化工学报 ›› 2024, Vol. 75 ›› Issue (12): 4532-4546.DOI: 10.11949/0438-1157.20240633
马浩天1( ), 荆体瑞1, 刘程程1, 玉散·吐拉甫2, 张喆2, 王一迪1(
), 荆体瑞1, 刘程程1, 玉散·吐拉甫2, 张喆2, 王一迪1( ), 王庆宏1, 陈春茂1, 徐春明1
), 王庆宏1, 陈春茂1, 徐春明1
收稿日期:2024-06-07
修回日期:2024-07-29
出版日期:2024-12-25
发布日期:2025-01-03
通讯作者:
王一迪
作者简介:马浩天(2000—),男,硕士研究生,mht16735367888@163.com
基金资助:
Haotian MA1( ), Tirui JING1, Chengcheng LIU1, Turap YUSAN2, Zhe ZHANG2, Yidi WANG1(
), Tirui JING1, Chengcheng LIU1, Turap YUSAN2, Zhe ZHANG2, Yidi WANG1( ), Qinghong WANG1, Chunmao CHEN1, Chunming XU1
), Qinghong WANG1, Chunmao CHEN1, Chunming XU1
Received:2024-06-07
Revised:2024-07-29
Online:2024-12-25
Published:2025-01-03
Contact:
Yidi WANG
摘要:
甲烷化学链重整是新型低碳的制氢技术,载氧体还原特性是决定化学链重整制氢产率的关键因素。重点探讨了不同比例Sr掺杂对LaFeO3还原特性的影响,通过表征FeO6八面体畸变和表面氧形态分析了Sr掺杂对氧空位浓度、晶格氧传递的作用。La0.8Sr0.2FeO3具有明显的FeO6八面体畸变,表现出最低的晶格氧还原温度750℃,恒温还原中La0.8Sr0.2FeO3的最大失重量为LaFeO3的2~3倍。动力拟合计算发现La1-x Sr x FeO3的还原过程均受成核核增长模型控制,同时受自催化模型影响,4种载氧体中,La0.8Sr0.2FeO3表现出最低的表观活化能。La0.8Sr0.2FeO3在水蒸气氧化实验中的增重速率约为LaFeO3的4倍,并且在20周期循环后保持原有物相不变。La1-x Sr x FeO3化学链重整与多周期稳定性的研究为甲烷化学链重整制氢材料选择和工艺优化提供了技术与数据支撑。
中图分类号:
马浩天, 荆体瑞, 刘程程, 玉散·吐拉甫, 张喆, 王一迪, 王庆宏, 陈春茂, 徐春明. Sr改性LaFeO3用于甲烷化学链重整的还原性能与动力学研究[J]. 化工学报, 2024, 75(12): 4532-4546.
Haotian MA, Tirui JING, Chengcheng LIU, Turap YUSAN, Zhe ZHANG, Yidi WANG, Qinghong WANG, Chunmao CHEN, Chunming XU. Study on reduction performance and kinetics of Sr-modified LaFeO3 for methane chemical looping reforming[J]. CIESC Journal, 2024, 75(12): 4532-4546.
| 函数名称 | 机理 | 函数编号 | g(α) | f(α) |
|---|---|---|---|---|
| Avrami-Erofeev方程 | 成核核增长 | A2 | [-ln(1-α)]1/2 | 2(1-α)[-ln(1-α)]1/2 |
| A3 | [-ln(1-α)]1/3 | 3(1-α)[-ln(1-α)]2/3 | ||
| A4 | [-ln(1-α)]1/4 | 4(1-α)[-ln(1-α)]3/4 | ||
| Prout-Tompkins方程 | 自催化模型 | B1 | ln[α/(1-α)] | α/(1-α) |
| Mampel Power法则 | 相界面反应(一维) | C1 | α | 1 |
| 收缩模型 | 收缩圆柱体(面积),相界面反应,圆柱形对称 | C2 | 1- (1-α)1/2 | 2(1-α)1/2 |
| 收缩球体(体积),相界面反应,球形对称 | C3 | 1- (1-α)1/3 | 3(1-α)2/3 | |
| Jander方程 | 一维扩散模型 | D1 | α2 | |
| 三维扩散,球形对称 | D3 | 1- | ||
| 反应级数模型 | 一级化学反应 | R1 | -ln(1-α) | 1-α |
| 二级化学反应 | R2 | [1/(1-α)]-1 | (1-α)2 | |
| 三级化学反应 | R3 | [1/(1-α)2]-1 | (1-α) 3 |
表1 机制函数的微分和积分表达式
Table 1 Differential and integral expressions of mechanism function
| 函数名称 | 机理 | 函数编号 | g(α) | f(α) |
|---|---|---|---|---|
| Avrami-Erofeev方程 | 成核核增长 | A2 | [-ln(1-α)]1/2 | 2(1-α)[-ln(1-α)]1/2 |
| A3 | [-ln(1-α)]1/3 | 3(1-α)[-ln(1-α)]2/3 | ||
| A4 | [-ln(1-α)]1/4 | 4(1-α)[-ln(1-α)]3/4 | ||
| Prout-Tompkins方程 | 自催化模型 | B1 | ln[α/(1-α)] | α/(1-α) |
| Mampel Power法则 | 相界面反应(一维) | C1 | α | 1 |
| 收缩模型 | 收缩圆柱体(面积),相界面反应,圆柱形对称 | C2 | 1- (1-α)1/2 | 2(1-α)1/2 |
| 收缩球体(体积),相界面反应,球形对称 | C3 | 1- (1-α)1/3 | 3(1-α)2/3 | |
| Jander方程 | 一维扩散模型 | D1 | α2 | |
| 三维扩散,球形对称 | D3 | 1- | ||
| 反应级数模型 | 一级化学反应 | R1 | -ln(1-α) | 1-α |
| 二级化学反应 | R2 | [1/(1-α)]-1 | (1-α)2 | |
| 三级化学反应 | R3 | [1/(1-α)2]-1 | (1-α) 3 |
| 参数 | LaFeO3 | La0.8Sr0.2FeO3 | La0.6Sr0.4FeO3 | La0.4Sr0.6FeO3 | |
|---|---|---|---|---|---|
| 键长/nm | Fe—O(1) | 0.20073 | 0.204536 | 0.2116 | 0.1941 |
| Fe—O(2) | 0.20099 | 0.212309 | 0.1960 | 0.1920 | |
| Fe—O(2) | 0.20020 | 0.180842 | 0.1960 | 0.1970 | |
| 键角/(°) | Fe—O(1)—Fe | 155.90 | 145.35 | 134.80 | 170.50 |
| Fe—O(2)—Fe | 157.01 | 169.06 | 166.20 | 177.00 | |
| 键长方差 | 0.0027 | 4.50 | 1.30 | 0.11 | |
表2 La1-x Sr x FeO3载氧体晶体结构参数
Table 2 Crystal structure parameters of La1-x Sr x FeO3 oxygen carrier
| 参数 | LaFeO3 | La0.8Sr0.2FeO3 | La0.6Sr0.4FeO3 | La0.4Sr0.6FeO3 | |
|---|---|---|---|---|---|
| 键长/nm | Fe—O(1) | 0.20073 | 0.204536 | 0.2116 | 0.1941 |
| Fe—O(2) | 0.20099 | 0.212309 | 0.1960 | 0.1920 | |
| Fe—O(2) | 0.20020 | 0.180842 | 0.1960 | 0.1970 | |
| 键角/(°) | Fe—O(1)—Fe | 155.90 | 145.35 | 134.80 | 170.50 |
| Fe—O(2)—Fe | 157.01 | 169.06 | 166.20 | 177.00 | |
| 键长方差 | 0.0027 | 4.50 | 1.30 | 0.11 | |
| 载氧体 | Fe2O3/% (质量分数) | La2O3/% (质量分数) | SrO/% (质量分数) | La/% (质量分数) | Sr/% (质量分数) | La/Sr 摩尔比 | 摩尔比 理论值 |
|---|---|---|---|---|---|---|---|
| LaFeO3 | 34.32 | 64.76 | — | 55.23 | — | — | — |
| La0.8Sr0.2FeO3 | 35.12 | 53.72 | 10.22 | 45.81 | 8.64 | 0.78/0.22 | 0.8/0.2 |
| La0.6Sr0.4FeO3 | 35.71 | 42.32 | 21.01 | 36.09 | 17.76 | 0.57/0.43 | 0.6/0.4 |
| La0.4Sr0.6FeO3 | 34.36 | 27.11 | 36.57 | 28.12 | 27.92 | 0.39/0.61 | 0.4/0.6 |
表3 La1-x Sr x FeO3载氧体XRF成分组成
Table 3 XRF compositional analysis of La1-x Sr x FeO3 oxygen carrier
| 载氧体 | Fe2O3/% (质量分数) | La2O3/% (质量分数) | SrO/% (质量分数) | La/% (质量分数) | Sr/% (质量分数) | La/Sr 摩尔比 | 摩尔比 理论值 |
|---|---|---|---|---|---|---|---|
| LaFeO3 | 34.32 | 64.76 | — | 55.23 | — | — | — |
| La0.8Sr0.2FeO3 | 35.12 | 53.72 | 10.22 | 45.81 | 8.64 | 0.78/0.22 | 0.8/0.2 |
| La0.6Sr0.4FeO3 | 35.71 | 42.32 | 21.01 | 36.09 | 17.76 | 0.57/0.43 | 0.6/0.4 |
| La0.4Sr0.6FeO3 | 34.36 | 27.11 | 36.57 | 28.12 | 27.92 | 0.39/0.61 | 0.4/0.6 |
| 载氧体 | 不同氧元素百分比/% | ||||
|---|---|---|---|---|---|
| O1(O2-) | O2 ( | O3 (—OH, | O4 (H2O) | Oads/Olatt | |
| LaFeO3 | 50.53 | 15.32 | 18.06 | 16.09 | 0.30 |
| La0.8Sr0.2FeO3 | 37.18 | 18.82 | 28.27 | 15.73 | 0.51 |
| La0.6Sr0.4FeO3 | 33.39 | 15.84 | 33.82 | 16.96 | 0.47 |
| La0.4Sr0.6FeO3 | 42.68 | 16.55 | 29.06 | 11.72 | 0.39 |
表4 La1-x Sr x FeO3载氧体氧元素O 1s XPS结果
Table 4 XPS results of oxygen element O 1s in La1-x Sr x FeO3 oxygen carrier
| 载氧体 | 不同氧元素百分比/% | ||||
|---|---|---|---|---|---|
| O1(O2-) | O2 ( | O3 (—OH, | O4 (H2O) | Oads/Olatt | |
| LaFeO3 | 50.53 | 15.32 | 18.06 | 16.09 | 0.30 |
| La0.8Sr0.2FeO3 | 37.18 | 18.82 | 28.27 | 15.73 | 0.51 |
| La0.6Sr0.4FeO3 | 33.39 | 15.84 | 33.82 | 16.96 | 0.47 |
| La0.4Sr0.6FeO3 | 42.68 | 16.55 | 29.06 | 11.72 | 0.39 |
| 载氧体 | 比表面积/ (m2/g) | 孔体积/ (10-3 cm3/g) | 平均孔径/nm |
|---|---|---|---|
| LaFeO3 | 15.27 | 2.4 | 6.41 |
| La0.8Sr0.2FeO3 | 19.35 | 3.7 | 10.99 |
| La0.6Sr0.4FeO3 | 17.91 | 3.2 | 5.88 |
| La0.4Sr0.6FeO3 | 12.11 | 1.7 | 4.51 |
表5 La1-x Sr x FeO3载氧体比表面积及孔隙数据
Table 5 Specific surface area and porosity data of La1-x Sr x FeO3 oxygen carriers
| 载氧体 | 比表面积/ (m2/g) | 孔体积/ (10-3 cm3/g) | 平均孔径/nm |
|---|---|---|---|
| LaFeO3 | 15.27 | 2.4 | 6.41 |
| La0.8Sr0.2FeO3 | 19.35 | 3.7 | 10.99 |
| La0.6Sr0.4FeO3 | 17.91 | 3.2 | 5.88 |
| La0.4Sr0.6FeO3 | 12.11 | 1.7 | 4.51 |
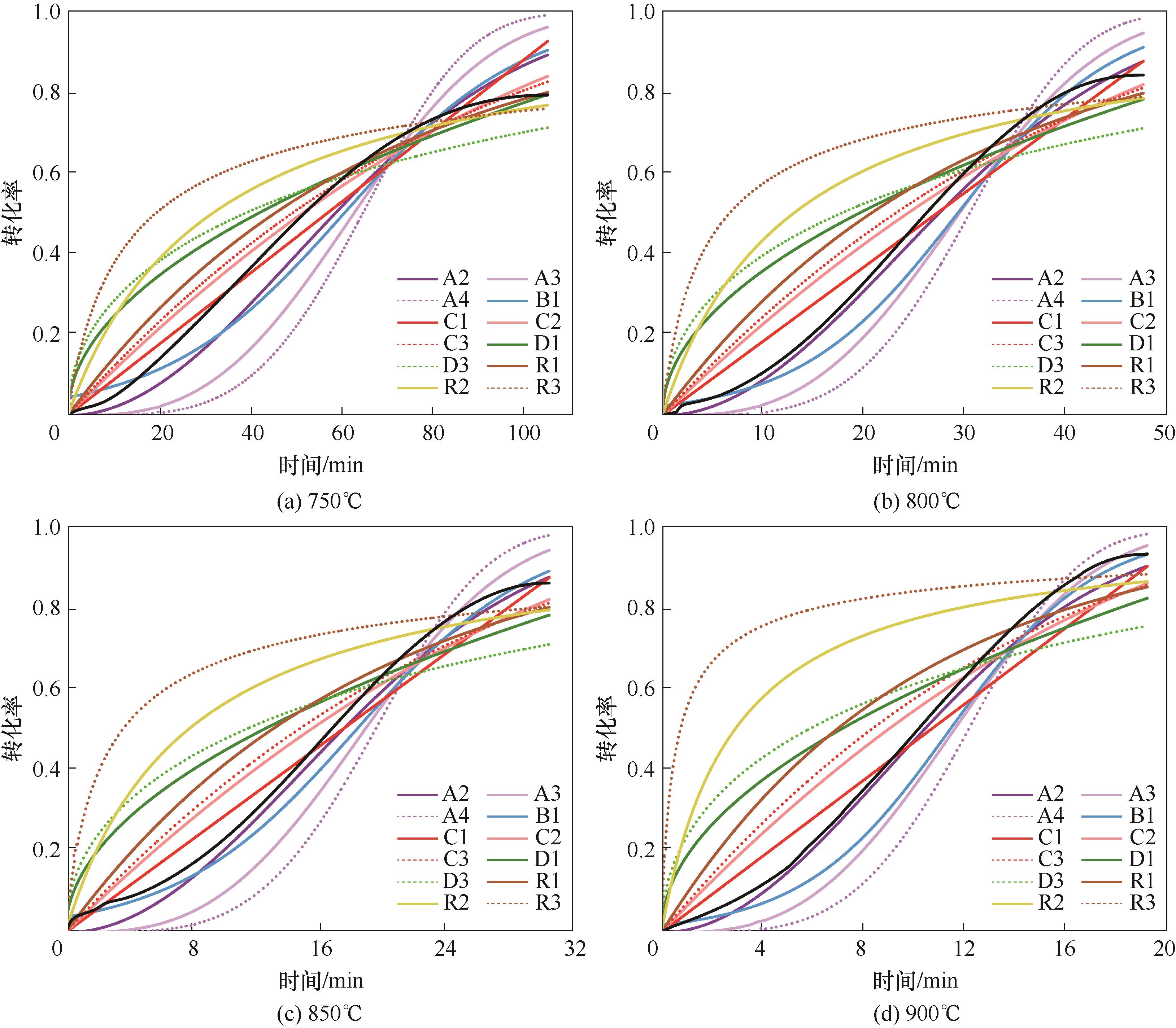
图7 不同温度下La0.8Sr0.2FeO3与CH4反应的常见动力学方程拟合曲线(彩色线为不同机理函数拟合结果,黑色线为实验结果)
Fig.7 Fitting curves of common kinetic equations for reaction of La0.8Sr0.2FeO3 with CH4 at different temperatures (colored lines represent fitting results of different mechanism functions, black lines represent experimental results)
| 载氧体 | 成核核增长模型 | 自催化枝状模型 | ||||
|---|---|---|---|---|---|---|
| 活化能/(kJ/mol) | 指前因子/s-1 | 相关系数 | 活化能/(kJ/mol) | 指前因子/s-1 | 相关系数 | |
| LaFeO3 | 159.454 | 1.337907×106 | 0.9217 | 164.87 | 1.35×107 | 0.9922 |
| La0.8Sr0.2FeO3 | 112.798 | 8.586×103 | 0.9933 | 135.93 | 4.024×105 | 0.9595 |
| La0.6Sr0.4FeO3 | 163.684 | 2×106 | 0.9927 | 167.27 | 1.09×107 | 0.9912 |
| La0.4Sr0.6FeO3 | 167.350 | 1.988×106 | 0.9961 | 144.91 | 8.4×105 | 0.9990 |
表6 La1-x Sr x FeO3载氧体在不同动力学模型中的主要动力学参数
Table 6 Main kinetic parameters of La1-x Sr x FeO3 oxygen carriers in different kinetic models
| 载氧体 | 成核核增长模型 | 自催化枝状模型 | ||||
|---|---|---|---|---|---|---|
| 活化能/(kJ/mol) | 指前因子/s-1 | 相关系数 | 活化能/(kJ/mol) | 指前因子/s-1 | 相关系数 | |
| LaFeO3 | 159.454 | 1.337907×106 | 0.9217 | 164.87 | 1.35×107 | 0.9922 |
| La0.8Sr0.2FeO3 | 112.798 | 8.586×103 | 0.9933 | 135.93 | 4.024×105 | 0.9595 |
| La0.6Sr0.4FeO3 | 163.684 | 2×106 | 0.9927 | 167.27 | 1.09×107 | 0.9912 |
| La0.4Sr0.6FeO3 | 167.350 | 1.988×106 | 0.9961 | 144.91 | 8.4×105 | 0.9990 |
| 载氧体 | 温度区间/K | 实验方法 | 模型 | Ea/(kJ/mol) | 文献 |
|---|---|---|---|---|---|
| LaFeO3 | 1023~1173 | 等温TGA | 成核核增长/自催化 | 159/165 | 本研究 |
| LaFeO3 | 1073~1223 | 等温质谱 | 成核核增长 | 151 | [ |
| MnFe2O4 | 1073~1173 | 等温TGA | 扩散控制 | 139 | [ |
| CeO2 | 873~1123 | 等温TCD | — | 137 | [ |
| CoWO4 | 1123~1223 | 等温TGA | 一级化学反应 | 221 | [ |
表7 文献中报道的载氧体还原动力学模型及活化能
Table 7 Reduction kinetic models and activation energies of oxygen carriers reported in literature
| 载氧体 | 温度区间/K | 实验方法 | 模型 | Ea/(kJ/mol) | 文献 |
|---|---|---|---|---|---|
| LaFeO3 | 1023~1173 | 等温TGA | 成核核增长/自催化 | 159/165 | 本研究 |
| LaFeO3 | 1073~1223 | 等温质谱 | 成核核增长 | 151 | [ |
| MnFe2O4 | 1073~1173 | 等温TGA | 扩散控制 | 139 | [ |
| CeO2 | 873~1123 | 等温TCD | — | 137 | [ |
| CoWO4 | 1123~1223 | 等温TGA | 一级化学反应 | 221 | [ |
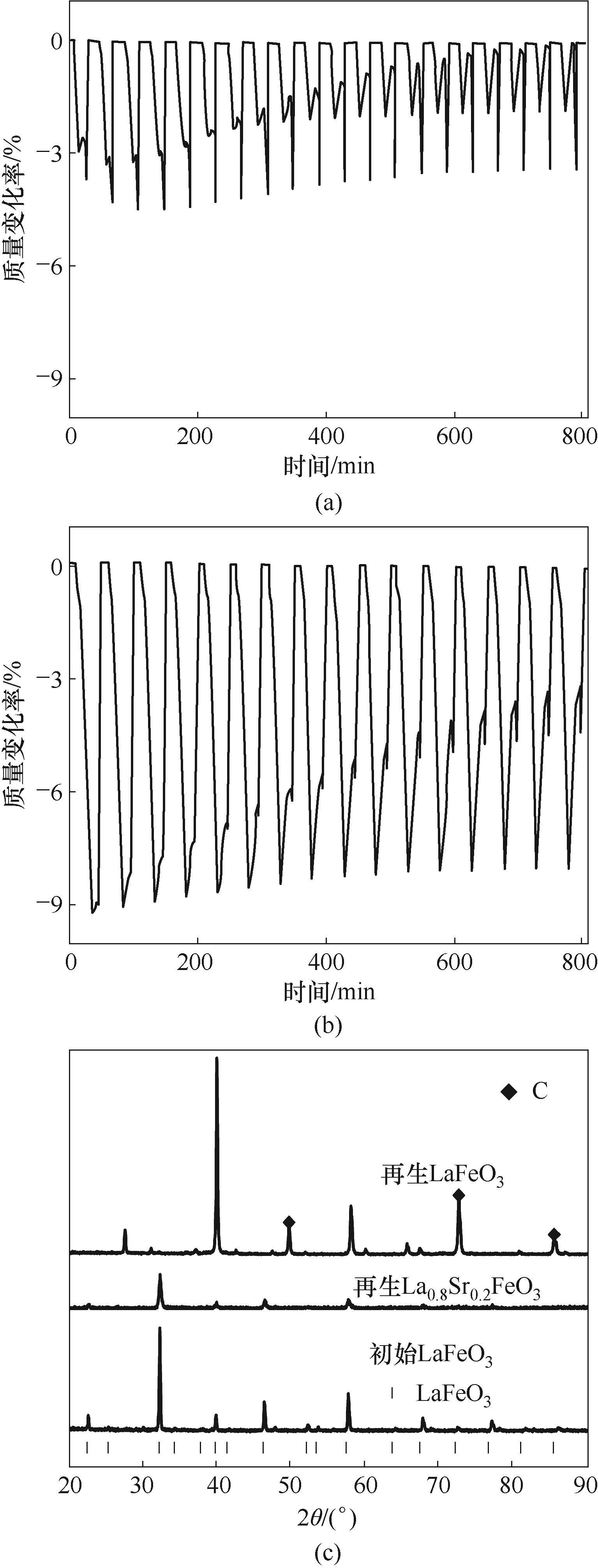
图11 LaFeO3 (a)、La0.8Sr0.2FeO3(b)在CH4还原-空气氧化过程中质量随时间的变化和再生载氧体与初始载氧体XRD谱图对比(c)
Fig.11 (a) Mass change of LaFeO3 during CH4 reduction-air oxidation process;(b) Mass change of La0.8Sr0.2FeO3 during CH4 reduction-air oxidation process; (c) XRD patterns comparison of regenerated and fresh oxygen carrier
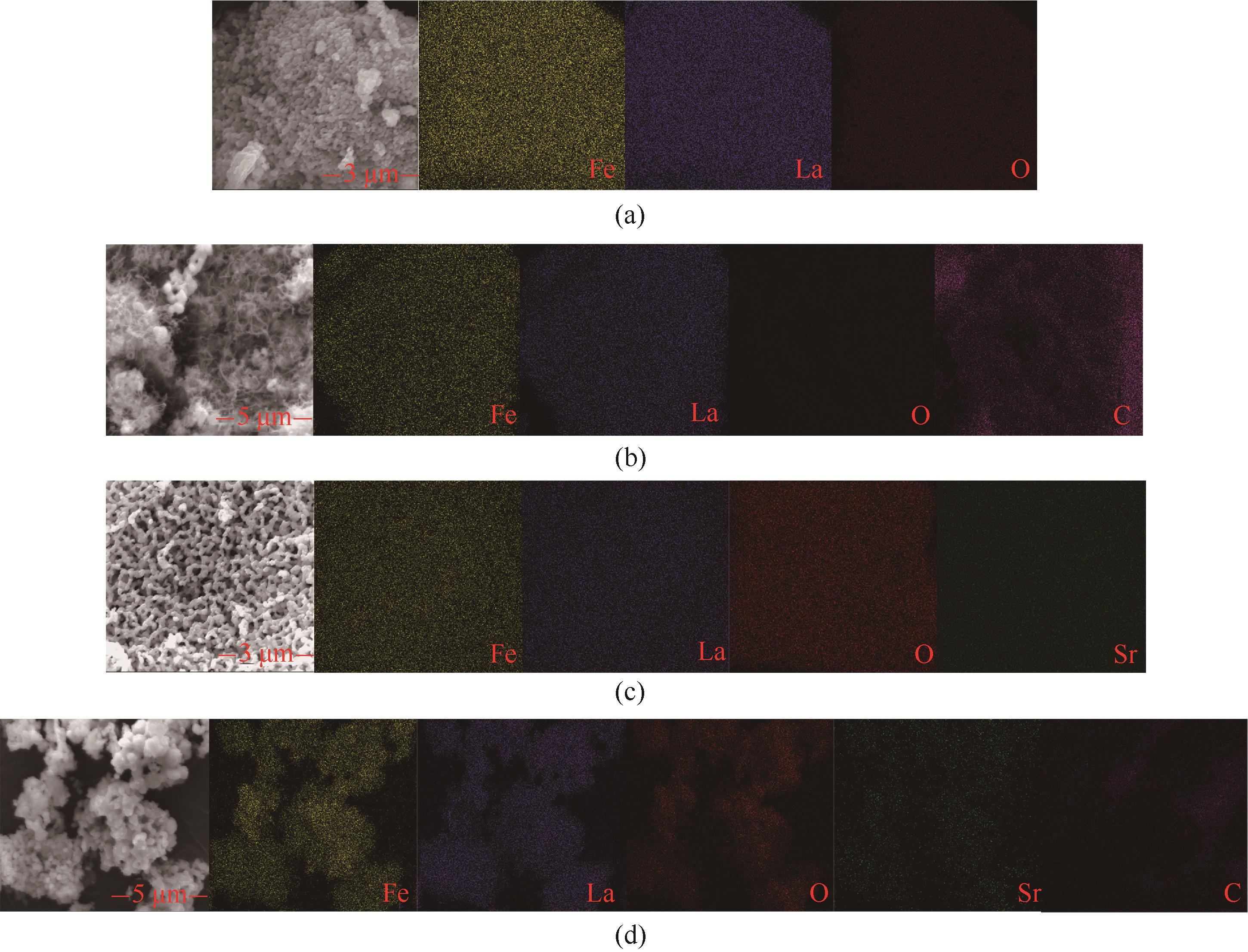
图12 初始的LaFeO3(a)、20周期后的LaFeO3(b)、初始的La0.8Sr0.2FeO3(c)和20周期后的La0.8Sr0.2FeO3(d)的SEM-Mapping谱图
Fig.12 SEM-Mapping of initial LaFeO3 (a), LaFeO3 after 20 cycles (b), initial La0.8Sr0.2FeO3 (c), La0.8Sr0.2FeO3 after 20 cycles (d)
| 1 | 符冠云. 氢能在我国能源转型中的地位和作用[J]. 中国煤炭, 2019, 45(10): 15-21. |
| Fu G Y. The status and role of hydrogen energy in China's energy transformation[J]. China Coal, 2019, 45(10): 15-21. | |
| 2 | Agency I E. Global Hydrogen Review 2022[M]. Paris: OECD, 2022. |
| 3 | 聂家波, 邓建悦. 燃料电池用氢气的制备工艺探讨[J]. 化工技术与开发, 2021, 50(8): 46-50. |
| Nie J B, Deng J Y. Discussion on preparation technology of hydrogen for fuel cell[J]. Technology & Development of Chemical Industry, 2021, 50(8): 46-50. | |
| 4 | Zhang H T, Sun Z X, Hu Y H. Steam reforming of methane: current states of catalyst design and process upgrading[J]. Renewable and Sustainable Energy Reviews, 2021, 149: 111330. |
| 5 | Luo M, Yi Y, Wang S Z, et al. Review of hydrogen production using chemical-looping technology[J]. Renewable and Sustainable Energy Reviews, 2018, 81: 3186-3214. |
| 6 | Liu R, Zhang X H, Liu T, et al. Dynamic oxygen migration and reaction over ceria-supported nickel oxides in chemical looping partial oxidation of methane[J]. Applied Catalysis B: Environmental, 2023, 328: 122478. |
| 7 | 付甜甜, 吐拉甫·玉散, 王邑维, 等. 镍修饰的铁基载氧体甲烷化学链制氢实验[J]. 燃烧科学与技术, 2020, 26(2): 125-132. |
| Fu T T, Turap Y S, Wang Y W, et al. Performance of iron-based oxygen carriers modified by NiO in chemical looping hydrogen generation process [J]. Journal of Combustion Science and Technology, 2020, 26(2): 125-132. | |
| 8 | Zhang X R, Wang J, Song Z L, et al. Co3O4-CeO2 for enhanced syngas by low-temperature methane conversion with CO2 utilization via a catalytic chemical looping process[J]. Fuel Processing Technology, 2023, 245: 107741. |
| 9 | Li F, Kim H R, Sridhar D, et al. Syngas chemical looping gasification process: oxygen carrier particle selection and performance[J]. Energy & Fuels, 2009, 23(8): 4182-4189. |
| 10 | Keller M, Fung J, Leion H, et al. Cu-impregnated alumina/silica bed materials for chemical looping reforming of biomass gasification gas[J]. Fuel, 2016, 180: 448-456. |
| 11 | Abad A, Mattisson T, Lyngfelt A, et al. Chemical-looping combustion in a 300 W continuously operating reactor system using a manganese-based oxygen carrier[J]. Fuel, 2006, 85(9): 1174-1185. |
| 12 | Dharanipragada N V R A, Buelens L C, Poelman H, et al. Mg-Fe-Al-O for advanced CO2 to CO conversion: carbon monoxide yield vs. oxygen storage capacity[J]. Journal of Materials Chemistry A, 2015, 3(31): 16251-16262. |
| 13 | Wang B W, Yan R, Zhao H B, et al. Investigation of chemical looping combustion of coal with CuFe2O4 oxygen carrier[J]. Energy & Fuels, 2011, 25(7): 3344-3354. |
| 14 | Ismail M, Liu W, Scott S A. The performance of Fe2O3-CaO oxygen carriers and the interaction of iron oxides with CaO during chemical looping combustion and H2 production[J]. Energy Procedia, 2014, 63: 87-97. |
| 15 | Hirabayashi D, Sakai Y, Yoshikawa T, et al. Mössbauer characterization of calcium–ferrite oxides prepared by calcining Fe2O3 and CaO[J]. Hyperfine Interactions, 2006, 167(1): 809-813. |
| 16 | Evdou A, Zaspalis V, Nalbandian L. Ferrites as redox catalysts for chemical looping processes[J]. Fuel, 2016, 165: 367-378. |
| 17 | Tang M C, Xu L, Fan M H. Progress in oxygen carrier development of methane-based chemical-looping reforming: a review[J]. Applied Energy, 2015, 151: 143-156. |
| 18 | Li Y L, Chen M Y, Jiang L, et al. Perovskites as oxygen storage materials for chemical looping partial oxidation and reforming of methane[J]. Physical Chemistry Chemical Physics, 2024, 26(3): 1516-1540. |
| 19 | Dai X P, Li R J, Yu C C, et al. Unsteady-state direct partial oxidation of methane to synthesis gas in a fixed-bed reactor using AFeO3 (A = La, Nd, Eu) perovskite-type oxides as oxygen storage[J]. The Journal of Physical Chemistry B, 2006, 110(45): 22525-22531. |
| 20 | Dai X P, Cheng J, Li Z Z, et al. Reduction kinetics of lanthanum ferrite perovskite for the production of synthesis gas by chemical-looping methane reforming[J]. Chemical Engineering Science, 2016, 153: 236-245. |
| 21 | Taylor F H, Buckeridge J, Catlow C R A. Screening divalent metals for A- and B-site dopants in LaFeO3 [J]. Chemistry of Materials, 2017, 29(19): 8147-8157. |
| 22 | He F, Li X N, Zhao K, et al. The use of La1- x Sr x FeO3 perovskite-type oxides as oxygen carriers in chemical-looping reforming of methane[J]. Fuel, 2013, 108: 465-473. |
| 23 | He F, Chen J, Liu S, et al. La1- x Sr x FeO3 perovskite-type oxides for chemical-looping steam methane reforming: identification of the surface elements and redox cyclic performance[J]. International Journal of Hydrogen Energy, 2019, 44(21): 10265-10276. |
| 24 | Ma Z, Lu Y G, Liu G F. Enhanced cyclic redox reactivity of Fe2O3/Al2O3 by Sr doping for chemical-looping combustion of solid fuels[J]. Fuel, 2022, 324: 124625. |
| 25 | Galwey A K. Meltingand thermal decompositions of solids[J]. Journal of Thermal Analysis and Calorimetry, 2007, 87(2): 601-615. |
| 26 | Feng Y C, Wang N N, Guo X, et al. Characteristics of dopant distribution and surface oxygen vacancy formation for modified Fe2O3 in chemical looping combustion[J]. Fuel, 2020, 276: 117942. |
| 27 | Zhang Y K, Zhao Y J, Yi Q, et al. NiO/κ-CeZrO4 functional oxygen carriers with Ni δ + and oxygen vacancy synergy for chemical looping partial oxidation reforming of methane[J]. Fuel Processing Technology, 2021, 219: 106875. |
| 28 | Wang Y J, Zheng Y E, Wang Y H, et al. Syngas production modified by oxygen vacancies over CeO2-ZrO2-CuO oxygen carrier via chemical looping reforming of methane[J]. Applied Surface Science, 2019, 481: 151-160. |
| 29 | 吕熠. 基于铁酸镧化学链转化焦油模型化合物制备合成气的研究[D]. 北京: 清华大学, 2022. |
| Lv Y. Study on syngas production from tar model compounds via chemical looping using lanthanum ferrite[D]. Beijing: Tsinghua University, 2022. | |
| 30 | Pecchi G, Reyes P, Zamora R, et al. Effect of the preparation method on the catalytic activity of La1- x Ca x FeO3 perovskite-type oxides[J]. Catalysis Today, 2008, 133: 420-427. |
| 31 | Shannon R D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides[J]. Acta Crystallographica Section A, 1976, 32(5): 751-767. |
| 32 | Hirata T. Oxygen position, octahedral distortion, and bond-valence parameter from bond lengths in Ti1- x Sn x O2 (0≤x≤1)[J]. Journal of the American Ceramic Society, 2000, 83(12): 3205-3207. |
| 33 | Zhu Y L, Zhou W, Yu J, et al. Enhancing electrocatalytic activity of perovskite oxides by tuning cation deficiency for oxygen reduction and evolution reactions[J]. Chemistry of Materials, 2016, 28(6): 1691-1697. |
| 34 | Gosavi P V, Biniwale R B. Pure phase LaFeO3 perovskite with improved surface area synthesized using different routes and its characterization[J]. Materials Chemistry and Physics, 2010, 119(1/2): 324-329. |
| 35 | Margellou A, Manos D, Petrakis D, et al. Activation of persulfate by LaFe1- x Co x O3 perovskite catalysts for the degradation of phenolics: effect of synthetic method and metal substitution[J]. Science of the Total Environment, 2022, 832: 155063. |
| 36 | Li P, Chen X Y, Li Y D, et al. A review on oxygen storage capacity of CeO2-based materials: influence factors, measurement techniques, and applications in reactions related to catalytic automotive emissions control[J]. Catalysis Today, 2019, 327: 90-115. |
| 37 | Rossetti I, Forni L. Catalytic flameless combustion of methane over perovskites prepared by flame-hydrolysis[J]. Applied Catalysis B: Environmental, 2001, 33(4): 345-352. |
| 38 | Zhang X H, Liu R, Liu T, et al. Redox catalysts for chemical looping methane conversion[J]. Trends in Chemistry, 2023, 5(7): 512-525. |
| 39 | Wei H J, Cao Y, Ji W J, et al. Lattice oxygen of La1- x Sr x MO3 (M=Mn, Ni) and LaMnO3- α F β perovskite oxides for the partial oxidation of methane to synthesis gas[J]. Catalysis Communications, 2008, 9(15): 2509-2514. |
| 40 | Rydén M, Lyngfelt A, Mattisson T, et al. Novel oxygen-carrier materials for chemical-looping combustion and chemical-looping reforming; La x Sr1- x Fe y Co1- y O3- δ perovskites and mixed-metal oxides of NiO, Fe2O3 and Mn3O4 [J]. International Journal of Greenhouse Gas Control, 2008, 2(1): 21-36. |
| 41 | Long Y H, Yang K, Gu Z H, et al. Hydrogen generation from water splitting over polyfunctional perovskite oxygen carriers by using coke oven gas as reducing agent[J]. Applied Catalysis B: Environmental, 2022, 301: 120778. |
| 42 | Huang Z, Gao N, Lin Y, et al. Exploring the migration and transformation of lattice oxygen during chemical looping with NiFe2O4 oxygen carrier[J]. Chemical Engineering Journal, 2022, 429: 132064. |
| 43 | Zhu K Y, Shi F, Zhu X F, et al. The roles of oxygen vacancies in electrocatalytic oxygen evolution reaction[J]. Nano Energy, 2020, 73: 104761. |
| 44 | Jaber J O, Probert S D. Pyrolysis and gasification kinetics of Jordanian oil-shales[J]. Applied Energy, 1999, 63(4): 269-286. |
| 45 | Zhou L P, Zhao H, Tong Y N, et al. Evolution of coke formation and its effect on β-zeolite in catalytic cracking[J]. Industrial & Engineering Chemistry Research, 2022, 61(48): 17440-17456. |
| 46 | Go K S, Son S R, Kim S D. Reaction kinetics of reduction and oxidation of metal oxides for hydrogen production[J]. International Journal of Hydrogen Energy, 2008, 33(21): 5986-5995. |
| 47 | Otsuka K, Wang Y, Nakamura M. Direct conversion of methane to synthesis gas through gas-solid reaction using CeO2-ZrO2 solid solution at moderate temperature[J]. Applied Catalysis A: General, 1999, 183(2): 317-324. |
| 48 | de los Ríos Castillo T, Salinas Gutiérrez J, López Ortiz A, et al. Global kinetic evaluation during the reduction of CoWO4 with methane for the production of hydrogen[J]. International Journal of Hydrogen Energy, 2013, 38(28): 12519-12526. |
| 49 | Chen L Y, Liu D C, Xue J, et al. Mechanism of phase segregation in ilmenite oxygen carriers for chemical-looping combustion[J]. Chemical Engineering Journal, 2024, 479: 147921. |
| [1] | 石美琳, 赵连达, 邓行健, 王静松, 左海滨, 薛庆国. 催化甲烷重整工艺的研究进展[J]. 化工学报, 2024, 75(S1): 25-39. |
| [2] | 赵焕娟, 包颖昕, 于康, 刘婧, 钱新明. 多元组分爆轰不稳定性定量实验研究[J]. 化工学报, 2024, 75(S1): 339-348. |
| [3] | 王军锋, 张俊杰, 张伟, 王家乐, 双舒炎, 张亚栋. 液相放电等离子体分解甲醇制氢:电极配置的优化[J]. 化工学报, 2024, 75(9): 3277-3286. |
| [4] | 罗欣怡, 徐强, 佘永璐, 聂腾飞, 郭烈锦. 光电分解水制氢气泡动力学特性及其传质机理研究[J]. 化工学报, 2024, 75(9): 3083-3093. |
| [5] | 徐宏标, 杨亮, 李子栋, 刘道平. 盐水微滴/泡沫铜复合体系中甲烷水合物生成动力学研究[J]. 化工学报, 2024, 75(9): 3287-3296. |
| [6] | 祝赫, 张仪, 齐娜娜, 张锴. 欧拉-欧拉双流体模型中颗粒黏性对液固散式流态化的影响[J]. 化工学报, 2024, 75(9): 3103-3112. |
| [7] | 丁湧, 李文建, 陈昭宇, 曹立辉, 刘轩铭, 任强强, 胡松, 向军. 废旧晶体硅光伏组件EVA有氧热解动力学与产物特性[J]. 化工学报, 2024, 75(9): 3310-3319. |
| [8] | 唐昊, 胡定华, 李强, 张轩畅, 韩俊杰. 抗加速度双切线弧流道内气泡动力学行为数值与可视化研究[J]. 化工学报, 2024, 75(9): 3074-3082. |
| [9] | 曾港, 陈林, 杨董, 袁海专, 黄彦平. 矩形通道内超临界CO2局部热流场可视化实验[J]. 化工学报, 2024, 75(8): 2831-2839. |
| [10] | 曹佳蕾, 孙立岩, 曾德望, 尹凡, 高子翔, 肖睿. 双流化床化学链制氢反应器的数值模拟[J]. 化工学报, 2024, 75(8): 2865-2874. |
| [11] | 丁家琦, 刘海涛, 赵普, 朱香凝, 王晓放, 谢蓉. 煤炭超临界水制氢反应器内多相流场智能滚动预测研究[J]. 化工学报, 2024, 75(8): 2886-2896. |
| [12] | 童永祺, 程杰, 林海, 陈曦, 赵海波. 10 MWth化学链燃烧反应装置的CPFD模拟[J]. 化工学报, 2024, 75(8): 2949-2959. |
| [13] | 罗莉, 陈文尧, 张晶, 钱刚, 周兴贵, 段学志. 氧化铝结构与表面性质调控及其催化甲醇脱水制二甲醚性能研究[J]. 化工学报, 2024, 75(7): 2522-2532. |
| [14] | 吴邦汉, 林定标, 陆海峰, 郭晓镭, 刘海峰. 竖直管气动物流传输系统管道压降和传送瓶输送特性[J]. 化工学报, 2024, 75(7): 2465-2473. |
| [15] | 马君霞, 李林涛, 熊伟丽. 基于Tri-training GPR的半监督软测量建模方法[J]. 化工学报, 2024, 75(7): 2613-2623. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号