化工学报 ›› 2024, Vol. 75 ›› Issue (11): 4237-4253.DOI: 10.11949/0438-1157.20240875
收稿日期:2024-08-02
修回日期:2024-09-20
出版日期:2024-11-25
发布日期:2024-12-26
通讯作者:
葛蔚
作者简介:常麒(1987—),男,博士后,qchang@ipe.ac.cn
基金资助:Received:2024-08-02
Revised:2024-09-20
Online:2024-11-25
Published:2024-12-26
Contact:
Wei GE
摘要:
提出了在填充流化床中以矿物碳酸化(MC)原位封存水煤气变换反应(WGSR)制备H2副产CO2的连续操作新工艺。除了借助WGSR的高温、高压、高湿环境强化MC,吸收剂粉末流化通过WGSR催化剂填充床的连续分离可应对产物层增厚导致的MC吸收速率快速衰减。基于晶粒尺度溶解扩散MC模型和WGSR表观动力学建立了该反应器的一维稳态活塞流模型,以此分析了750 MW整体气化联合循环(IGCC)装置中应用此新工艺的技术经济性。基于模拟获得的CO2、MC吸收剂粉末转化率及给定能耗、碳税等计算CO2减排费用表明:将MC吸收剂的碳酸化产物作为水泥辅助性凝胶材料(SCM)产生附加值是此技术商业化的前提;考虑CO2、MC吸收剂粉末转化率与研磨能耗,宜采用10 μm左右的MC吸收剂细粉末。
中图分类号:
常麒, 葛蔚. 填充流化床集成水煤气变换与二氧化碳矿化的模拟分析[J]. 化工学报, 2024, 75(11): 4237-4253.
Qi CHANG, Wei GE. Simulation study on the integration of water-gas shift and CO2-mineralization in a packed fluidized bed[J]. CIESC Journal, 2024, 75(11): 4237-4253.
| 吸收剂 | 摩尔分数/% | (mol/m3) | (mol/m3) | (m2/s) | (J/(mol·K)) | ||
|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | |||||
| 硅灰石 | 50 | 50 | 0 | 0.0467 | 0.0233 | 8.09×10-10 | 39.43 |
| 炉渣 | 50 | 37.5 | 12.5 | 0.0467 | 0.0175 | 1.00×10-9 | 40.97 |
| 煤灰 | 37.5 | 47.5 | 15 | 0.0444 | 0.0100 | 1.52×10-9 | 44.01 |
| 石英(参考) | 0 | 100 | 0 | 0.0386 | 0 | 1.13×10-5 | 108.72 |
表1 吸收剂摩尔组成与MC模型参数
Table 1 Sorbent molar compositions and MC model parameters
| 吸收剂 | 摩尔分数/% | (mol/m3) | (mol/m3) | (m2/s) | (J/(mol·K)) | ||
|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | |||||
| 硅灰石 | 50 | 50 | 0 | 0.0467 | 0.0233 | 8.09×10-10 | 39.43 |
| 炉渣 | 50 | 37.5 | 12.5 | 0.0467 | 0.0175 | 1.00×10-9 | 40.97 |
| 煤灰 | 37.5 | 47.5 | 15 | 0.0444 | 0.0100 | 1.52×10-9 | 44.01 |
| 石英(参考) | 0 | 100 | 0 | 0.0386 | 0 | 1.13×10-5 | 108.72 |
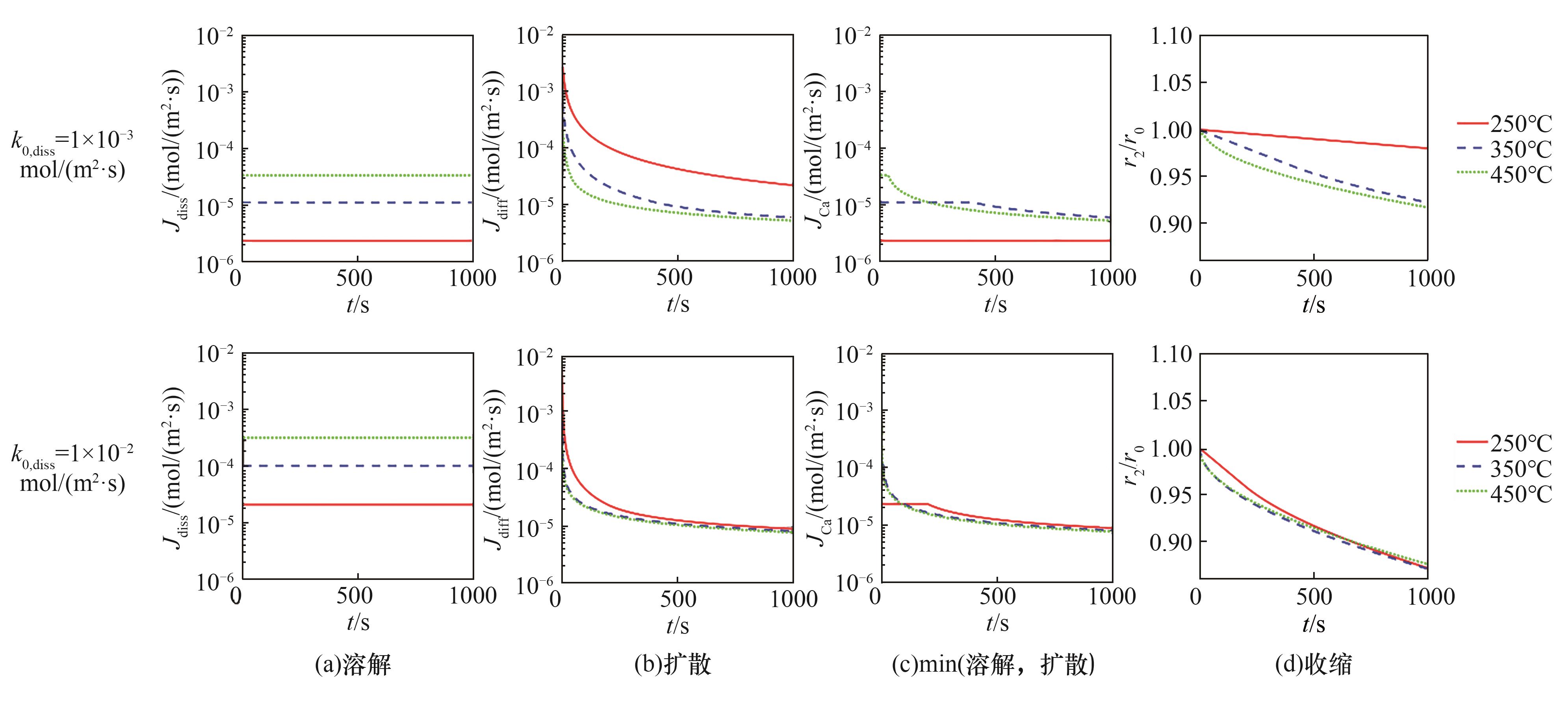
图6 Arrhenius方程指前因子对单个吸收剂粉末随时间的扩散、溶解、两者最小通量及收缩的影响(54.28 atm, ds=10 μm)
Fig.6 Effect of the pre-exponential factor of the Arrhenius equation on the diffusion, dissipation, and their minimum flux and shrinkage versus time for an isolated sorbent powder at 54.28 atm and ds=10 μm
| 数值 | |
|---|---|
| 压碎能耗/(kWh/t) | 2 |
| 磨碎能耗/(kWh/t) | |
| 至75 μm | 11 |
| 从75 μm至38 μm | 70 |
| 从38 μm至10 μm | 150 |
| 吸收剂原料价格/(CNY/t) | 100 |
| 电价/(CNY/MWh) | 450 |
| CO2碳税/(CNY/t) | 80 |
| 生产单位量传统水泥的CO2排放量(t/t) | 0.6 |
| 水泥价格/(CNY/t) | 450 |
表2 技术-经济分析中取自文献的参数[8,57]
Table 2 Parameters taken from literature in the techno-economic analysis[8,57]
| 数值 | |
|---|---|
| 压碎能耗/(kWh/t) | 2 |
| 磨碎能耗/(kWh/t) | |
| 至75 μm | 11 |
| 从75 μm至38 μm | 70 |
| 从38 μm至10 μm | 150 |
| 吸收剂原料价格/(CNY/t) | 100 |
| 电价/(CNY/MWh) | 450 |
| CO2碳税/(CNY/t) | 80 |
| 生产单位量传统水泥的CO2排放量(t/t) | 0.6 |
| 水泥价格/(CNY/t) | 450 |
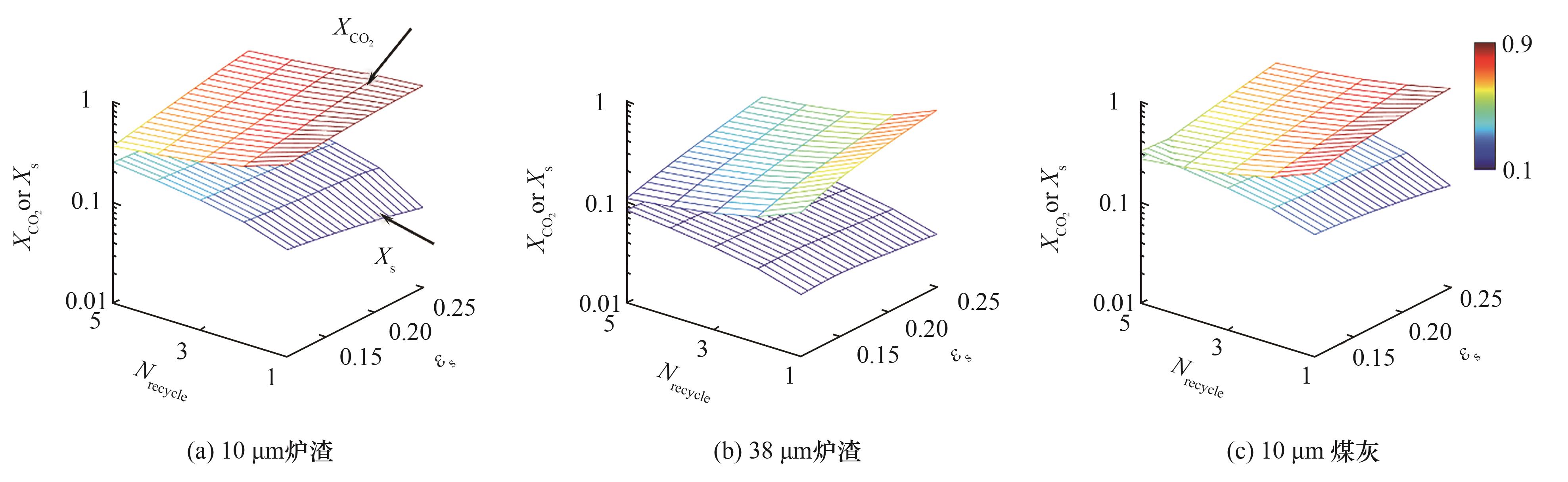
图10 对10、38 µm炉渣与10 µm煤灰,吸收剂粉末相含率、循环次数对CO2与吸收剂转化率的影响
Fig.10 Effect of sorbent holdup and recycling number on CO2 and sorbent conversions for 10, 38 µm slag and 10 µm fly ash
| 吸收剂粉末 | Xs | ||||||
|---|---|---|---|---|---|---|---|
| 0.05 | 0.10 | 0.15 | 0.20 | 0.25 | 0.30 | ||
| 炉渣(10 μm) | εs | 0.25 | 0.145 | 0.243 | 0.243 | — | — |
| Nrecycle | 1 | 1 | 3 | 5 | — | — | |
| 炉渣(38 μm) | εs | 0.194 | — | — | — | — | — |
| Nrecycle | 2 | — | — | — | — | — | |
| 煤灰(10 μm) | εs | 0.25 | 0.25 | 0.145 | 0.243 | 0.194 | 0.152 |
| Nrecycle | 1 | 1 | 1 | 3 | 4 | 5 | |
表3 不同吸收剂粉末转化率要求下的经济性最优化的(εs, Nrecycle)操作组合
Table 3 Economic optimization of (εs, Nrecycle) operation combinations under different sorbent powder conversion requirements
| 吸收剂粉末 | Xs | ||||||
|---|---|---|---|---|---|---|---|
| 0.05 | 0.10 | 0.15 | 0.20 | 0.25 | 0.30 | ||
| 炉渣(10 μm) | εs | 0.25 | 0.145 | 0.243 | 0.243 | — | — |
| Nrecycle | 1 | 1 | 3 | 5 | — | — | |
| 炉渣(38 μm) | εs | 0.194 | — | — | — | — | — |
| Nrecycle | 2 | — | — | — | — | — | |
| 煤灰(10 μm) | εs | 0.25 | 0.25 | 0.145 | 0.243 | 0.194 | 0.152 |
| Nrecycle | 1 | 1 | 1 | 3 | 4 | 5 | |
| 形状 | dp/m | εp | ω1 | ω2 | εs,dyn | fkk |
|---|---|---|---|---|---|---|
| 球 | 0.01 | 0.62 | 150 | 1.75 | 0.2291φ | -0.1528/Fr2+74.13/Fr0.7309 |
| 拉西环 | 0.003 | 0.29 | 0 | 3.2323 | 0.0829φ | 1.47/Fr1.17 |
| 矩鞍 | 0.0063 | 0.344 | 15.86 | 0 | 0.4485φ | 0.119/Fr1.64 |
表A1 不同颗粒形状的几何与流动参数[34-35]
Table A1 Geometric and the hydrodynamic parameters for different pellet shapes[34-35]
| 形状 | dp/m | εp | ω1 | ω2 | εs,dyn | fkk |
|---|---|---|---|---|---|---|
| 球 | 0.01 | 0.62 | 150 | 1.75 | 0.2291φ | -0.1528/Fr2+74.13/Fr0.7309 |
| 拉西环 | 0.003 | 0.29 | 0 | 3.2323 | 0.0829φ | 1.47/Fr1.17 |
| 矩鞍 | 0.0063 | 0.344 | 15.86 | 0 | 0.4485φ | 0.119/Fr1.64 |
| 吸收剂 | 摩尔分数/% | ρs/(kg/m3) | ut/(m/s), umf/(10-3 m/s) | ||||
|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | ds=10 μm | ds=38 μm | ds=75 μm | ||
| 硅灰石 | 50 | 50 | 0 | 2710.3 | 0.0113, 0.046 | 0.0906, 0.662 | 0.1981, 2.577 |
| 炉渣 | 50 | 37.5 | 12.5 | 2967.1 | 0.0118, 0.051 | 0.0972, 0.726 | 0.2108, 2.825 |
| 煤灰 | 37.5 | 47.5 | 15 | 2892.9 | 0.0118, 0.049 | 0.0953, 0.707 | 0.2072, 2.753 |
表A2 吸收剂摩尔组成与密度,54.28 atm条件下的粉末终端沉降速度、起始流化速度
Table A2 Sorbent molar compositions and density, terminal settling and minimum fluidization velocities at 54.28 atm
| 吸收剂 | 摩尔分数/% | ρs/(kg/m3) | ut/(m/s), umf/(10-3 m/s) | ||||
|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | ds=10 μm | ds=38 μm | ds=75 μm | ||
| 硅灰石 | 50 | 50 | 0 | 2710.3 | 0.0113, 0.046 | 0.0906, 0.662 | 0.1981, 2.577 |
| 炉渣 | 50 | 37.5 | 12.5 | 2967.1 | 0.0118, 0.051 | 0.0972, 0.726 | 0.2108, 2.825 |
| 煤灰 | 37.5 | 47.5 | 15 | 2892.9 | 0.0118, 0.049 | 0.0953, 0.707 | 0.2072, 2.753 |
| Type | kWGSR/ mol/(kg·s· | EWGSR/ (J/mol) | ω1 | ω2 | ω3 | ω4 | ρp/ (kg/m3) | θ |
|---|---|---|---|---|---|---|---|---|
| LTC | 0.82×105 | 47400 | 1 | 1 | 0 | 0 | 5904 | 0.55 |
| HTC | 0.47×106 | 88000 | 0.9 | 0.31 | -0.156 | -0.05 | 2476 | 0.5 |
表B1 WGSR动力学参数[54-55]
Table B1 WGSR kinetic parameters[54-55]
| Type | kWGSR/ mol/(kg·s· | EWGSR/ (J/mol) | ω1 | ω2 | ω3 | ω4 | ρp/ (kg/m3) | θ |
|---|---|---|---|---|---|---|---|---|
| LTC | 0.82×105 | 47400 | 1 | 1 | 0 | 0 | 5904 | 0.55 |
| HTC | 0.47×106 | 88000 | 0.9 | 0.31 | -0.156 | -0.05 | 2476 | 0.5 |
| 1 | Rahman A, Farrok O, Haque M M. Environmental impact of renewable energy source based electrical power plants: solar, wind, hydroelectric, biomass, geothermal, tidal, ocean, and osmotic[J]. Renewable and Sustainable Energy Reviews, 2022, 161: 112279. |
| 2 | Liu R, Wang X L, Gao S W. CO2 capture and mineralization using carbide slag doped fly ash[J]. Greenhouse Gases: Science and Technology, 2020, 10(1): 103-115. |
| 3 | Wang T. An overview of IGCC systems[M]//Integrated Gasification Combined Cycle (IGCC) Technologies. Amsterdam: Elsevier, 2017: 1-80. |
| 4 | Sikarwar V S, Pfeifer C, Ronsse F, et al. Progress in in-situ CO2-sorption for enhanced hydrogen production[J]. Progress in Energy and Combustion Science, 2022, 91: 101008. |
| 5 | Lackner K S, Wendt C H, Butt D P, et al. Carbon dioxide disposal in carbonate minerals[J]. Energy, 1995, 20(11): 1153-1170. |
| 6 | Seifritz W. CO2 disposal by means of silicates[J]. Nature, 1990, 345: 486. |
| 7 | Srivastava S, Cerutti M, Nguyen H, et al. Carbonated steel slags as supplementary cementitious materials: reaction kinetics and phase evolution[J]. Cement and Concrete Composites, 2023, 142: 105213. |
| 8 | Strunge T, Renforth P, van der Spek M. Towards a business case for CO2 mineralisation in the cement industry[J]. Communications Earth & Environment, 2022, 3: 59. |
| 9 | Pan S Y, Chen Y H, Fan L S, et al. CO2 mineralization and utilization by alkaline solid wastes for potential carbon reduction[J]. Nature Sustainability, 2020, 3: 399-405. |
| 10 | Mazzella A, Errico M, Spiga D. CO2 uptake capacity of coal fly ash: influence of pressure and temperature on direct gas-solid carbonation[J]. Journal of Environmental Chemical Engineering, 2016, 4(4): 4120-4128. |
| 11 | Ukwattage N L, Ranjith P G, Wang S H. Investigation of the potential of coal combustion fly ash for mineral sequestration of CO2 by accelerated carbonation[J]. Energy, 2013, 52: 230-236. |
| 12 | 包炜军, 李会泉, 张懿. 温室气体CO2矿物碳酸化固定研究进展[J]. 化工学报, 2007, 58(1): 1-9. |
| Bao W J, Li H Q, Zhang Y. Progress in carbon dioxide sequestration by mineral carbonation[J]. Journal of Chemical Industry and Engineering (China), 2007, 58(1): 1-9. | |
| 13 | Zevenhoven R, Fagerlund J, Songok J K. CO2 mineral sequestration: developments toward large-scale application[J]. Greenhouse Gases: Science and Technology, 2011, 1(1): 48-57. |
| 14 | Ben Ghacham A, Cecchi E, Pasquier L C, et al. CO2 sequestration using waste concrete and anorthosite tailings by direct mineral carbonation in gas-solid-liquid and gas-solid routes[J]. Journal of Environmental Management, 2015, 163: 70-77. |
| 15 | 任京伟, 王涛, 陈雨雷, 等. CO2矿化研究现状及应用潜力[J]. 地球科学, 2020, 45(7): 2413-2425. |
| Ren J W, Wang T, Chen Y L, et al. Research status and application potential of CO2 mineralization[J]. Earth Science, 2020, 45(7): 2413-2425. | |
| 16 | Liu W Z, Teng L M, Rohani S, et al. CO2 mineral carbonation using industrial solid wastes: a review of recent developments[J]. Chemical Engineering Journal, 2021, 416: 129093. |
| 17 | 王中辉, 苏胜, 尹子骏, 等. CO2矿化及吸收-矿化一体化(IAM)方法研究进展[J]. 化工进展, 2021, 40(4): 2318-2327. |
| Wang Z H, Su S, Yin Z J, et al. Research progress of CO2 mineralization and integrated absorption-mineralization(IAM) method[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2318-2327. | |
| 18 | 王秋华, 吴嘉帅, 张卫风. 碱性工业固废矿化封存二氧化碳研究进展[J]. 化工进展, 2023, 42(3): 1572-1582. |
| Wang Q H, Wu J S, Zhang W F. Research progress of alkaline industrial solid wastes mineralization for carbon dioxide sequestration[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1572-1582. | |
| 19 | Wang C B, Jia L F, Tan Y W, et al. Carbonation of fly ash in oxy-fuel CFB combustion[J]. Fuel, 2008, 87(7): 1108-1114. |
| 20 | Ji L, Yu H, Wang X L, et al. CO2 sequestration by direct mineralisation using fly ash from Chinese Shenfu coal[J]. Fuel Processing Technology, 2017, 156: 429-437. |
| 21 | Liu W, Su S, Xu K, et al. CO2 sequestration by direct gas–solid carbonation of fly ash with steam addition[J]. Journal of Cleaner Production, 2018, 178: 98-107. |
| 22 | Patel A, Basu P, Acharya B. An investigation into partial capture of CO2 released from a large coal/petcoke fired circulating fluidized bed boiler with limestone injection using its fly and bottom ash[J]. Journal of Environmental Chemical Engineering, 2017, 5(1): 667-678. |
| 23 | Gadikota G. Multiphase carbon mineralization for the reactive separation of CO2 and directed synthesis of H2 [J]. Nature Reviews Chemistry, 2020, 4(2): 78-89. |
| 24 | 田森林, 李晨, 赵群, 等. 一种利用硅酸盐实时碳化固定水气变换过程二氧化碳的方法: 114751372A[P]. 2022-07-15. |
| Tian S L, Li C, Zhao Q, et al. A method for real-time carbonization of silicates to fix carbon dioxide during the water-gas shift process: 114751372A[P]. 2022-07-15. | |
| 25 | Sutherland J P, Vassilatos G, Kubota H, et al. The effect of packing on a fluidized bed[J]. AIChE Journal, 1963, 9(4): 437-441. |
| 26 | van der Ham A G J, Prins W, van Swaaij W P M. A small-scale regularly packed circulating fluidized bed (Part Ⅰ): Hydrodynamics[J]. Powder Technology, 1994, 79(1): 17-28. |
| 27 | Claus G, Vergnes F, Le Goff P. Hydrodynamic study of gas and solid flow through a screen-packing[J]. The Canadian Journal of Chemical Engineering, 1976, 54(3): 143-147. |
| 28 | Veneman R, Hilbers T, Brilman D W F, et al. CO2 capture in a continuous gas-solid trickle flow reactor[J]. Chemical Engineering Journal, 2016, 289: 191-202. |
| 29 | Cherbański R, Molga E. Sorption-enhanced steam-methane reforming with simultaneous sequestration of CO2 on fly ashes—proof of concept and simulations for gas-solid-solid trickle flow reactor[J]. Chemical Engineering and Processing - Process Intensification, 2018, 124: 37-49. |
| 30 | Gao K, Iliuta M C. Trends and advances in the development of coal fly ash-based materials for application in hydrogen-rich gas production: a review[J]. Journal of Energy Chemistry, 2022, 73: 485-512. |
| 31 | Predojević Z J, Petrović D L, Duduković A P. Pressure drop in a countercurrent gas-flowing solids-packed bed contactor[J]. Industrial & Engineering Chemistry Research, 2001, 40(25): 6039-6043. |
| 32 | Xie Z Z, Wang S, Shen Y S. CFD-DEM modelling of the migration of fines in suspension flow through a solid packed bed[J]. Chemical Engineering Science, 2021, 231: 116261. |
| 33 | Henry C, Minier J P, Brambilla S. Particle resuspension: challenges and perspectives for future models[J]. Physics Reports, 2023, 1007: 1-98. |
| 34 | Song X Q, Wang Z W, Jin Y, et al. Gas-solids circulating fluidization in a packed bed[J]. Powder Technology, 1995, 83(2): 127-131. |
| 35 | Panic B, Krol L, Dankmeyer-Laczny J. The investigations of gas-powder two phase flow in packed bed[J]. Steel Research, 2000, 71(8): 271-276. |
| 36 | Ding Y L, He Y R, Cong N T, et al. Hydrodynamics and heat transfer of gas–solid two-phase mixtures flowing through packed beds—a review[J]. Progress in Natural Science, 2008, 18(10): 1185-1196. |
| 37 | Živković L A, Pohar A, Likozar B, et al. Reactor conceptual design by optimization for hydrogen production through intensified sorption- and membrane-enhanced water-gas shift reaction[J]. Chemical Engineering Science, 2020, 211: 115174. |
| 38 | Majérus O, Lehuédé P, Biron I, et al. Glass alteration in atmospheric conditions: crossing perspectives from cultural heritage, glass industry, and nuclear waste management[J]. npj Materials Degradation, 2020, 4: 27. |
| 39 | Abdolhosseini Qomi M J, Miller Q R S, Zare S, et al. Molecular-scale mechanisms of CO2 mineralization in nanoscale interfacial water films[J]. Nature Reviews Chemistry, 2022, 6(9): 598-613. |
| 40 | Oelkers E H. General kinetic description of multioxide silicate mineral and glass dissolution[J]. Geochimica et Cosmochimica Acta, 2001, 65(21): 3703-3719. |
| 41 | Oelkers E H, Golubev S V, Chairat C, et al. The surface chemistry of multi-oxide silicates[J]. Geochimica et Cosmochimica Acta, 2009, 73(16): 4617-4634. |
| 42 | Li Z S, Liu Y, Cai N S. Understanding the enhancement effect of high-temperature steam on the carbonation reaction of CaO with CO2 [J]. Fuel, 2014, 127: 88-93. |
| 43 | Wang H, Li Z S, Cai N S. Multiscale model for steam enhancement effect on the carbonation of CaO particle[J]. Chemical Engineering Journal, 2020, 394: 124892. |
| 44 | Zheng K, Zhang Z T, Yang F H, et al. Molecular dynamics study of the structural properties of calcium aluminosilicate slags with varying Al2O3/SiO2 ratios[J]. ISIJ International, 2012, 52(3): 342-349. |
| 45 | Delaye J M, Le Gac A, Macaluso S, et al. Investigation of alumino-silicate glasses by coupling experiments and simulations (Part Ⅰ): Structures[J]. Journal of Non-Crystalline Solids, 2021, 567: 120936. |
| 46 | Yadav S, Mehra A. Mathematical modelling and experimental study of carbonation of wollastonite in the aqueous media[J]. Journal of CO2 Utilization, 2019, 31: 181-191. |
| 47 | Rimstidt J D, Dove P M. Mineral/solution reaction rates in a mixed flow reactor: Wollastonite hydrolysis[J]. Geochimica et Cosmochimica Acta, 1986, 50(11): 2509-2516. |
| 48 | Ptáček P, Nosková M, Brandštetr J, et al. Mechanism and kinetics of wollastonite fibre dissolution in the aqueous solution of acetic acid[J]. Powder Technology, 2011, 206(3): 338-344. |
| 49 | Cummings K, Lanford W A, Feldmann M. Weathering of glass in moist and polluted air[J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 1998, 136: 858-862. |
| 50 | Sessegolo L, Verney-Carron A, Valle N, et al. Alteration of potash-lime silicate glass in atmospheric medium: study of mechanisms and kinetics using 18O and D isotopes[J]. Journal of Non-Crystalline Solids, 2021, 570: 121020. |
| 51 | DDBST GmbH. Saturated Vapor Pressure[EB/OL]. [2024-10-06]. . |
| 52 | Froment G F, Bischoff K B, De Wilde J. Chemical Reactor Analysis and Design[M]. 2nd ed. New York: John Wiley & Sons Inc., 1990. |
| 53 | El-Bazi W, Bideq M, El-Abidi A, et al. Numerical study of a water gas shift fixed bed reactor operating at low pressures[J]. Bulletin of Chemical Reaction Engineering & Catalysis, 2022, 17(2): 304-321. |
| 54 | Choi Y, Stenger H G. Water gas shift reaction kinetics and reactor modeling for fuel cell grade hydrogen[J]. Journal of Power Sources, 2003, 124(2): 432-439. |
| 55 | Hla S S, Park D, Duffy G J, et al. Kinetics of high-temperature water-gas shift reaction over two iron-based commercial catalysts using simulated coal-derived syngases[J]. Chemical Engineering Journal, 2009, 146(1): 148-154. |
| 56 | Adams T A, Barton P I. A dynamic two-dimensional heterogeneous model for water gas shift reactors[J]. International Journal of Hydrogen Energy, 2009, 34(21): 8877-8891. |
| 57 | Gerdemann S J, O'Connor W K, Dahlin D C, et al. Ex situ aqueous mineral carbonation[J]. Environmental Science & Technology, 2007, 41(7): 2587-2593. |
| 58 | Kats A. Hydrogen in alpha-quartz[J]. Philips Research Reports, 1962, 17: 133-279. |
| 59 | Farver J R. Oxygen and hydrogen diffusion in minerals[J]. Reviews in Mineralogy and Geochemistry, 2010, 72(1): 447-507. |
| 60 | Brady J B, Cherniak D J. Diffusion in minerals: an overview of published experimental diffusion data[J]. Reviews in Mineralogy and Geochemistry, 2010, 72(1): 899-920. |
| 61 | Smets B M J, Lommen T P A. The leaching of sodium containing glasses: ion exchange or diffusion of molecular water?[J]. Journal de Physique Colloques, 1982, 43(C9): 649-652. |
| 62 | Kuroda M, Tachibana S, Sakamoto N, et al. Water diffusion in silica glass through pathways formed by hydroxyls[J]. American Mineralogist, 2018, 103(3): 412-417. |
| 63 | Schott J, Pokrovsky O S, Spalla O, et al. Formation, growth and transformation of leached layers during silicate minerals dissolution: the example of wollastonite[J]. Geochimica et Cosmochimica Acta, 2012, 98: 259-281. |
| 64 | Xiao R, Jiang X, Zhang M M, et al. Analytical investigation of phase assemblages of alkali-activated materials in CaO-SiO2-Al2O3 systems: the management of reaction products and designing of precursors[J]. Materials & Design, 2020, 194: 108975. |
| 65 | Francesconi J A, Mussati M C, Aguirre P A. Analysis of design variables for water-gas-shift reactors by model-based optimization[J]. Journal of Power Sources, 2007, 173(1): 467-477. |
| 66 | Giuliano A, Poletto M, Barletta D. Pure hydrogen co-production by membrane technology in an IGCC power plant with carbon capture[J]. International Journal of Hydrogen Energy, 2018, 43(41): 19279-19292. |
| [1] | 杨勇, 祖子轩, 李煜坤, 王东亮, 范宗良, 周怀荣. T型圆柱形微通道内CO2碱液吸收数值模拟[J]. 化工学报, 2024, 75(S1): 135-142. |
| [2] | 王军锋, 张俊杰, 张伟, 王家乐, 双舒炎, 张亚栋. 液相放电等离子体分解甲醇制氢:电极配置的优化[J]. 化工学报, 2024, 75(9): 3277-3286. |
| [3] | 罗欣怡, 徐强, 佘永璐, 聂腾飞, 郭烈锦. 光电分解水制氢气泡动力学特性及其传质机理研究[J]. 化工学报, 2024, 75(9): 3083-3093. |
| [4] | 曹佳蕾, 孙立岩, 曾德望, 尹凡, 高子翔, 肖睿. 双流化床化学链制氢反应器的数值模拟[J]. 化工学报, 2024, 75(8): 2865-2874. |
| [5] | 丁家琦, 刘海涛, 赵普, 朱香凝, 王晓放, 谢蓉. 煤炭超临界水制氢反应器内多相流场智能滚动预测研究[J]. 化工学报, 2024, 75(8): 2886-2896. |
| [6] | 马旭, 滕亚栋, 刘杰, 王宇璐, 张鹏, 张莲海, 姚万龙, 展静, 吴青柏. 喷雾法水合物法捕集分离烟道气中CO2[J]. 化工学报, 2024, 75(5): 2001-2016. |
| [7] | 王成秀, 宋大山, 李之辉, 杨潇, 蓝兴英, 高金森, 徐春明. Geldart C类脱硫灰颗粒在环流耦合提升管内稳定流动特性[J]. 化工学报, 2024, 75(4): 1485-1496. |
| [8] | 臧雅晴, 张益钧, 王金钊, 王倩, 李殿卿, 冯俊婷, 段雪. 基于反应耦合的低能耗水合氯化钙脱水制无水氯化钙[J]. 化工学报, 2024, 75(4): 1508-1518. |
| [9] | 王沛, 段睿明, 张广儒, 金万勤. 光热驱动的膜分离生物甲烷制氢过程建模与仿真分析[J]. 化工学报, 2024, 75(3): 967-973. |
| [10] | 刘志鹏, 赵长颖, 吴睿, 张智昊. 基于水电解制氢的梯度多孔传输层中气液流动可视化实验研究[J]. 化工学报, 2024, 75(2): 520-530. |
| [11] | 向千禧, 杨小康, 孙嘉琦, 谢峰, 邵志刚. 质子交换膜水电解池分布特性研究[J]. 化工学报, 2024, 75(11): 4359-4368. |
| [12] | 向腾龙, 王治红, 汪贵, 李龙. 液化天然气冷能梯级利用的多功能集成系统研究[J]. 化工学报, 2024, 75(10): 3401-3413. |
| [13] | 孙琼, 杨富鑫, 谭厚章, 王晓坡. 低共熔溶剂捕集烟气CO2模拟研究[J]. 化工学报, 2024, 75(10): 3705-3717. |
| [14] | 赵璐, 吴涵, 刘宪云. 负载型钌催化剂用于氨硼烷水解制氢反应[J]. 化工学报, 2024, 75(10): 3639-3650. |
| [15] | 赵若晗, 黄蒙蒙, 朱春英, 付涛涛, 高习群, 马友光. 缩口T型微通道内纳米流体吸收CO2的流动与传质研究[J]. 化工学报, 2024, 75(1): 221-230. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号
