化工学报 ›› 2021, Vol. 72 ›› Issue (1): 555-568.DOI: 10.11949/0438-1157.20201071
收稿日期:2020-07-31
修回日期:2020-10-05
出版日期:2021-01-05
发布日期:2021-01-05
通讯作者:
石琪
作者简介:赵宇(1994—),男,硕士研究生,基金资助:
ZHAO Yu( ),SHI Qi(
),SHI Qi( ),DONG Jinxiang
),DONG Jinxiang
Received:2020-07-31
Revised:2020-10-05
Online:2021-01-05
Published:2021-01-05
Contact:
SHI Qi
摘要:
在生物质催化炼制呋喃化合物的过程中,通常得到的是低浓度糠醛(Fur)和5-羟甲基糠醛(5-HMF)的混合物。基于Fur和5-HMF都是椭圆形分子,因此通过设计构筑和调控椭圆形孔窗的吸附剂可以实现Fur和5-HMF的筛分分离。选用二价钴盐和三种烷基取代基团逐渐增大的咪唑配体(2-乙基咪唑/2-eIm、2-丙基咪唑/2-pIm和2-丁基咪唑/2-bIm)合成了三种椭圆形孔窗尺寸逐渐减小的ANA拓扑ZIFs材料:ANA-[Co(eIm)2]、ANA-[Co(pIm)2]和ANA-[Co(bIm)2]。首先解析了这三种ZIFs材料的晶体结构,并对其进行了PXRD、水蒸气吸附、N2吸脱附和SEM等基本表征,然后采用静态吸附和动态柱吸附研究了这三种材料对Fur和5-HMF的吸附分离性能。静态吸附、单组分动态柱吸附以及综合速率模型模拟计算结果显示:ANA-[Co(pIm)2]狭窄的椭圆形孔窗与Fur分子尺寸接近,但小于5-HMF,使得Fur分子可以吸附进入椭圆形孔窗,而5-HMF分子几乎不能通过。进一步在双组分Fur/5-HMF(5%/5%,质量分数)动态柱吸附中,ANA-[Co(pIm)2]对Fur的吸附量为91.7 mg·g-1,不吸附5-HMF。因此,通过改变咪唑配体取代基团精细调控ZIFs椭圆形孔窗尺寸,并利用ZIFs椭圆形孔窗的位阻效应实现了Fur和5-HMF的筛分分离。
中图分类号:
赵宇, 石琪, 董晋湘. ZIFs椭圆形孔窗的精细调控及糠醛/5-羟甲基糠醛吸附分离性能研究[J]. 化工学报, 2021, 72(1): 555-568.
ZHAO Yu, SHI Qi, DONG Jinxiang. Fine adjustment of elliptical windows of ZIFs and performances of adsorptive separation of furfural/5-hydroxymethylfurfural[J]. CIESC Journal, 2021, 72(1): 555-568.
ZIFs (CCDC number) | Empirical formula | Molecular weight | Crystal system | Space group | Cell parameter a /? | Density/ (g·cm-3) | Window/?2 | SBET / (m2·g-1) | Pore volume/(cm3·g-1) |
|---|---|---|---|---|---|---|---|---|---|
ANA-[Co(eIm)2] (2017658) | C10H14CoN4 | 249.18 | cubic | 26.581(2) | 1.058 | 7.4×4.9/4.2 | 610 | 0.268 | |
ANA-[Co(pIm)2] (2017659) | C12H18CoN4 | 277.23 | cubic | 26.606(2) | 1.173 | 7.5×3.1/4.2 | 333 | 0.139 | |
ANA-[Co(bIm)2] (2017657) | C14H22CoN4 | 305.28 | cubic | 26.340(3) | 1.332 | 7.5×1.3/4.1 | — | — |
表1 ZIFs材料的晶体结构参数
Table 1 Crystal structure parameters of ZIFs materials
ZIFs (CCDC number) | Empirical formula | Molecular weight | Crystal system | Space group | Cell parameter a /? | Density/ (g·cm-3) | Window/?2 | SBET / (m2·g-1) | Pore volume/(cm3·g-1) |
|---|---|---|---|---|---|---|---|---|---|
ANA-[Co(eIm)2] (2017658) | C10H14CoN4 | 249.18 | cubic | 26.581(2) | 1.058 | 7.4×4.9/4.2 | 610 | 0.268 | |
ANA-[Co(pIm)2] (2017659) | C12H18CoN4 | 277.23 | cubic | 26.606(2) | 1.173 | 7.5×3.1/4.2 | 333 | 0.139 | |
ANA-[Co(bIm)2] (2017657) | C14H22CoN4 | 305.28 | cubic | 26.340(3) | 1.332 | 7.5×1.3/4.1 | — | — |
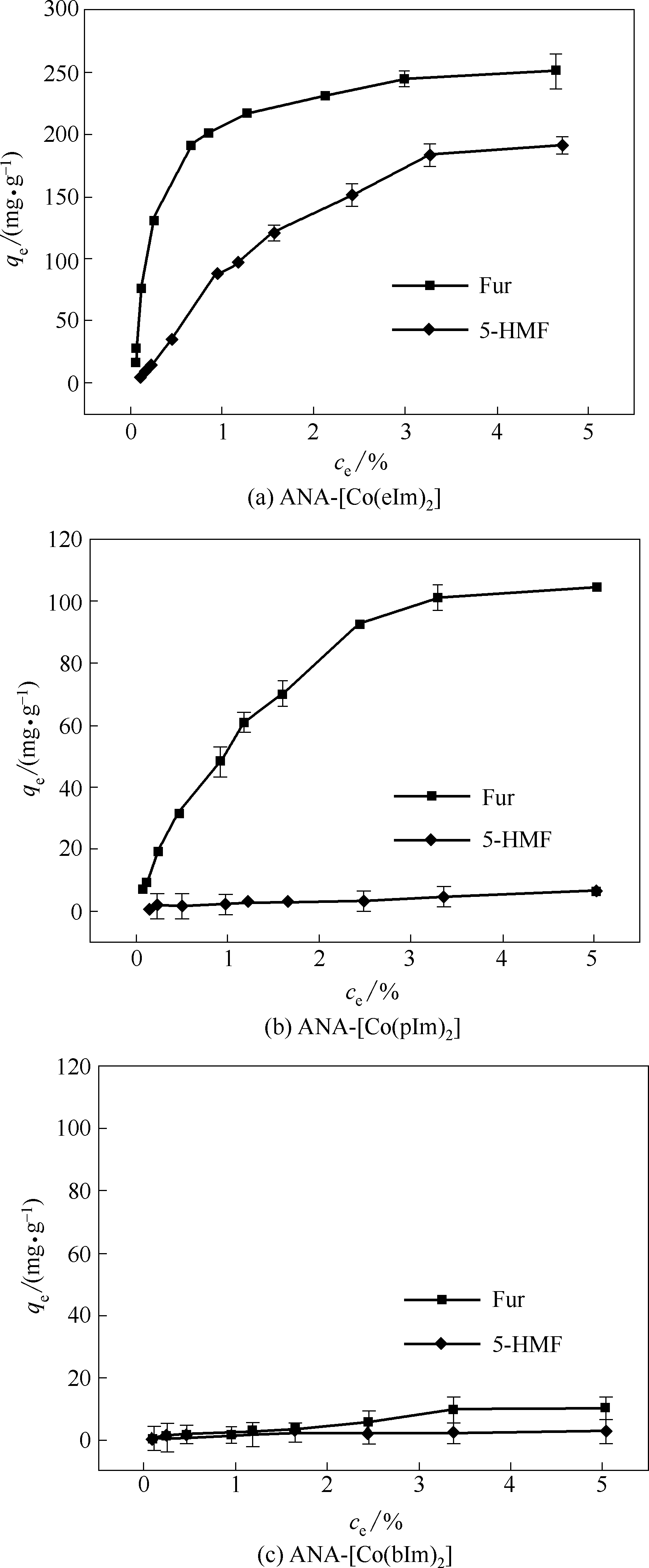
图4 25℃下ZIFs对单组分Fur和5-HMF的静态吸附等温线(误差棒对应每个点的标准偏差)
Fig.4 Static adsorption isotherms of ZIFs for single-component Fur and 5-HMF at the temperature of 25℃ (Error bars correspond to the standard deviation at each point)
| ZIFs | Langmuir model | Freundlich model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fur | 5-HMF | Fur | 5-HMF | |||||||||
KL/ (L·mg-1) | qmax/(mg·g-1) | R2 | KL/ (L·mg-1) | qmax/(mg·g-1) | R2 | KF/(mg·g-1) | n | R2 | KF/ (mg·g-1) | n | R2 | |
| ANA-[Co(eIm)2] | 3.144 | 271.8 | 0.981 | 0.367 | 318.1 | 0.981 | 176.153 | 3.108 | 0.841 | 79.946 | 1.569 | 0.941 |
| ANA-[Co(pIm)2] | 0.622 | 145.2 | 0.991 | 0.344 | 9.2 | 0.901 | 52.038 | 1.968 | 0.952 | 2.376 | 1.710 | 0.942 |
| ANA-[Co(bIm)2] | 0.064 | 44.1 | 0.950 | 1.014 | 3.1 | 0.958 | 2.672 | 1.145 | 0.945 | 1.403 | 2.263 | 0.937 |
表2 Langmuir和Freundlich吸附模型对单组分静态吸附拟合的结果
Table 2 Langmuir adsorption and Freundlich adsorption model for single-component batch adsorption fitting results
| ZIFs | Langmuir model | Freundlich model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fur | 5-HMF | Fur | 5-HMF | |||||||||
KL/ (L·mg-1) | qmax/(mg·g-1) | R2 | KL/ (L·mg-1) | qmax/(mg·g-1) | R2 | KF/(mg·g-1) | n | R2 | KF/ (mg·g-1) | n | R2 | |
| ANA-[Co(eIm)2] | 3.144 | 271.8 | 0.981 | 0.367 | 318.1 | 0.981 | 176.153 | 3.108 | 0.841 | 79.946 | 1.569 | 0.941 |
| ANA-[Co(pIm)2] | 0.622 | 145.2 | 0.991 | 0.344 | 9.2 | 0.901 | 52.038 | 1.968 | 0.952 | 2.376 | 1.710 | 0.942 |
| ANA-[Co(bIm)2] | 0.064 | 44.1 | 0.950 | 1.014 | 3.1 | 0.958 | 2.672 | 1.145 | 0.945 | 1.403 | 2.263 | 0.937 |

图5 25℃下ZIFs对单组分Fur和5-HMF的动态穿透曲线(填料层:25 cm;柱径:0.4 cm;流量:0.05 ml·min-1)
Fig.5 Breakthrough curves of single-component Fur and 5-HMF on ZIFs at the temperature of 25℃ (length: 25 cm; diameter: 0.4 cm; rate: 0.05 ml·min-1)
| GRM parameter | ANA-[Co(eIm)2] | ANA-[Co(pIm)2] | ANA-[Co(bIm)2] | |||
|---|---|---|---|---|---|---|
| Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | |
| Dax /(cm2·min-1) | 5.730×10-4 | 1.567×10-4 | 4.820×10-4 | 1.773×10-4 | 1.720×10-4 | 4.675×10-4 |
| Kfilm/(cm·min-1) | 2.740×10-2 | 2.360×10-2 | 3.510×10-2 | 1.350×10-2 | 3.490×10-2 | 1.900×10-2 |
| Dpore /(cm2·min-1) | 2.176×10-3 | 2.054×10-4 | 1.534×10-3 | 8.035×10-5 | 8.712×10-5 | 8.006×10-5 |
表3 综合速率模型对单组分动态柱吸附的拟合结果
Table 3 Fitting results of the general rate model (GRM) for single-component dynamic column adsorption
| GRM parameter | ANA-[Co(eIm)2] | ANA-[Co(pIm)2] | ANA-[Co(bIm)2] | |||
|---|---|---|---|---|---|---|
| Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | |
| Dax /(cm2·min-1) | 5.730×10-4 | 1.567×10-4 | 4.820×10-4 | 1.773×10-4 | 1.720×10-4 | 4.675×10-4 |
| Kfilm/(cm·min-1) | 2.740×10-2 | 2.360×10-2 | 3.510×10-2 | 1.350×10-2 | 3.490×10-2 | 1.900×10-2 |
| Dpore /(cm2·min-1) | 2.176×10-3 | 2.054×10-4 | 1.534×10-3 | 8.035×10-5 | 8.712×10-5 | 8.006×10-5 |
| Item | ANA-[Co(eIm)2] /(mg·g-1) | ANA-[Co(pIm)2] /(mg·g-1) | ANA-[Co(bIm)2]/(mg·g-1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2% | 5% | 2% | 5% | 2% | 5% | |||||||
| Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | |
| single-component batch adsorption | 222.3 | 129.3 | 251.4 | 190.8 | 77.7 | 3.3 | 105.1 | 6.5 | 4.4 | 1.9 | 10.1 | 2.8 |
| dynamic column adsorption | ||||||||||||
| single | 212.8 | 125.8 | 245.3 | 195.8 | 76.5 | 3.9 | 93.8 | 7.3 | 4.2 | 0.1 | 9.9 | 1.5 |
| binary | 208.9 | 2.7 | 239.1 | 2.3 | 77.1 | — | 91.7 | — | 4.1 | — | 9.1 | — |
表4 单组分静态吸附和动态柱吸附的吸附量汇总
Table 4 Summary of adsorption capacity of single-component batch adsorption and dynamic column adsorption
| Item | ANA-[Co(eIm)2] /(mg·g-1) | ANA-[Co(pIm)2] /(mg·g-1) | ANA-[Co(bIm)2]/(mg·g-1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2% | 5% | 2% | 5% | 2% | 5% | |||||||
| Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | Fur | 5-HMF | |
| single-component batch adsorption | 222.3 | 129.3 | 251.4 | 190.8 | 77.7 | 3.3 | 105.1 | 6.5 | 4.4 | 1.9 | 10.1 | 2.8 |
| dynamic column adsorption | ||||||||||||
| single | 212.8 | 125.8 | 245.3 | 195.8 | 76.5 | 3.9 | 93.8 | 7.3 | 4.2 | 0.1 | 9.9 | 1.5 |
| binary | 208.9 | 2.7 | 239.1 | 2.3 | 77.1 | — | 91.7 | — | 4.1 | — | 9.1 | — |
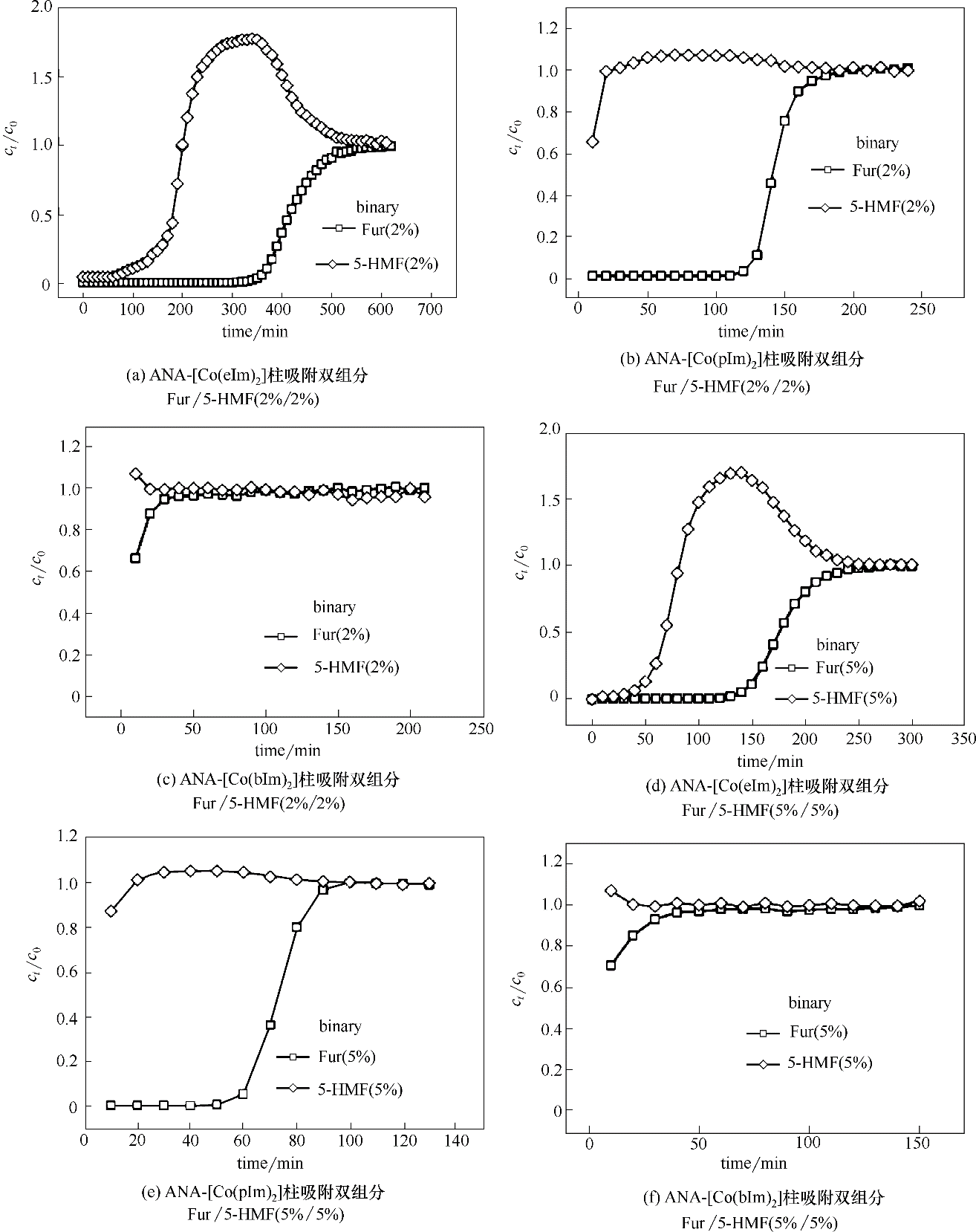
图6 25℃下ZIFs对双组分Fur/5-HMF的动态穿透曲线(填料层:25 cm;柱径:0.4 cm;流量:0.05 ml·min-1)
Fig.6 Breakthrough curves of binary-component Fur/5-HMF diluted in water on ZIFs at the temperature of 25℃(length: 25 cm; diameter: 0.4 cm; rate: 0.05 ml·min-1)
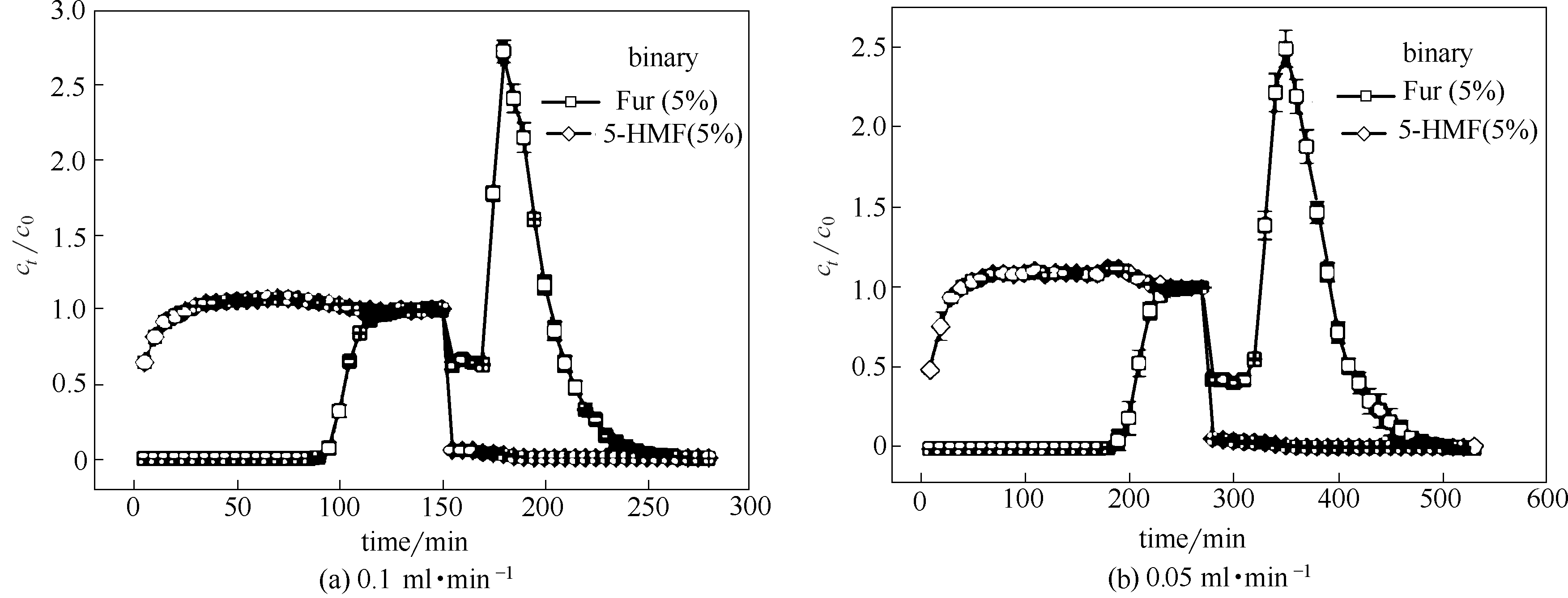
图7 不同流量下ANA-[Co(pIm)2]对双组分Fur/5-HMF(5%/5%)的动态柱吸脱附曲线(填料层:33 cm,柱径:0.6 cm,吸附温度:25℃,脱附温度:40℃;误差棒对应每个点的标准偏差)
Fig.7 Adsorption-desorption breakthrough curves of binary-component Fur/5-HMF (5%/5%) diluted in water on ANA-[Co(pIm)2] at different flow rates (length: 33 cm, diameter: 0.6 cm, adsorption temperature: 25℃, desorption temperature: 40℃; error bars correspond to the standard deviation at each point)
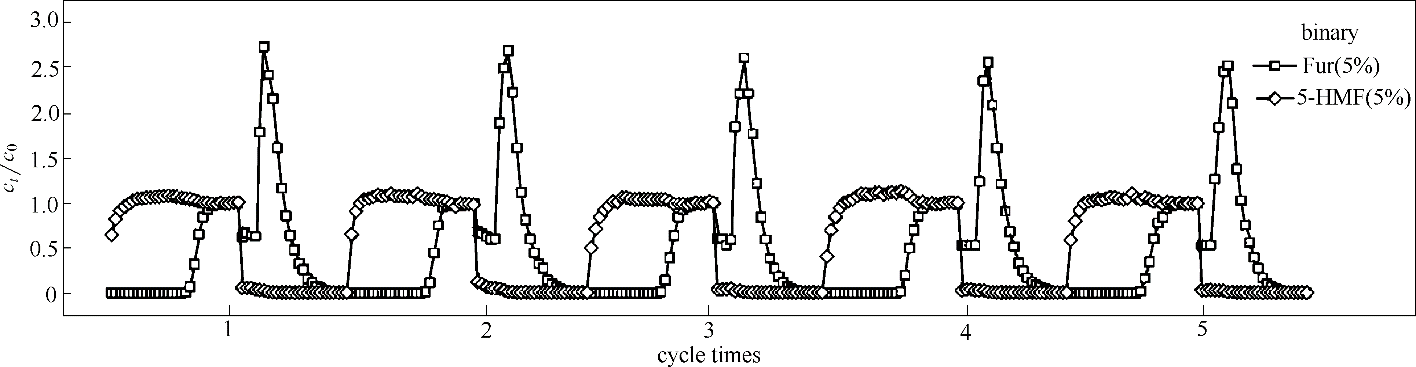
图8 0.1 ml·min-1流量下ANA-[Co(pIm)2]对双组分Fur/5-HMF(5%/5%)循环5次的动态柱吸脱附曲线(填料层:33 cm,柱径:0.6 cm,吸附温度:25℃,脱附温度:40℃)
Fig.8 Adsorption-desorption breakthrough curves of binary-component Fur/5-HMF (5%/5%) diluted in water on ANA-[Co(pIm)2] in 5 adsorption-desorption cycles at a flow rate of 0.1 ml·min-1 (length: 33 cm, diameter: 0.6 cm, adsorption temperature: 25℃, desorption temperature: 40℃)
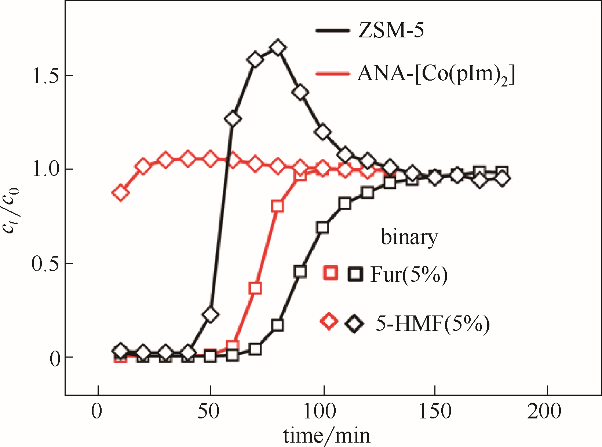
图10 25℃下ZSM-5和ANA-[Co(pIm)2]对双组分Fur/5-HMF(5%/5%)的动态穿透曲线(填料层:25 cm;柱径:0.4 cm,流量:0.05 ml·min-1)
Fig.10 Breakthrough curves of binary-component Fur/5-HMF (5%/5%) diluted in water on ZSM-5 and ANA-[Co(pIm)2] at the temperature of 25℃(length: 25 cm, diameter: 0.4 cm, rate: 0.05 ml·min-1)
| Adsorbent | qFur/(mg·g-1) | q5-HMF /(mg·g-1) | SFur,5-HMF |
|---|---|---|---|
| ZSM-5 | 112.7 | 30.1 | 3.7 |
| MAF-5 | 220.0 | 1.9 | 115.8 |
| ANA-[Co(eIm)2] | 239.1 | 2.3 | 104.0 |
| ANA-[Co(pIm)2] | 91.7 | — | — |
表5 ZSM-5,MAF-5[21],ANA-[Co(eIm)2]和ANA-[Co(pIm)2]的动态柱吸附量和吸附选择性汇总
Table 5 Summary of adsorption capacity and selectivity of dynamic column adsorption for ZSM-5, MAF-5[21], ANA-[Co(eIm)2] and ANA-[Co(pIm)2]
| Adsorbent | qFur/(mg·g-1) | q5-HMF /(mg·g-1) | SFur,5-HMF |
|---|---|---|---|
| ZSM-5 | 112.7 | 30.1 | 3.7 |
| MAF-5 | 220.0 | 1.9 | 115.8 |
| ANA-[Co(eIm)2] | 239.1 | 2.3 | 104.0 |
| ANA-[Co(pIm)2] | 91.7 | — | — |
| 1 | Ragauskas A J, Williams C K, Davison B H, et al. The path forward for biofuels and biomaterials[J]. Science, 2006, 311(5760): 484-489. |
| 2 | Zhang X G, Wilson K, Lee A F. Heterogeneously catalyzed hydrothermal processing of C5-C6 sugars[J]. Chemical Reviews, 2016, 116(19): 12328-12368. |
| 3 | Bozell J J, Petersen G R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy's “Top 10” revisited[J]. Green Chemistry, 2010, 12(4): 539-554. |
| 4 | Xu S Q, Pan D H, Wu Y F, et al. Direct conversion of wheat straw components into furan compounds using a highly efficient and reusable SnCl2-PTA/β zeolite catalyst[J]. Industrial & Engineering Chemistry Research, 2019, 58(22): 9276-9285. |
| 5 | Widsten P, Murton K, West M. Production of 5-hydroxymethylfurfural and furfural from a mixed saccharide feedstock in biphasic solvent systems[J]. Industrial Crops & Products, 2018, 119: 237-242. |
| 6 | 聂一凡, 候其东, 李维尊, 等. 糠醛的水解制备和应用研究进展[J]. 化工进展, 2019, 38(5): 2164-2178. |
| Nie Y F, Hou Q D, Li W Z, et al. Advances in production furfural via hydrolysis and application of furfural[J]. Chemical Industry and Engineering Progress, 2019, 38(5): 2164-2178. | |
| 7 | van Putten R J, van der Waal J C, de Jong E D, et al. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources[J]. Chemical Reviews, 2013, 113(3): 1499-1597. |
| 8 | Zhang K, Agrawal M, Harper J, et al. Removal of the fermentation inhibitor, furfural, using activated carbon in cellulosic-ethanol production[J]. Industrial & Engineering Chemistry Research, 2011, 50(24): 14055-14060. |
| 9 | Rajabbeigi N, Ranjan R, Tsapatsis M, et al. Selective adsorption of HMF on porous carbons from fructose/DMSO mixtures[J]. Microporous and Mesoporous Materials, 2012, 158: 253-256. |
| 10 | Jia X M, Li X Y, Liu Z, et al. Adsorption process and mechanism for furfural separation with macroporous resin[J]. Desalination and Water Treatment, 2014, 56(8): 1-11. |
| 11 | Ijzer A C, Vriezekolk E, Rolevink E, et al. Performance analysis of aromatic adsorptive resins for the effective removal of furan derivatives from glucose[J]. Journal of Chemical Technology & Biotechnology, 2015, 90(1): 101-109. |
| 12 | Anbia M, Mohammadi N. A nanoporous adsorbent for removal of furfural from aqueous solutions[J]. Desalination, 2009, 249(1): 150-153. |
| 13 | Ranjan R, Thust S, Gounaris C E, et al. Adsorption of fermentation inhibitors from lignocellulosic biomass hydrolyzates for improved ethanol yield and value-added product recovery[J]. Microporous and Mesoporous Materials, 2009, 122(1): 143-148. |
| 14 | Huang X C, Lin Y Y, Zhang J P, et al. Ligand-directed strategy for zeolite-type metal-organic frameworks: zinc (II) imidazolates with unusual zeolitic topologies[J]. Angewandte Chemie International Edition, 2006, 45(10): 1557-1559. |
| 15 | Banerjee R, Phan A, Wang B, et al. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture[J]. Science, 2008, 319(5865): 939-943. |
| 16 | Li J R, Sculley J P, Zhou H C. Metal-organic frameworks for separations[J]. Chemical Reviews, 2012, 112(2): 869-932. |
| 17 | 王可可, 李亮莎, 黄宏亮, 等. 铪金属-有机骨架材料的孔尺寸调控及其吸附性能[J]. 化工学报, 2014, 65(5): 1696-1705. |
| Wang K K, Li L S, Huang H L, et al. Control of pore size in Hf-based metal-organic frameworks and exploration of their adsorption properties[J]. CIESC Journal, 2014, 65(5): 1696-1705. | |
| 18 | 高春苹, 石琪, 董晋湘.憎水性金属多氮唑骨架材料(MAF-6)对糠醛/水吸附分离性能的研究[J].化工进展, 2017, 36(9): 3429-3435. |
| Gao C P, Shi Q, Dong J X. Study of the adsorptive separation performance of hydrophobic metal azolate framework(MAF-6) for furfural/water system[J]. Chemical Industry and Engineering Progress, 2017, 36(9): 3429-3435. | |
| 19 | Jin H, Li Y S, Liu X L, et al. Recovery of HMF from aqueous solution by zeolitic imidazolate frameworks[J]. Chemical Engineering Science, 2015, 124: 170-178. |
| 20 | Baerlocher C, McCusker L B, Olson D H. Atlas of Zeolite Framework Types[M]. Elsevier, 2007. |
| 21 | Zhao Y, Xu J, Wang J, et al. Adsorptive separation of furfural/5-hydroxymethylfurfural in MAF-5 with ellipsoidal pores[J]. Industrial & Engineering Chemistry Research, 2020, 59 (25): 11734-11742. |
| 22 | Jin H, Li Y S, Yang W S. Adsorption of biomass-derived polyols onto metal-organic frameworks from aqueous solutions[J]. Industrial & Engineering Chemistry Research, 2018, 57(35): 11963-11969. |
| 23 | Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum[J]. Journal of the American Chemical Society, 1918, 40(9): 1361-1403. |
| 24 | Foo K Y, Hameed B H. Insights into the modeling of adsorption isotherm systems[J]. Chemical Engineering Journal, 2010, 156(1): 2-10. |
| 25 | Schute K, Louven Y, Detoni C, et al. Selective liquid phase adsorption of biogenic HMF on hydrophobic spherical activated carbons[J]. Chemie Ingenieur Technik, 2016, 88(3): 355-362. |
| 26 | Yang Q, Runge T. Cross-linked polyethylenimine for selective adsorption and effective recovery of lignocellulose-derived organic acids and aldehydes[J]. ACS Sustainable Chemistry & Engineering, 2018, 7(1): 933-943. |
| 27 | Zheng J Y, Pan B Y, Xiao J X, et al. Experimental and mathematical simulation of noncompetitive and competitive adsorption dynamic of formic acid-levulinic acid-5- hydroxymethylfurfural from single, binary, and ternary systems in a fixed-bed column of SY-01 resin[J]. Industrial & Engineering Chemistry Research, 2018, 57: 8518-8528. |
| 28 | Zhu A X, Lin R B, Qi X L, et al. Zeolitic metal azolate frameworks (MAFs) from ZnO/Zn(OH)2 and monoalkyl-substituted imidazoles and 1, 2, 4-triazoles: efficient syntheses and properties[J]. Microporous and Mesoporous Materials, 2012, 157: 42-49. |
| 29 | 李长海, 石洪仁, 唐洪宇.弱碱性树脂处理β-萘磺酸废水的吸附分离热力学性能[J].化工学报, 2004, 55(7): 1117-1123. |
| Li C H, Shi H R, Tang H Y. Adsorption separation of β-naphthalenesulphonic acid wastewater on weakly basic resin and thermodynamics [J]. Journal of Chemical Industry and Engineering(China), 2004, 55(7): 1117-1123. | |
| 30 | Sulaymon A H, Ahmed K W. Competitive adsorption of furfural and phenolic compounds onto activated carbon in fixed bed column[J]. Environmental Science & Technology, 2008, 42(2): 392-397. |
| 31 | Lin X Q, Qi G X, Shi S L, et al. Estimation of fixed-bed column parameters and mathematical modeling of breakthrough behaviors for adsorption of levulinic acid from aqueous solution using SY-01 resin[J]. Separation and Purification Technology, 2017, 174: 222-231. |
| 32 | Lin X Q, Wu J L, Jin X H, et al. Selective separation of biobutanol from acetone-butanol-ethanol fermentation broth by means of sorption methodology based on a novel macroporous resin[J].Biotechnology Progress, 2012, 28(4): 962-972. |
| 33 | 尚华, 白洪灏, 刘佳奇, 等. CH4-N2在自支撑颗粒型Silicalite-1上的吸附分离及PSA模拟[J]. 化工学报, 2020, 71(5): 2088-2098. |
| Shang H, Bai H H, Liu J Q, et al. PSA simulation and adsorption separation of CH4-N2 by self-supporting pellets Silicalite-1[J]. CIESC Journal, 2020, 71(5): 2088-2098. | |
| 34 | Weil J, Dien B S, Bothast R J, et al. Removal of fermentation inhibitors formed during pretreatment of biomass by polymeric adsorbents[J]. Industrial & Engineering Chemistry Research, 2002, 41(24): 6132-6138. |
| 35 | Schute K, Louven Y, Detoni C, et al. Selective liquid phase adsorption of biogenic HMF on hydrophobic spherical activated carbons[J]. Chemie Ingenieur Technik, 2016, 88(3): 355-362. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [5] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [6] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [7] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [8] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [9] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [10] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [11] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [12] | 文兆伦, 李沛睿, 张忠林, 杜晓, 侯起旺, 刘叶刚, 郝晓刚, 官国清. 基于自热再生的隔壁塔深冷空分工艺设计及优化[J]. 化工学报, 2023, 74(7): 2988-2998. |
| [13] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [14] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [15] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号