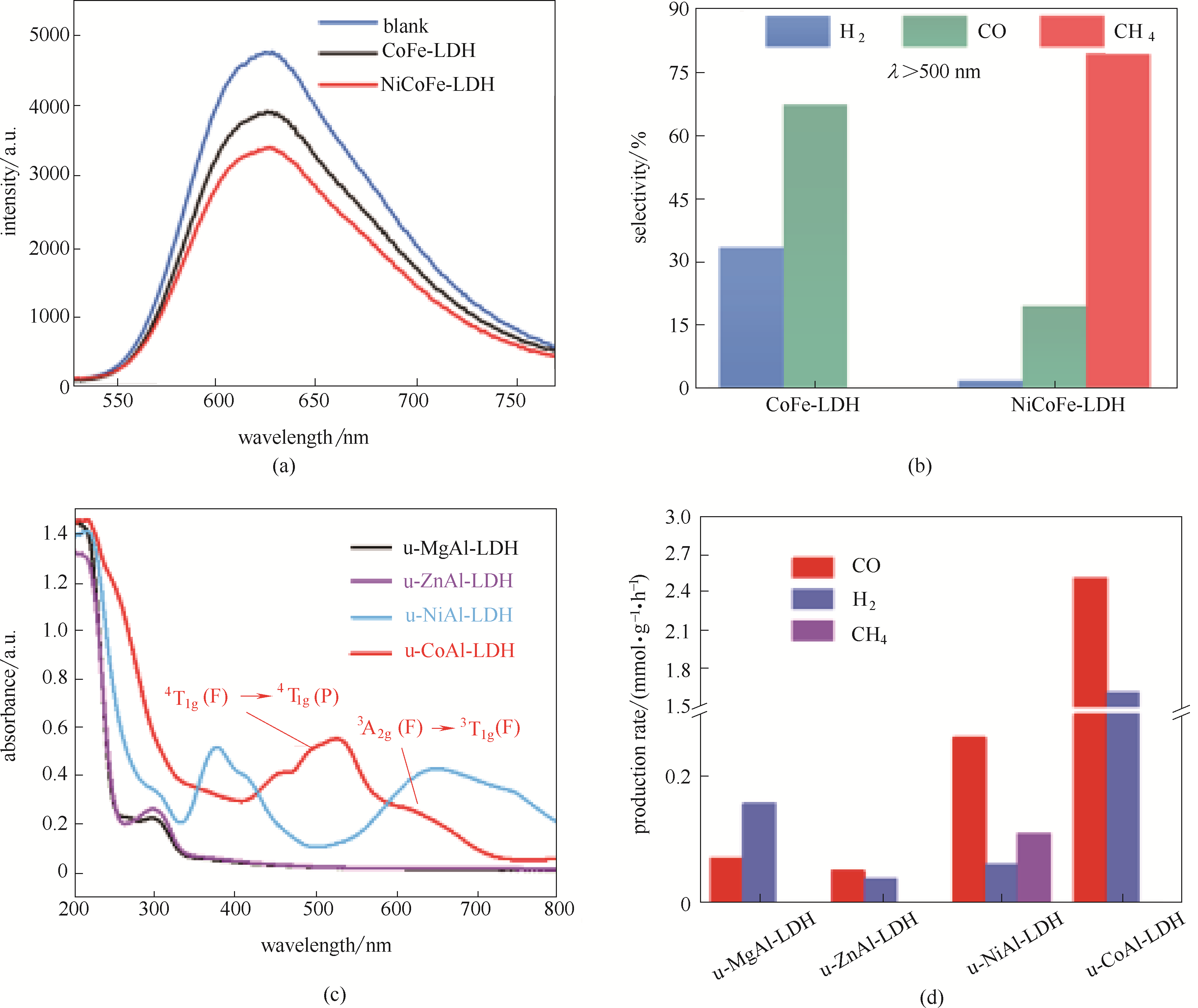
图13 CoFe-LDH及NiCoFe-LDH材料室温下光致发光光谱图(a); 波长>500 nm条件下CoFe-LDH与NiCoFe-LDH催化CO2还原性能对比(b)[90]; 不同种类Al基LDHs紫外可见光谱(c); 不同种类Al基LDHs可见光催化CO2生成CO, H2及CH4还原性能对比(d)[91]
Fig.13 Room-temperature photoluminescence (PL) spectra of CoFe-LDH and NiCoFe-LDH (a); Selectivity of CH4, CO, and H2 under irradiation above 500 nm for CoFe-LDH and NiCoFe-LDH (b)[90]; UV-vis spectra for the various u-MAl-LDH photocatalysts (c); Production rates of CO, H2, and CH4 on various u-MAl-LDH photocatalysts in CO2PR under visible light (d)[91]

图14 不同浓度LDH/MoS2催化剂调控合成气比例示意图(a); 不同LDH/MoS2催化剂浓度催化CO2还原反应产率及选择性(b)[94]; 不同插层阴离子NiAl-LDH对于CO2还原过程产物选择性示意图(c)[95]; CeO2不同比例负载MgAl-LDH催化选择性(d)[96]
Fig.14 Schematic illustration of photocatalytic CO2 reduction to tunable syngas on CoAl-LDH/MoS2 heterostructures with different catalyst concentrations (a); LDH/MoS2 nanocomposite yield and selectivity of CO and H2 in CO2PR with different concentrations (b)[94]; Schematic diagram of the selectivity of photocatalytic CO2 reduction by different anion intercalated NiAl-LDH (c)[95]; Selectivity of LDH and Ce-x (d)[96]
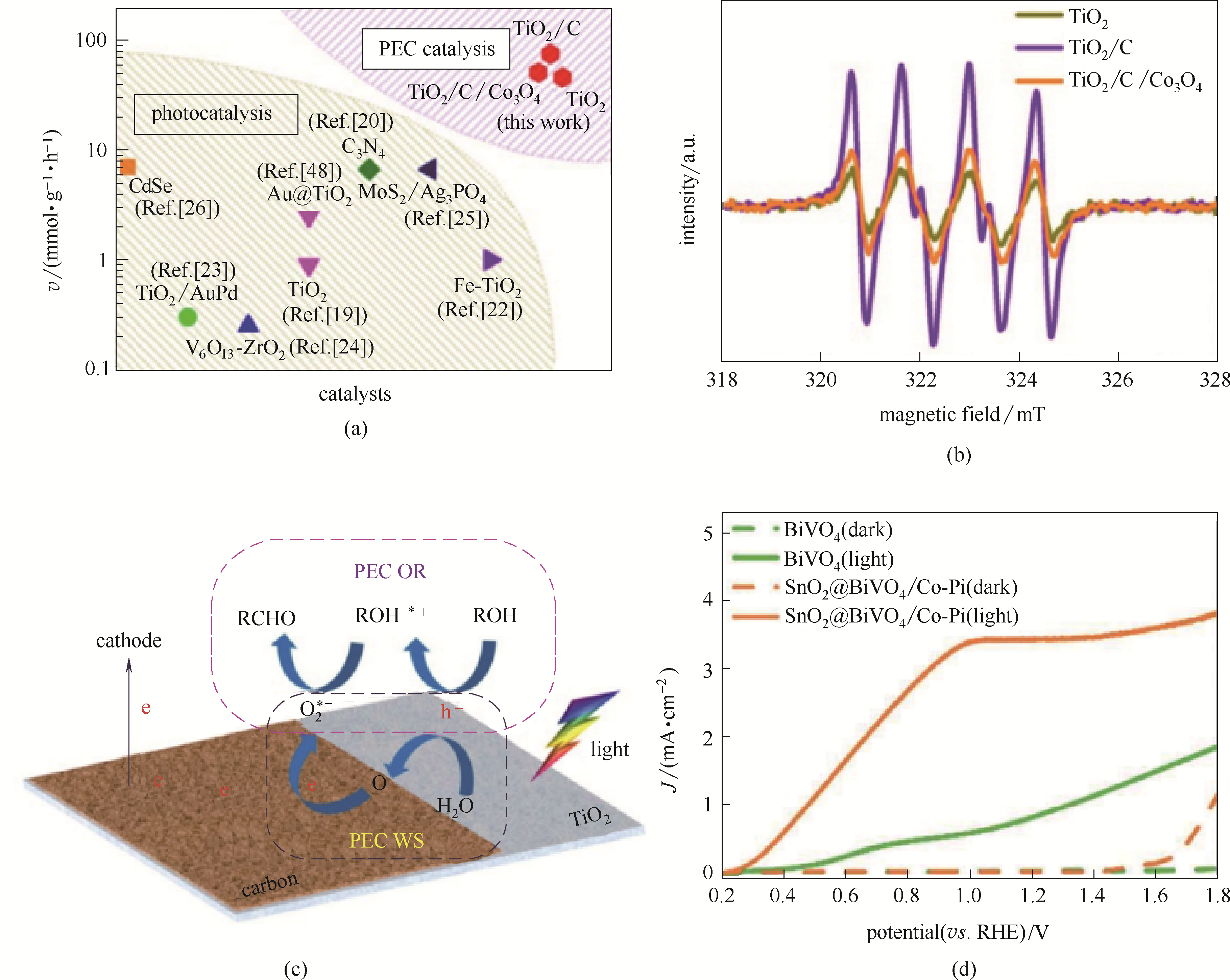
图15 本文中PEC催化速率与前人光催化过程反应速率对比(图中文献号是参考文献[97]中的文献号)(a); DMPO自旋电子捕获剂检测TiO2、TiO2/C和TiO2/C/Co3O4中·O2-结果(b); PEC WS-OR耦合氧化过程示意图(c)[97]; SnO2@BiVO4/Co-Pi光照条件下催化尿素氧化光电流曲线(d)[98]
Fig.15 Comparison of reaction rate between PEC catalysis in this work and photocatalysis reported previously (the Ref. numbers in the figure were Ref. numbers of the Ref.[97])(a); DMPO spin-trapping ESR spectra recorded for DMPO-·O2- over TiO2, TiO2/C, and TiO2/C/Co3O4 sample, respectively (b); Schematic illustration for the PEC WS-OR coupling process (c)[97]; Photocurrent-potential curves under illumination of urea oxidation (d)[98]

图16 存在O缺陷的ZnTi-LDH材料结构示意图(a); ZnTi-LDH能带结构示意图(b); 苯酚产率结果(c); 催化剂及反应气氛调控对苯酚产率影响(d); DMPO自旋捕获以检测·OH(e); DMPO自旋捕获以检测·O2-(f)[99]
Fig.16 Supercell model of ZnTi-LDH layer doped with VO vacancies(a); Schematic band diagrams (b); Reaction time profiles of phenol (c); Conversion of benzene oxidation (d); 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as spin-trapping agent under UV-vis light irradiation to detect ·OH (e) and·O2- (f), respectively[99]

图17 HMF氧化流程及中间产物(a);糠醛氧化耦合水分解催化过程电解池示意图(b);催化过程中HMF及其氧化产物浓度随时间的变化(c);NiFe-LDH生长于碳纸表面作为催化材料的LSV测试结果(d)[102]
Fig.17 HMF oxidation process and products(a); Schematic diagram of the electrochemical system used for the overall cell reactions (b); Concentration changes of HMF and its oxidation products with the time of chronoamperometric tests (c); LSV curves of the NiFe-LDH nanosheet growth on carbon fiber paper (d)[102]
| 1 | Yao S, Zhang X, Zhou W, et al. Atomic-layered Au clusters on α-MoC as catalysts for the low-temperature water-gas shift reaction [J]. Science, 2017, 357: 389-393. |
| 2 | Luo J, Im J H, Mayer M T, et al. Water photolysis at 12.3% efficiency via perovskite photovoltaics and earth-abundant catalysts [J]. Science, 2014, 345: 1593-1596. |
| 3 | Tang C, Cheng N, Pu Z, et al. NiSe nanowire film supported on nickel foam: an efficient and stable 3D bifunctional electrode for full water splitting [J]. Angew. Chem. Int. Ed., 2015, 54(32): 9351-9355. |
| 4 | Wang J, Li Z, Li X, et al. Photocatalytic hydrogen evolution from glycerol and water over nickel-hybrid cadmium sulfide quantum dots under visible-light irradiation [J]. ChemSusChem, 2014, 7(5): 1468-1475. |
| 5 | Wang J, Cui W, Liu Q, et al. Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting [J]. Adv. Mater., 2016, 28(2): 215-230. |
| 6 | Hou Y, Lohe M R, Zhang J, et al. Vertically oriented cobalt selenide/NiFe layered-double-hydroxide nanosheets supported on exfoliated graphene foil: an efficient 3D electrode for overall water splitting [J]. Energy Environ. Sci., 2016, 9(2): 478-483. |
| 7 | Liu P, Yang S, Zhang B, et al. Defect-rich ultrathin cobalt-iron layered double hydroxide for electrochemical overall water splitting [J]. ACS Appl. Mater. Interface, 2016, 8(50): 34474-33481. |
| 8 | Wang Y, Xie C, Zhang Z, et al. In situ exfoliated, N-doped, and edge-rich ultrathin layered double hydroxides nanosheets for oxygen evolution reaction [J]. Adv. Funct. Mater., 2018, 28(4): 1870119. |
| 9 | Wang Y, Zhang Y, Liu Z, et al. Layered double hydroxide nanosheets with multiple vacancies obtained by dry exfoliation as highly efficient oxygen evolution electrocatalysts [J]. Angew. Chem. Int. Ed., 2017, 56(21): 5867-5871. |
| 10 | Li W, Jiang N, Hu B, et al. Electrolyzer design for flexible decoupled water splitting and organic upgrading with electron reservoirs [J]. Chem., 2018, 4(3): 637-649. |
| 11 | Hughes M D, Xu Y J, Jenkins P, et al. Tunable gold catalysts for selective hydrocarbon oxidation under mild conditions [J]. Nature, 2005, 437(7062): 1132-1135. |
| 12 | Fukuzumi S, Ohkubo K. Selective photocatalytic reactions with organic photocatalysts [J]. Chem. Sci., 2013, 4(2): 561-574. |
| 13 | Ide Y, Torii M, Sano T, et al. Layered silicate as an excellent partner of a TiO2 photocatalyst for efficient and selective green fine-chemical synthesis [J]. J. Am. Chem. Soc., 2013, 135(32): 11784-11786. |
| 14 | Han G, Jin Y H, Burgess R A, et al. Visible-light-driven valorization of biomass intermediates integrated with H2 production catalyzed by ultrathin Ni/CdS nanosheets [J]. J. Am. Chem. Soc., 2017, 139(44): 15584-11587. |
| 15 | Wang D, Wang M, Li Z, et al. Fe-based metal-organic frameworks for highly selective photocatalytic benzene hydroxylation to phenol [J]. ACS Catal., 2015, 5(11): 6852-6857. |
| 16 | Zheng J, Lyu Y, Qiao M, et al. Photoelectrochemical synthesis of ammonia on the aerophilic-hydrophilic heterostructure with 37.8% efficiency [J]. Chem., 2019, 5(3): 617-633. |
| 17 | Fan G, Li F, Evans D G, et al. Catalytic applications of layered double hydroxides: recent advances and perspectives [J]. Chem. Soc. Rev., 2014, 43(20): 7040-7066. |
| 18 | 王瑞瑞,赵有璟,邵明飞, 等. 层状双金属氢氧化物用于催化水氧化的研究进展 [J]. 化工学报, 2016, 67(1): 54-72. |
| Wang R R, Zhao Y J, Shao M F, et al. Recent progresses in water oxidation over layered double hydroxide catalysts [J]. CIESC Journal, 2016, 67(1): 54-72. | |
| 19 | Zhang F, Xiang X, Li F, et al. Layered double hydroxides as catalytic materials: recent development [J]. Catal. Surv. Asia, 2008, 12(4): 253-265. |
| 20 | Wang Q, O'Hare D. Recent advances in the synthesis and application of layered double hydroxides (LDH) nanosheets [J]. Chem. Rev., 2012, 112: 4124-4155. |
| 21 | Zhao Y, Zhao Y, Waterhouse G I N, et al. Layered-double-hydroxide nanosheets as efficient visible-light-driven photocatalysts for dinitrogen fixation [J]. Adv. Mater., 2017, 29(42): 1703828. |
| 22 | Zhao Y, Waterhouse G I N, Chen G, et al. Two-dimensional-related catalytic materials for solar-driven conversion of COx into valuable chemical feedstocks [J]. Chem. Soc. Rev., 2019, 48(7): 1972-2010. |
| 23 | Hong W T, Risch M, Stoerzinger K A, et al. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis [J]. Energy Environ. Sci., 2015, 8(5): 1404-1427. |
| 24 | Gao R, Yan D. Recent development of Ni/Fe‐based micro/nanostructures toward photo/electrochemical water oxidation [J]. Adv. Energy Mater., 2020, 10(11): 1900954. |
| 25 | Ping J, Wang Y, Lu Q, et al. Self-assembly of single-layer CoAl-layered double hydroxide nanosheets on 3D graphene network used as highly efficient electrocatalyst for oxygen evolution reaction [J]. Adv. Mater., 2016, 28(35): 7640-7645. |
| 26 | Qiao B, Wang A, Yang X, et al. Single-atom catalysis of CO oxidation using Pt1/FeOx [J]. Nat. Chem., 2011, 3(8): 634-641. |
| 27 | Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode [J]. Nature, 1972, 238: 37-38. |
| 28 | Silva C G, Bouizi Y, Fornés V, et al. Layered double hydroxides as highly efficient photocatalysts for visible light oxygen generation from water [J]. J. Am. Chem. Soc., 2009, 131: 13833-13839. |
| 29 | Zhao Y, Chen P, Zhang B, et al. Highly dispersed TiO6 units in a layered double hydroxide for water splitting [J]. Chem. Eur. J., 2012, 18: 11949–11958. |
| 30 | Zhao Y, Li B, Wang Q, et al. NiTi-layered double hydroxides nanosheets as efficient photocatalysts for oxygen evolution from water using visible light [J]. Chem. Sci., 2014, 5(3): 951-958. |
| 31 | Li B, Zhao Y, Zhang S, et al. Visible-light-responsive photocatalysts toward water oxidation based on NiTi-layered double hydroxide/reduced graphene oxide composite materials [J]. ACS. Appl. Mater. Interfaces, 2013, 5(20): 10233-10239. |
| 32 | Gunjakar J L, Kim T W, Kim H N, et al. Mesoporous layer-by-layer ordered nanohybrids of layered double hydroxide and layered metal oxide: highly active visible light photocatalysts with improved chemical stability [J]. J. Am. Chem. Soc., 2011, 133(38): 14998-15007. |
| 33 | Wang C, Ma B, Xu S, et al. Visible-light-driven overall water splitting with a largely-enhanced efficiency over a Cu2O@ZnCr-layered double hydroxide photocatalyst [J]. Nano Energy, 2017, 32: 463-469. |
| 34 | Wang C, Ma B, Cao X, et al. Bridge-type interface optimization on a dual-semiconductor heterostructure toward high performance overall water splitting [J]. J. Mater. Chem. A, 2018, 6(17): 7871-7876. |
| 35 | Zhou P, Wang Y, Xie C, et al. Acid-etched layered double hydroxides with rich defects for enhancing the oxygen evolution reaction [J]. Chem. Commun., 2017, 53(86): 11778-11781. |
| 36 | Huang L, Chen R, Xie C, et al. Rapid cationic defect and anion dual-regulated layered double hydroxides for efficient water oxidation [J]. Nanoscale, 2018, 10(28): 13638-13644. |
| 37 | Gao Z, Liu J, Chen X, et al. Engineering NiO/NiFe LDH intersection to bypass scaling relationship for oxygen evolution reaction via dynamic tridimensional adsorption of intermediates [J]. Adv. Mater., 2019, 31(11): e1804769. |
| 38 | Zhang J, Liu J, Xi L, et al. Single-atom Au/NiFe layered double hydroxide electrocatalyst: probing the origin of activity for oxygen evolution reaction [J]. J. Am. Chem. Soc., 2018, 140(11): 3876-3879. |
| 39 | Xiong X, Cai Z, Zhou D, et al. A highly-efficient oxygen evolution electrode based on defective nickel-iron layered double hydroxide [J]. Sci. China Mater., 2018, 61(7): 939-947. |
| 40 | 李天, 郝晓杰, 白莎, 等. 单层类水滑石纳米片的可控合成及规模生产展望[J]. 物理化学学报, 2020, 36: 1912005. |
| Li T, Hao X J, Bai S, et al. Controllable synthesis and scale-up production prospect of monolayer layered double hydroxide nanosheets[J]. Acta Phys. Chim. Sin., 2020, 36: 1912005. | |
| 41 | Zhao Y, Zhang X, Jia X, et al. Sub-3 nm ultrafine monolayer layered double hydroxide nanosheets for electrochemical water oxidation [J]. Adv. Energy Mater., 2018, 8(18):1703585. |
| 42 | Zhu H, Zhang J, Yanzhang R, et al. When cubic cobalt sulfide meets layered molybdenum disulfide: a core-shell system toward synergetic electrocatalytic water splitting [J]. Adv. Mater., 2015, 27(32): 4752-4759. |
| 43 | Li Y, Zhang H, Jiang M, et al. Ternary NiCoP nanosheet arrays: an excellent bifunctional catalyst for alkaline overall water splitting [J]. Nano Res., 2016, 9(8): 2251-2259. |
| 44 | Zhou L, Jiang S, Liu Y, et al. Ultrathin CoNiP@layered double hydroxides core–shell nanosheets arrays for largely enhanced overall water splitting [J]. ACS Appl. Energy Mater., 2018, 1(2): 623-631. |
| 45 | Jia X, Zhao Y, Chen G, et al. Ni3FeN nanoparticles derived from ultrathin NiFe-layered double hydroxide nanosheets: an efficient overall water splitting electrocatalyst[J]. Adv. Energy Mater., 2016, 6(10): 1502585. |
| 46 | Xiao K, Zhou L, Shao M, et al. Fabrication of (Ni,Co)0.85Se nanosheet arrays derived from layered double hydroxides toward largely enhanced overall water splitting [J]. J. Mater. Chem. A, 2018, 6(17): 7585-7591. |
| 47 | Yang H, Chen Z, Guo P, et al. B-doping-induced amorphization of LDH for large-current-density hydrogen evolution reaction [J]. Appl. Catal. B: Environ., 2020, 261: 118240. |
| 48 | Fan H, Chen W, Chen G, et al. Plasma-heteroatom-doped Ni-V-Fe trimetallic phospho-nitride as high-performance bifunctional electrocatalyst [J]. Appl. Catal. B: Environ., 2020, 268: 118440. |
| 49 | Artero V, Chavarot-Kerlidou M, Fontecave M, et al. Splitting water with cobalt [J]. Angew. Chem. Int. Ed., 2011, 50(32): 7238-7266. |
| 50 | Zhang R, Shao M, Xu S, et al. Photo-assisted synthesis of zinc-iron layered double hydroxides/TiO2 nanoarrays toward highly-efficient photoelectrochemical water splitting [J]. Nano Energy, 2017, 33: 21-28. |
| 51 | Liu J, Xu S, Li Y, et al. Facet engineering of WO3 arrays toward highly efficient and stable photoelectrochemical hydrogen generation from natural seawater [J]. Appl. Catal. B: Environ., 2020, 264: 118540. |
| 52 | Guo J, Mao C, Zhang R, et al. Reduced titania@layered double hydroxide hybrid photoanodes for enhanced photoelectrochemical water oxidation [J]. J. Mater. Chem. A, 2017, 5(22): 11016-11025. |
| 53 | Shao M, Ning F, Wei M, et al. Hierarchical nanowire arrays based on ZnO core-layered double hydroxide shell for largely enhanced photoelectrochemical water splitting [J]. Adv. Funct. Mater., 2014, 24(5): 580-586. |
| 54 | Long X, Wang C, Wei S, et al. Layered double hydroxide onto perovskite oxide-decorated ZnO nanorods for modulation of carrier transfer behavior in photoelectrochemical water oxidation [J]. ACS Appl. Mater. Interfaces, 2020, 12(2): 2452-2459. |
| 55 | Hage R, Lienke A. Applications of transition-metal catalysts to textile and wood-pulp bleaching [J]. Angew. Chem. Int. Ed., 2005, 45(2): 206-222. |
| 56 | Campos-Martin J M, Blanco-Brieva G, Fierro J L, et al. Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process [J]. Angew. Chem. Int. Ed., 2006, 45(42): 6962-6984. |
| 57 | Cho S, Jang J W, Park Y B, et al. An exceptionally facile method to produce layered double hydroxides on a conducting substrate and their application for solar water splitting without an external bias [J]. Energy Environ. Sci., 2014, 7(7): 2301-2307. |
| 58 | Bi S, Li J, Zhong Q, et al. Low-cost CoFe2O4/biomass carbon hybrid from metal-enriched sulfate reducing bacteria as an electrocatalyst for water oxidation [J]. RSC Adv., 2018, 8(40): 22799-22805. |
| 59 | Jafari Foruzin L, Rezvani Z, Nejati K, et al. High quantum efficiency of photocatalytic water oxidation over the TiO2/MMO nanocomposite under visible-light irradiation [J]. J. Mol. Liq., 2019, 12: 2452-2459. |
| 60 | Wang R, Pan K, Han D, et al. Solar-driven H2O2 generation from H2O and O2 using earth-abundant mixed-metal oxide@carbon nitride photocatalysts [J]. ChemSusChem, 2016, 9(17): 2470-2479. |
| 61 | Han D, Xiang X, Yang J, et al. In-situ conversion and catalytic properties of mixed-metal oxide catalysts for photosynthesis of hydrogen peroxide [J]. Sci. Sin. Chim., 2017, 47(4): 465-473. |
| 62 | Sharma A S, Kaur H, Shah D, et al. Selective oxidation of alcohols by supported gold nanoparticles: recent advances [J]. RSC Adv., 2016, 6(34): 28688-28727. |
| 63 | Cho S H, Kim J Y, Kwak J. Recent advances in the transition metal-catalyzed two-fold oxidative C—H bond activation strategy for C—C and C—N bond formation [J]. Chem. Soc. Rev., 2011, 40(10): 5068-5083. |
| 64 | Galvis H M T, de Jong K P. Catalysts for production of lower olefins from synthesis gas: a review [J]. ACS Catal., 2013, 3(9): 2130-2149. |
| 65 | Yang C, Zhao H, Hou Y, et al. Fe5C2 nanoparticles: a facile bromide-induced synthesis and as an active phase for Fischer-Tropsch synthesis [J]. J. Am. Chem. Soc., 2012, 134(38): 15814-15821. |
| 66 | Gao W, Zhao Y, Chen H, et al. Core-shell Cu@(CuCo-alloy)/Al2O3 catalysts for the synthesis of higher alcohols from syngas [J]. Green Chem., 2015, 17(3): 1525-1534. |
| 67 | Eggenhuisen T M, Munnik P, Talsma H, et al. Freeze-drying for controlled nanoparticle distribution in Co/SiO2 Fischer-Tropsch catalysts [J]. J. Catal., 2013, 297: 306-313. |
| 68 | Hirsa M, Johannes H, Chaitanya B, et al. Supported iron nanoparticles as catalysts for sustainable production of lower olefins [J]. Science, 2012, 335: 835-838. |
| 69 | Li Y, Gao W, Peng M, et al. Interfacial Fe5C2-Cu catalysts toward low-pressure syngas conversion to long-chain alcohols [J]. Nat. Commun., 2020, 11(1): 61-68. |
| 70 | Zhao Y, Zhao B, Liu J, et al. Oxide-modified nickel photocatalysts for the production of hydrocarbons in visible light [J]. Angew. Chem. Int. Ed., 2016, 55(13): 4215-4219. |
| 71 | Li Z, Liu J, Zhao Y, et al. Co-based catalysts derived from layered-double-hydroxide nanosheets for the photothermal production of light olefins [J]. Adv. Mater., 2018, 30(31): e1800527. |
| 72 | Zhao Y, Li Z, Li M, et al. Reductive transformation of layered-double-hydroxide nanosheets to Fe-based heterostructures for efficient visible-light photocatalytic hydrogenation of CO [J]. Adv. Mater., 2018, 30: e1803127. |
| 73 | Chen G, Gao R, Zhao Y, et al. Alumina-supported CoFe alloy catalysts derived from layered-double-hydroxide nanosheets for efficient photothermal CO2 hydrogenation to hydrocarbons [J]. Adv. Mater., 2018, 30(3): 1704663. |
| 74 | Guo Y, Hu J, Wan L, et al. Nanostructured materials for electrochemical energy conversion and storage devices [J]. Adv. Mater., 2008, 20(15): 2878-2887. |
| 75 | Wallace G G, Chen J, Li D, et al. Nanostructured carbon electrodes [J]. J. Mater. Chem., 2010, 20(18): 3553-3562. |
| 76 | Shao M, Ning F, Zhao J, et al. Hierarchical layered double hydroxide microspheres with largely enhanced performance for ethanol electrooxidation [J]. Adv. Funct. Mater., 2013, 23(28): 3513-3518. |
| 77 | Li L, Dou L, Zhang H, et al. Layered double hydroxide supported gold nanoclusters by glutathione-capped Au nanoclusters precursor method for highly efficient aerobic oxidation of alcohols [J]. Nanoscale, 2014, 6(7): 3753-3763. |
| 78 | Fang W, Zhang Q, Chen J, et al. Gold nanoparticles on hydrotalcites as efficient catalysts for oxidant-free dehydrogenation of alcohols [J]. Chem. Commun., 2010, 46(9): 1547-1549. |
| 79 | Wang C, Xie Z, de Krafft K E, et al. Doping metal-organic frameworks for water oxidation, carbon dioxide reduction, and organic photocatalysis [J]. J. Am. Chem. Soc., 2011, 133(34): 13445-13454. |
| 80 | Fu Y, Sun D, Chen Y, et al. An amine-functionalized titanium metal-organic framework photocatalyst with visible-light-induced activity for COL reduction [J]. Angew. Chem. Int. Ed., 2012, 51(14): 3364-3367. |
| 81 | Li Q, Li X, Wageh S, et al. CdS/graphene nanocomposite photocatalysts [J]. Adv. Energy Mater., 2015, 5: 1500010. |
| 82 | Zhang J, Wang Y, Jin J, et al. Efficient visible-light photocatalytic hydrogen evolution and enhanced photostability of core/shell CdS/g-C3N4 nanowires [J]. ACS Appl. Mater. Interfaces, 2013, 5(20): 10317-10324. |
| 83 | 张晓晴,徐艳,杨春辉,等.原位共沉淀法制备Ni-Mg-Al-LDHs/γ-Al2O3催化前驱体在甲烷二氧化碳重整反应体系中的性能评价[J]. 物理化学学报, 2015, 31(5): 948-954. |
| Zhang X Q, Xu Y, Yang C H, et al. In-situ co-precipitation of Ni-Mg-Al-LDH catalytic precursor on γ-Al2O3 for dry reforming of methane: synthesis and evaluation [J]. Acta Phys. -Chim. Sin., 2015, 31(5): 948-954. | |
| 84 | Zhu M, Ge Q, Zhu X. Catalytic reduction of CO2 to CO via reverse water gas shift reaction: recent advances in the design of active and selective supported metal catalysts [J]. Trans. Tianjin Univ., 2020,26(3): 172-187. |
| 85 | Zhao Y, Chen G, Bian T, et al. Defect-rich ultrathin ZnAl-layered double hydroxide nanosheets for efficient photoreduction of CO2 to CO with water [J]. Adv. Mater., 2015, 27(47): 7824-7831. |
| 86 | Tan L, Xu S M, Wang Z, et al. Highly selective photoreduction of CO2 with suppressing H2 evolution over monolayer layered double hydroxide under irradiation above 600 nm [J]. Angew. Chem. Int. Ed., 2019, 58(34): 11860-11867. |
| 87 | Zhao Y, Liu C, Wang C, et al. Sulfur vacancy-promoted highly selective electrosynthesis of functionalized aminoarenes via transfer hydrogenation of nitroarenes with H2O over a Co3S4-x nanosheet cathode [J]. CCS Chem., 2020, 2: 507-515. |
| 88 |
Wu Y, Liu C, Wang C, et al. Selective transfer semihydrogenation of alkynes with H2O (D2O) as the H (D) source over a Pd-P cathode [J]. Angew. Chem. Int. Ed., 2020. doi: 10.1002/anie.202009757.
DOI URL |
| 89 | Wang Z, Xu S, Tan L, et al. 600 nm-driven photoreduction of CO2 through the topological transformation of layered double hydroxides nanosheets [J]. Appl. Catal. B Environ., 2020, 270: 11884. |
| 90 | Hao X, Tan L, Xu Y, et al. Engineering active Ni sites in ternary layered double hydroxide nanosheets for a highly selective photoreduction of CO2 to CH4 under irradiation above 500 nm [J]. Ind. Eng. Chem. Res., 2020, 59(7): 3008-3015. |
| 91 | Bai S, Wang Z, Tan L, et al. 600 nm irradiation-induced efficient photocatalytic CO2 reduction by ultrathin layered double hydroxide nanosheets [J]. Ind. Eng. Chem. Res., 2020, 59(13): 5848-5857. |
| 92 | Davis B. Fischer-Tropsch synthesis: reaction mechanisms for iron catalysts [J]. Catal. Today, 2009, 141(1/2): 25-33. |
| 93 | Wang X, Wang Z, Bai Y, et al. Tuning the selectivity of photoreduction of CO2 to syngas over Pd/layered double hydroxide nanosheets under visible light up to 600 nm [J]. J. Energy Chem., 2020, 46: 1-7. |
| 94 | Qiu C, Hao X, Tan L, et al. 500 nm induced tunable syngas synthesis from CO2 photoreduction by controlling heterojunction concentration [J]. Chem. Commun., 2020, 56(40): 5354-5357. |
| 95 | Kipkorip P, Tan L, Ren J, et al. Intercalation effect in NiAl-layered double hydroxide nanosheets for CO2 reduction under visible light [J]. Chem. Res. Chinese Universities, 2020, 36(1): 127-133. |
| 96 |
Tan L, Kipkorip P, Ren J, et al. Photocatalytic syngas synthesis from CO2 to H2O using ultrafine CeO2-decorated layered double hydroxide nanosheets under visible light up to 600 nm [J]. Front. Chem. Sci. Eng., 2020. doi: 10.1007/S11705-020-1947-4.
DOI URL |
| 97 | Zhang R, Shao M, Li Z, et al. Photoelectrochemical catalysis toward selective anaerobic oxidation of alcohols [J]. Chem. Eur. J., 2017, 23(34): 8142-8147. |
| 98 | Liu J, Li J, Shao M, et al. Directed synthesis of SnO2@BiVO4/Co-Pi photoanode for highly efficient photoelectrochemical water splitting and urea oxidation [J]. J. Mater. Chem. A, 2019, 7(11): 6327-6336. |
| 99 | Li J, Xu Y, Ding Z, et al. Photocatalytic selective oxidation of benzene to phenol in water over layered double hydroxide: a thermodynamic and kinetic perspective [J]. Chem. Eng. J., 2020, 388: 124248. |
| 100 | You B, Jiang N, Liu X, et al. Simultaneous H2 generation and biomass upgrading in water by an efficient noble-metal-free bifunctional electrocatalyst [J]. Angew. Chem. Int. Ed., 2016, 55(34): 9913-9917. |
| 101 | You B, Liu X, Jiang N, et al. A general strategy for decoupled hydrogen production from water splitting by integrating oxidative biomass valorization [J]. J. Am. Chem. Soc., 2016, 138(41): 13639-13646. |
| 102 | Liu W J, Dang L, Xu Z, et al. Electrochemical oxidation of 5-hydroxymethylfurfural with NiFe layered double hydroxide (LDH) nanosheet catalysts [J]. ACS Catal., 2018, 8(6): 5533-5541. |
| [1] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [2] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [3] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [4] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [5] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [6] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [7] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [8] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [9] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [10] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [11] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [12] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [13] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [14] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [15] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号