化工学报 ›› 2021, Vol. 72 ›› Issue (10): 5183-5195.DOI: 10.11949/0438-1157.20210506
收稿日期:2021-04-13
修回日期:2021-07-04
出版日期:2021-10-05
发布日期:2021-10-05
通讯作者:
刘月明
作者简介:黄鑫(1995—),男,硕士研究生,基金资助:
Xin HUANG( ),Yuxia LIN,Binghui YAN,Yueming LIU(
),Yuxia LIN,Binghui YAN,Yueming LIU( )
)
Received:2021-04-13
Revised:2021-07-04
Online:2021-10-05
Published:2021-10-05
Contact:
Yueming LIU
摘要:
低碳烯烃(
中图分类号:
黄鑫,林玉霞,阎炳会,刘月明. 失活TS-1高效催化
Xin HUANG,Yuxia LIN,Binghui YAN,Yueming LIU. Deactivated TS-1 as an efficient catalyst for catalytic cracking of butene to propene[J]. CIESC Journal, 2021, 72(10): 5183-5195.
| Zeolite | Acidity① | Si/Ti② | Si/Al2② | Pore properties③ | Size④/ nm | |||||
|---|---|---|---|---|---|---|---|---|---|---|
WA/ (mmol/g) | MSA/ (mmol/g) | Total/ (mmol/g) | MSA/WA | ABET/ (m2/g) | Vtotal/ (cm3/g) | Vmic/ (cm3/g) | ||||
| MTS-1 | ~0 | ~0 | ~0 | – | 41 | 500 | 0.42 | 0.20 | ~250 | |
| De-TS-1 | 0.19 | 0.08 | 0.27 | 0.42 | 23 | 342 | 0.33 | 0.14 | <100 | |
| ZSM-5(70) | 0.32 | 0.45 | 0.77 | 1.41 | 63 | 428 | 0.70 | 0.15 | ~500 | |
表1 MTS-1、De-TS-1及ZSM-5(70)分子筛酸性质和孔结构之间的比较
Table 1 The comparison of acid properties and pore structure among MTS-1, De-TS-1 and ZSM-5(70)
| Zeolite | Acidity① | Si/Ti② | Si/Al2② | Pore properties③ | Size④/ nm | |||||
|---|---|---|---|---|---|---|---|---|---|---|
WA/ (mmol/g) | MSA/ (mmol/g) | Total/ (mmol/g) | MSA/WA | ABET/ (m2/g) | Vtotal/ (cm3/g) | Vmic/ (cm3/g) | ||||
| MTS-1 | ~0 | ~0 | ~0 | – | 41 | 500 | 0.42 | 0.20 | ~250 | |
| De-TS-1 | 0.19 | 0.08 | 0.27 | 0.42 | 23 | 342 | 0.33 | 0.14 | <100 | |
| ZSM-5(70) | 0.32 | 0.45 | 0.77 | 1.41 | 63 | 428 | 0.70 | 0.15 | ~500 | |

图3 MTS-1、De-TS-1及ZSM-5(70)分子筛的氨气程序升温脱附谱图和吡啶红外谱图
Fig.3 NH3-TPD and Py-IR spectra of MTS-1, De-TS-1 and ZSM-5(70)(Desorption temperature was controlled at 373 K in Fig.(b))
| Zeolite | Conversion( %(mol) | Selectivity/% (mol) | P/E/ (mol/mol) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H2 | Arom. | |||||||||
| MTS-1 | ~0.50 | — | — | — | — | — | — | — | — | — |
| De-TS-1 | 42.19 | 4.22 | 47.87 | 34.29 | 5.69 | 0.19+0.05 | 6.84 | 0.29 | 0.47 | 11.34 |
| C-CAT | 46.41 | 4.74 | 49.71 | 31.42 | 6.03 | 0.13+0.03 | 7.23 | 0.21 | 0.39 | 10.48 |
表2 MTS-1,De-TS-1及C-CAT催化剂催化C4=裂解反应所得的原料转化率和产物分布
Table 2 The result of butene conversion and product distribution produced from catalytic cracking of butene over MTS-1, De-TS-1 and C-CAT
| Zeolite | Conversion( %(mol) | Selectivity/% (mol) | P/E/ (mol/mol) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H2 | Arom. | |||||||||
| MTS-1 | ~0.50 | — | — | — | — | — | — | — | — | — |
| De-TS-1 | 42.19 | 4.22 | 47.87 | 34.29 | 5.69 | 0.19+0.05 | 6.84 | 0.29 | 0.47 | 11.34 |
| C-CAT | 46.41 | 4.74 | 49.71 | 31.42 | 6.03 | 0.13+0.03 | 7.23 | 0.21 | 0.39 | 10.48 |
| No. | Sample | Smicro①/ (m2/g) | Sexter①/ (m2/g) | Vmicro①/ (cm3/g) | Vmeso①/ (cm3/g) | Vtotal①/ (cm3/g) | Si/Ti② |
|---|---|---|---|---|---|---|---|
| 1 | De-TS-1 | 309 | 33 | 0.136 | 0.189 | 0.325 | 23 |
| 2 | AT-0.5 | 338 | 55 | 0.146 | 0.221 | 0.367 | 26 |
| 3 | AT-1.0 | 344 | 51 | 0.150 | 0.230 | 0.380 | 28 |
| 4 | AT-1.5 | 335 | 59 | 0.147 | 0.217 | 0.364 | 25 |
| 5 | AT-2.0 | 341 | 54 | 0.148 | 0.229 | 0.377 | 25 |
表3 De-TS-1及其不同硝酸浓度酸洗样的孔结构性质
Table 3 The pore properties of De-TS-1 and the corresponding HNO3 modification samples
| No. | Sample | Smicro①/ (m2/g) | Sexter①/ (m2/g) | Vmicro①/ (cm3/g) | Vmeso①/ (cm3/g) | Vtotal①/ (cm3/g) | Si/Ti② |
|---|---|---|---|---|---|---|---|
| 1 | De-TS-1 | 309 | 33 | 0.136 | 0.189 | 0.325 | 23 |
| 2 | AT-0.5 | 338 | 55 | 0.146 | 0.221 | 0.367 | 26 |
| 3 | AT-1.0 | 344 | 51 | 0.150 | 0.230 | 0.380 | 28 |
| 4 | AT-1.5 | 335 | 59 | 0.147 | 0.217 | 0.364 | 25 |
| 5 | AT-2.0 | 341 | 54 | 0.148 | 0.229 | 0.377 | 25 |

图6 De-TS-1及其不同硝酸浓度酸洗样的吡啶红外谱图
Fig.6 Py-IR spectra of De-TS-1 and the corresponding samples with HNO3 modification(Desorption temperature was controlled at 373 K)
| No. | Sample | 1540 cm-1/1880 cm-1 | 1447 cm-1/1880 cm-1 |
|---|---|---|---|
| 1 | De-TS-1 | 0.021 | 0.152 |
| 2 | AT-0.5 | 0.028 | 0.171 |
| 3 | AT-1.0 | 0.031 | 0.157 |
| 4 | AT-1.5 | 0.025 | 0.171 |
| 5 | AT-2.0 | 0.024 | 0.164 |
表4 De-TS-1及其不同硝酸浓度酸洗样的Br?nsted和Lewis酸酸量
Table 4 The amount of Br?nsted and Lewis acid sites of De-TS-1 and the corresponding samples with HNO3 modification
| No. | Sample | 1540 cm-1/1880 cm-1 | 1447 cm-1/1880 cm-1 |
|---|---|---|---|
| 1 | De-TS-1 | 0.021 | 0.152 |
| 2 | AT-0.5 | 0.028 | 0.171 |
| 3 | AT-1.0 | 0.031 | 0.157 |
| 4 | AT-1.5 | 0.025 | 0.171 |
| 5 | AT-2.0 | 0.024 | 0.164 |

图7 De-TS-1及其酸洗样催化C4=裂解反应所得的原料转化率和产物分布随硝酸浓度的变化关系
Fig.7 The relationship between butene conversion and product distribution produced from catalytic cracking of butene over samples and HNO3 concentration

图10 De-TS-1、De-TS-1-AT-1及其不同K/Ti比下K+交换样的氨气程序升温脱附谱图
Fig.10 NH3-TPD spectra of De-TS-1, De-TS-1-AT-1 and its potassium ionmodified samples under different K/Ti ratio
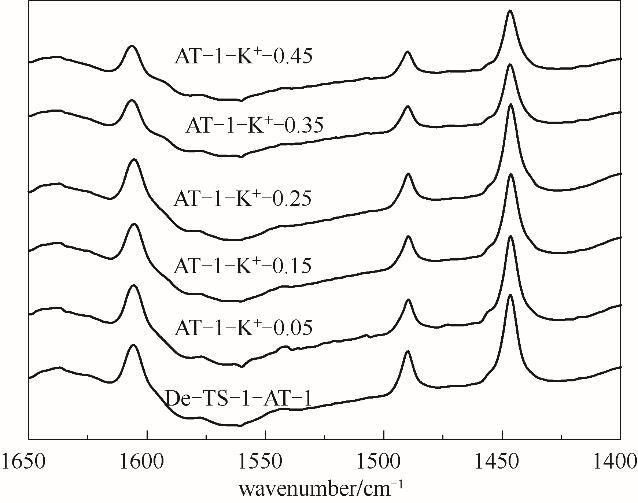
图11 De-TS-1-AT-1及其不同K/Ti下K+交换样的吡啶红外谱图
Fig.11 Py-IR spectra of De-TS-1-AT-1 and its potassium ionmodified samples under different K/Ti ratio (Desorption temperature was controlled at 373 K)
| No. | Sample | 1540 cm-1/1880 cm-1 | 1446 cm-1/1880 cm-1 |
|---|---|---|---|
| 1 | De-TS-1-AT-1 | 0.031 | 0.157 |
| 2 | AT-1-K+-0.05 | 0.023 | 0.158 |
| 3 | AT-1-K+-0.15 | 0.019 | 0.153 |
| 4 | AT-1-K+-0.25 | 0.017 | 0.155 |
| 5 | AT-1-K+-0.35 | 0.014 | 0.153 |
| 6 | AT-1-K+-0.45 | 0.011 | 0.155 |
表5 De-TS-1-AT-1及其不同K/Ti比下K+交换样的Br?nsted和Lewis酸酸量
Table 5 The amount of Br?nsted and Lewis acid sites of De-TS-1-AT-1 and its potassium ionmodified samples under different K/Ti ratio
| No. | Sample | 1540 cm-1/1880 cm-1 | 1446 cm-1/1880 cm-1 |
|---|---|---|---|
| 1 | De-TS-1-AT-1 | 0.031 | 0.157 |
| 2 | AT-1-K+-0.05 | 0.023 | 0.158 |
| 3 | AT-1-K+-0.15 | 0.019 | 0.153 |
| 4 | AT-1-K+-0.25 | 0.017 | 0.155 |
| 5 | AT-1-K+-0.35 | 0.014 | 0.153 |
| 6 | AT-1-K+-0.45 | 0.011 | 0.155 |

图12 De-TS-1-AT-1及其不同K/Ti下K+改性样的真空红外光谱图
Fig.12 Vacuum infrared spectroscopy of De-TS-1-AT-1 and its potassium ion modified samples under different K/Ti ratio
| No. | Sample | Cracking of | Oxidation of 1-hexene② | Alcoholysis of PO③ | ||||
|---|---|---|---|---|---|---|---|---|
| Conv.( | Yield.( % | Conv.(1-hex.)/% | Sel.(epo.)/ % | Conv.(PO)/ % | Sel.(PPM)/ % | Sel.(SPM)/ % | ||
| 1 | De-TS-1 | 42.19 | 20.20 | 12.1 | 97.0 | 77.5 | 24.9 | 75.1 |
| 2 | De-TS-1-AT-1 | 59.37 | 31.82 | 16.6 | 82.9 | 88.7 | 19.7 | 80.3 |
| 3 | AT-1-K+-0.05 | 40.40 | 18.31 | 16.9 | 86.0 | 72.5 | 20.5 | 79.5 |
| 4 | AT-1-K+-0.15 | 33.09 | 14.38 | 17.5 | 85.5 | 67.7 | 19.9 | 80.1 |
| 5 | AT-1-K+-0.25 | 26.81 | 11.20 | 17.8 | 83.0 | 55.1 | 17.6 | 82.4 |
| 6 | AT-1-K+-0.35 | 21.38 | 8.43 | 17.9 | 83.9 | 49.0 | 17.0 | 83.0 |
| 7 | AT-1-K+-0.45 | 16.89 | 6.67 | 17.7 | 82.9 | 38.7 | 16.3 | 83.7 |
表6 De-TS-1、De-TS-1-AT-1及其不同K/Ti比下K+交换样催化各项反应的反应结果
Table 6 The activities in catalytic cracking of C4=, oxidation of 1-hexene and alcoholysis of PO over De-TS-1, De-TS-1-AT-1 and its potassium ion modified samples under different K/Ti ratio
| No. | Sample | Cracking of | Oxidation of 1-hexene② | Alcoholysis of PO③ | ||||
|---|---|---|---|---|---|---|---|---|
| Conv.( | Yield.( % | Conv.(1-hex.)/% | Sel.(epo.)/ % | Conv.(PO)/ % | Sel.(PPM)/ % | Sel.(SPM)/ % | ||
| 1 | De-TS-1 | 42.19 | 20.20 | 12.1 | 97.0 | 77.5 | 24.9 | 75.1 |
| 2 | De-TS-1-AT-1 | 59.37 | 31.82 | 16.6 | 82.9 | 88.7 | 19.7 | 80.3 |
| 3 | AT-1-K+-0.05 | 40.40 | 18.31 | 16.9 | 86.0 | 72.5 | 20.5 | 79.5 |
| 4 | AT-1-K+-0.15 | 33.09 | 14.38 | 17.5 | 85.5 | 67.7 | 19.9 | 80.1 |
| 5 | AT-1-K+-0.25 | 26.81 | 11.20 | 17.8 | 83.0 | 55.1 | 17.6 | 82.4 |
| 6 | AT-1-K+-0.35 | 21.38 | 8.43 | 17.9 | 83.9 | 49.0 | 17.0 | 83.0 |
| 7 | AT-1-K+-0.45 | 16.89 | 6.67 | 17.7 | 82.9 | 38.7 | 16.3 | 83.7 |

图13 De-TS-1、De-TS-1-AT-1及De-TS-1-AT-1-K+-0.05催化C4=裂解反应的反应稳定性
Fig.13 Reaction stability of catalytic cracking of butene over De-TS-1, De-TS-1-AT-1 and De-TS-1-AT-1-K+-0.05
| 1 | 陈硕, 王定博, 吉媛媛, 等. 丙烯为目的产物的技术进展[J]. 石油化工, 2011, 40(2): 217-224. |
| Chen S, Wang D B, Ji Y Y, et al. Development in on-purpose propylene technology[J]. Petrochemical Technology, 2011, 40(2): 217-224. | |
| 2 | 白尔铮, 胡云光. 四种增产丙烯催化工艺的技术经济比较[J]. 工业催化, 2003, 11(5): 7-12. |
| Bai E Z, Hu Y G. Techno-economics of four types of propylene promotion catalytic processes[J]. Industrial Catalysis, 2003, 11(5): 7-12. | |
| 3 | Sekiguchi M, Takamatsu Y. Process for producing propylene and aromatic hydrocarbons, and producing apparatus therefor: US8034987[P]. 2011-10-11. |
| 4 | Voskoboynikov T V, Pelekh A Y, Senetar J J. OCP catalyst with improved steam tolerance: US8609567[P]. 2013-12-17. |
| 5 | 滕加伟, 谢在库. 无黏结剂复合孔分子筛催化烯烃裂解制丙烯技术[J]. 中国科学: 化学, 2015, 45(5): 533-540. |
| Teng J W, Xie Z K. Novel binder-less hierarchical ZSM-5 catalyst for olefins catalytic cracking to produce propylene[J]. Science China Chemistry, 2015, 45(5): 533-540. | |
| 6 | 马会霞, 周峰, 武光, 等. 多级孔HZSM-5分子筛催化快速热解生物质制芳烃[J]. 化工学报, 2020, 71(11): 5200-5207. |
| Ma H X, Zhou F, Wu G, et al. Catalytic fast pyrolysis of biomass to aromatics over hierarchical HZSM-5[J]. CIESC Journal, 2020, 71(11): 5200-5207. | |
| 7 | 金放, 刘铁良, 王先桥, 等. 工业氟硅酸合成钛硅介孔分子筛催化环己烯环氧化[J]. 化工学报, 2016, 67(10): 4176-4186. |
| Jin F, Liu T L, Wang X Q, et al. Synthesis of mesoporous titanosilicates from industrial by-product hexafluosilicic acid and application for catalytic cyclohexene epoxidation[J]. CIESC Journal, 2016, 67(10): 4176-4186. | |
| 8 | 冯利利, 卢书培, 齐兴义, 等. Me-OMS-1s分子筛催化叔丁基过氧化氢分解制备叔丁醇[J]. 化工学报, 2015, 66(10): 3965-3970. |
| Feng L L, Lu S P, Qi X Y, et al. Catalytic decomposition of tert-butyl hydroperoxide into tert-butyl alcohol over Me-OMS-1s molecular sieves[J]. CIESC Journal, 2015, 66(10): 3965-3970. | |
| 9 | Zhu X X, Liu S L, Song Y Q, et al. Catalytic cracking of C4 alkenes to propene and ethene: influences of zeolites pore structures and Si/Al2 ratios[J]. Applied Catalysis A: General, 2005, 288(1/2): 134-142. |
| 10 | Zeng P H, Liang Y, Ji S F, et al. Preparation of phosphorus-modified PITQ-13 catalysts and their performance in 1-butene catalytic cracking[J]. Journal of Energy Chemistry, 2014, 23(2): 193-200. |
| 11 | Meusinger J, Corma A. Influence of zeolite composition and structure on hydrogen transfer reactions from hydrocarbons and from hydrogen[J]. Journal of Catalysis, 1996, 159(2): 353-360. |
| 12 | Shen K X, Huang X, Wang J, et al. Synthesis of FER/MFI composite zeolite using isopropylamine as a structure-directing agent[J]. Microporous and Mesoporous Materials, 2020, 297: 110027. |
| 13 | Lin L F, Qiu C F, Zhuo Z X, et al. Acid strength controlled reaction pathways for the catalytic cracking of 1-butene to propene over ZSM-5[J]. Journal of Catalysis, 2014, 309: 136-145. |
| 14 | Lin L F, Zhao S F, Zhang D W, et al. Acid strength controlled reaction pathways for the catalytic cracking of 1-pentene to propene over ZSM-5[J]. ACS Catalysis, 2015, 5(7): 4048-4059. |
| 15 | Zhao S F, Yang D, Zhang X W, et al. ZSM-5 with controllable acidity as an efficient catalyst for a highly adjustable propene/ethene ratio in the 1-butene cracking[J]. Chemical Communications, 2016, 52(75): 11191-11194. |
| 16 | Iwase Y, Sakamoto Y, Shiga A, et al. Shape-selective catalysis determined by the volume of a zeolite cavity and the reaction mechanism for propylene production by the conversion of butene using a proton-exchanged zeolite[J]. The Journal of Physical Chemistry C, 2012, 116(8): 5182-5196. |
| 17 | Epelde E, Ibañez M, Aguayo A T, et al. Differences among the deactivation pathway of HZSM-5 zeolite and SAPO-34 in the transformation of ethylene or 1-butene to propylene[J]. Microporous and Mesoporous Materials, 2014, 195: 284-293. |
| 18 | Arudra P, Bhuiyan T I, Akhtar M N, et al. Silicalite-1 as efficient catalyst for production of propene from 1-butene[J]. ACS Catalysis, 2014, 4(11): 4205-4214. |
| 19 | Hattori H, Arudra P, Abdalla A, et al. Infrared study of silanol groups on dealuminated high silica MFI zeolite to correlate different types of silanol groups with activity for conversion of 1-butene to propene[J]. Catalysis Letters, 2020, 150(3): 771-780. |
| 20 | Zhao G L, Teng J W, Xie Z K, et al. Effect of phosphorus on HZSM-5 catalyst for C4-olefin cracking reactions to produce propylene[J]. Journal of Catalysis, 2007, 248(1): 29-37. |
| 21 | Xue N H, Chen X K, Nie L, et al. Understanding the enhancement of catalytic performance for olefin cracking: hydrothermally stable acids in P/HZSM-5[J]. Journal of Catalysis, 2007, 248(1): 20-28. |
| 22 | Epelde E, Santos J I, Florian P, et al. Controlling coke deactivation and cracking selectivity of MFI zeolite by H3PO4 or KOH modification[J]. Applied Catalysis A: General, 2015, 505: 105-115. |
| 23 | Xu R F, Liu J X, Liang C C, et al. Effect of alkali metal ion modification on the catalytic performance of nano-HZSM-5 zeolite in butene cracking[J]. Journal of Fuel Chemistry and Technology, 2011, 39(6): 449-454. |
| 24 | Li J W, Ma H F, Sun Q W, et al. Effect of iron and phosphorus on HZSM-5 in catalytic cracking of 1-butene[J]. Fuel Processing Technology, 2015, 134: 32-38. |
| 25 | 姚旭婷, 黄鑫, 林玉霞, 等. 失活TS-1高效催化环己烯水合生成环己醇的研究[J]. 化学学报, 2020, 78(10): 1111-1119. |
| Yao X T, Huang X, Lin Y X, et al. Deactivated TS-1 as efficient catalyst for hydration of cyclohexene to cyclohexanol[J]. Acta Chimica Sinica, 2020, 78(10): 1111-1119. | |
| 26 | 卓佐西, 林龙飞, 邓秀娟, 等. 钛硅分子筛催化环己酮液相氨肟化固定床工艺[J]. 催化学报,2013, 34(3): 604-611. |
| Zhuo Z X, Lin L F, Deng X J, et al. Fixed-bed process of liquid-phase ammoximation of cyclohexanone over titanosilicates[J]. Chinese Journal of Catalysis, 2013, 34(3): 604-611. | |
| 27 | Taramasso M, Perego G, Notari B. Preparation of porous crystalline synthetic material comprised of silicon and titanium oxides: US4410501[P]. 1983-10-18. |
| 28 | Nijhuis T A, Huizinga B J, Makkee M, et al. Direct epoxidation of propene using gold dispersed on TS-1 and other titanium-containing supports[J]. Industrial & Engineering Chemistry Research, 1999, 38(3): 884-891. |
| 29 | 孙斌. 环己酮氨肟化反应过程中钛硅分子筛的溶解流失研究[J]. 石油炼制与化工, 2005, 36(11): 54-58. |
| Sun B. Study on dissolution erosion of titanium silicalite zeolite in cyclohehanone ammoximation[J]. Petroleum Processing and Petrochemicals, 2005, 36(11): 54-58. | |
| 30 | Su J, Xiong G, Zhou J C, et al. Amorphous Ti species in titanium silicalite-1: structural features, chemical properties, and inactivation with sulfosalt[J]. Journal of Catalysis, 2012, 288: 1-7. |
| 31 | Fang X Q, Wang Q, Zheng A M, et al. Fluorine-planted titanosilicate with enhanced catalytic activity in alkene epoxidation with hydrogen peroxide[J]. Catalysis Science & Technology, 2012, 2(12): 2433. |
| 32 | Wang Y, Liu Y M, Li X H, et al. Intermolecular condensation of ethylenediamine to 1, 4-diazabicyclo[2, 2, 2]octane over TS-1 catalysts[J]. Journal of Catalysis, 2009, 266(2): 258-267. |
| 33 | Vayssilov G N. Structural and physicochemical features of titanium silicalites[J]. Catalysis Reviews, 1997, 39(3): 209-251. |
| 34 | Blasco T, Camblor M A, Corma A, et al. The state of Ti in titanoaluminosilicates isomorphous with zeolite.beta[J]. Journal of the American Chemical Society, 1993, 115(25): 11806-11813. |
| 35 | Notari B. Titanium silicalites[J]. Catalysis Today, 1993, 18(2): 163-172. |
| 36 | Liu Z F, Davis R J. Investigation of the structure of microporous Ti-Si mixed oxides by X-ray, UV reflectance, FT-Raman, and FT-IR spectroscopies[J]. The Journal of Physical Chemistry, 1994, 98(4): 1253-1261. |
| 37 | 刘银乾, 李永祥, 吴巍, 等. 环己酮氨肟化反应体系中TS-1分子筛失活原因的研究[J]. 石油炼制与化工, 2002, 33(5): 41-45. |
| Liu Y Q, Li Y X, Wu W, et al. Study on deactivation behavior of ts-1 molecular sieve in cyclohexanone ammoximation[J]. Petroleum Processing and Petrochemicals, 2002, 33(5): 41-45. | |
| 38 | Itoh M, Hattori H, Tanabe K. The acidic properties of TiO2-SiO2 and its catalytic activities for the amination of phenol, the hydration of ethylene and the isomerization of butene[J]. Journal of Catalysis, 1974, 35(2): 225-231. |
| 39 | Post J G, van Hooff J H C. Acidity and activity of H-ZSM-5 measured with NH3-t.p.d. and n-hexane cracking[J]. Zeolites, 1984, 4(1): 9-14. |
| 40 | Farneth W E, Gorte R J. Methods for characterizing zeolite acidity[J]. Chemical Reviews, 1995, 95(3): 615-635. |
| 41 | Al-Dughaither A S, de Lasa H. HZSM-5 zeolites with different SiO2/Al2O3 ratios. Characterization and NH3 desorption kinetics[J]. Industrial & Engineering Chemistry Research, 2014, 53(40): 15303-15316. |
| 42 | Corma A, Orchillés A V. Current views on the mechanism of catalytic cracking[J]. Microporous and Mesoporous Materials, 2000, 35/36: 21-30. |
| 43 | Emeis C A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts[J]. Journal of Catalysis, 1993, 141(2): 347-354. |
| 44 | Barzetti T, Selli E, Moscotti D, et al. Pyridine and ammonia as probes for FTIR analysis of solid acid catalysts[J]. Journal of the Chemical Society, Faraday Transactions, 1996, 92(8): 1401. |
| 45 | den Hollander M A, Wissink M, Makkee M, et al. Gasoline conversion: reactivity towards cracking with equilibrated FCC and ZSM-5 catalysts[J]. Applied Catalysis A: General, 2002, 223(1/2): 85-102. |
| 46 | Wu P, Tatsumi T, Komatsu T, et al. A novel titanosilicate with MWW structure(Ⅰ): Hydrothermal synthesis, elimination of extraframework titanium, and characterizations[J]. The Journal of Physical Chemistry B, 2001, 105(15): 2897-2905. |
| 47 | Liu H, Lu G Z, Guo Y L, et al. Effect of pretreatment on properties of TS-1/diatomite catalyst for hydroxylation of phenol by H2O2 in fixed-bed reactor[J]. Catalysis Today, 2004, 93/94/95: 353-357. |
| 48 | Qi Y Y, Ye C B, Zhuang Z, et al. Preparation and evaluation of titanium silicalite-1 utilizing pretreated titanium dioxide as a titanium source[J]. Microporous and Mesoporous Materials, 2011, 142(2/3): 661-665. |
| 49 | 阎炳会. C4=/C5=催化裂解C2=/C3=导向生成反应路径的研究[D]. 上海: 华东师范大学, 2019. |
| Yan B H. Study on the reaction pathway of C4=/C5= catalytic cracking to C2=/C3= [D]. Shanghai: East China Normal University, 2019. | |
| 50 | Wu L Z, Zhao S F, Lin L F, et al. In-depth understanding of acid catalysis of solvolysis of propene oxide over titanosilicates and titanosilicate/H2O2 systems[J]. Journal of Catalysis, 2016, 337: 248-259. |
| [1] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [2] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [3] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [4] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [5] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [6] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [7] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [8] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [9] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [10] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [11] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [12] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [13] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [14] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [15] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号