化工学报 ›› 2025, Vol. 76 ›› Issue (2): 438-453.DOI: 10.11949/0438-1157.20241138
董举1( ), 余留洋1, 贾晟哲1, 史连军2,3, 王诗瀚2,3, 胡国涛2,3, 汤伟伟1(
), 余留洋1, 贾晟哲1, 史连军2,3, 王诗瀚2,3, 胡国涛2,3, 汤伟伟1( ), 王静康1, 龚俊波1(
), 王静康1, 龚俊波1( )
)
收稿日期:2024-10-15
修回日期:2024-11-14
出版日期:2025-02-25
发布日期:2025-03-10
通讯作者:
汤伟伟,龚俊波
作者简介:董举(2002—),男,硕士研究生,dongju@tju.edu.cn
基金资助:
Ju DONG1( ), Liuyang YU1, Shengzhe JIA1, Lianjun SHI2,3, Shihan WANG2,3, Guotao HU2,3, Weiwei TANG1(
), Liuyang YU1, Shengzhe JIA1, Lianjun SHI2,3, Shihan WANG2,3, Guotao HU2,3, Weiwei TANG1( ), Jingkang WANG1, Junbo GONG1(
), Jingkang WANG1, Junbo GONG1( )
)
Received:2024-10-15
Revised:2024-11-14
Online:2025-02-25
Published:2025-03-10
Contact:
Weiwei TANG, Junbo GONG
摘要:
电子级磷酸作为电子工业常用的一种超高纯试剂,广泛应用于大屏幕液晶显示器和超大规模集成电路等微电子工业中的湿法蚀刻和清洗。随着电子元器件加工精度的迭代更新,对电子级磷酸中杂质含量与微粒的要求也在日益提高。综述了电子级磷酸深度净化的常用方法,重点阐述了结晶精制技术在电子级磷酸深度净化中的优势和应用进展,总结了结晶法在磷酸深度净化过程中的杂质包藏与迁移机制,并概述了结晶纯化的过程强化手段。最后,对电子级磷酸结晶精制技术的发展作出了前景展望。
中图分类号:
董举, 余留洋, 贾晟哲, 史连军, 王诗瀚, 胡国涛, 汤伟伟, 王静康, 龚俊波. 电子级磷酸的结晶精制技术发展现状与研究进展[J]. 化工学报, 2025, 76(2): 438-453.
Ju DONG, Liuyang YU, Shengzhe JIA, Lianjun SHI, Shihan WANG, Guotao HU, Weiwei TANG, Jingkang WANG, Junbo GONG. Current status and research progress of crystallization technology of electronic grade phosphoric acid[J]. CIESC Journal, 2025, 76(2): 438-453.
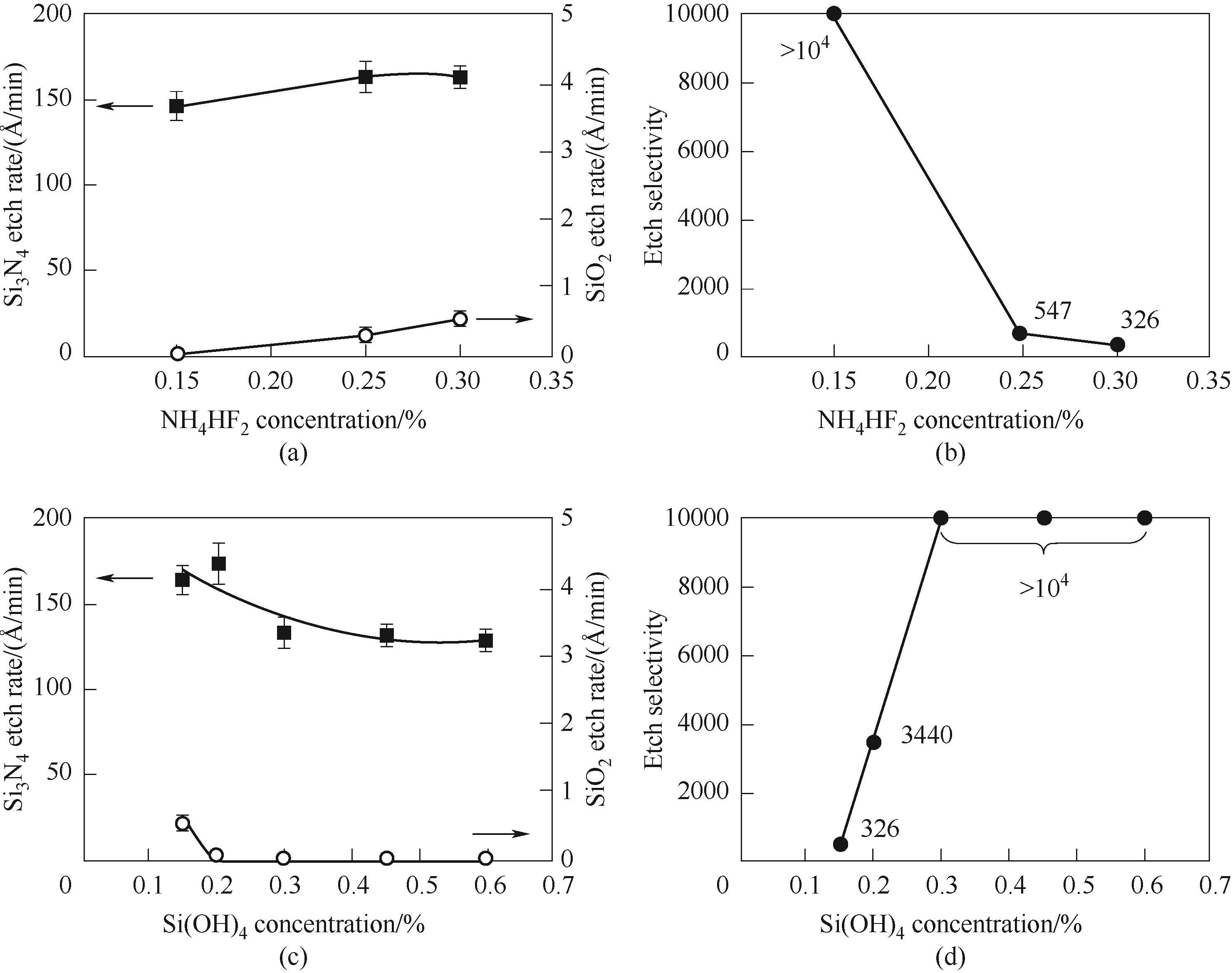
图2 Si3N4与SiO2膜的蚀刻速率(a)与Si3N4对SiO2的刻蚀选择性(b)随NH4HF2在含有质量分数0.15% Si(OH)4的H3PO4溶液中浓度的变化;Si3N4与SiO2膜的蚀刻速率(c)与Si3N4对SiO2的刻蚀选择性(d)随Si(OH)4在含有质量分数0.30% NH4HF2的H3PO4溶液中浓度的变化[11](1 Å =0.1 nm)
Fig.2 Etch rates of Si3N4 and SiO2 films (a) and etch selectivity of Si3N4 to SiO2 (b) with the addition of NH4HF2 in the presence of 0.15%(mass) of Si(OH)4 in H3PO4; Etch rates of Si3N4 and SiO2 films (c) and etch selectivity of Si3N4 to SiO2 (d) with the concentration of Si(OH)4 in the presence of 0.30%(mass) of NH4HF2 in H3PO4[11]
| 项目 | 指标 | |
|---|---|---|
| E1 | E2 | |
磷酸(H3PO4)质量分数 (85%)/(%) | 85~87 | 85~87 |
易氧化物(以H3PO4计) 质量分数/% | ≤0.005 | ≤0.001 |
| 硝酸盐( | ≤5 | ≤0.5 |
| 硫酸盐( | ≤10 | ≤5 |
| 氯化物(Cl-)质量分数/(mg/kg) | ≤1 | ≤0.5 |
| 铝(Al)/(μg/kg) | ≤200 | ≤50 |
| 硼(B)/(μg/kg) | — | ≤50 |
| 锑(Sb)/(μg/kg) | ≤3000 | ≤300 |
| 砷(As)/(μg/kg) | ≤100 | ≤20 |
| 钡(Ba)/(μg/kg) | ≤100 | ≤20 |
| 镉(Cd)/(μg/kg) | ≤100 | ≤20 |
| 钙(Ca)/(μg/kg) | ≤1000 | ≤50 |
| 铬(Cr)/(μg/kg) | ≤100 | ≤20 |
| 钴(Co)/(μg/kg) | ≤100 | ≤20 |
| 铜(Cu)/(μg/kg) | ≤50 | ≤20 |
| 镓(Ga)/(μg/kg) | ≤100 | ≤10 |
| 金(Au)/(μg/kg) | ≤100 | ≤10 |
| 铁(Fe)/(μg/kg) | ≤300 | ≤50 |
| 铅(Pb)/(μg/kg) | ≤100 | ≤20 |
| 锂(Li)/(μg/kg) | ≤100 | ≤10 |
| 镁(Mg)/(μg/kg) | ≤100 | ≤20 |
| 锰(Mn)/(μg/kg) | ≤100 | ≤20 |
| 镍(Ni)/(μg/kg) | ≤100 | ≤20 |
| 钾(K)/(μg/kg) | ≤100 | ≤20 |
| 银(Ag)/(μg/kg) | ≤100 | ≤20 |
| 钠(Na)/(μg/kg) | ≤500 | ≤50 |
| 锡(Sn)/(μg/kg) | — | ≤10 |
| 锶(Sr)/(μg/kg) | ≤100 | ≤20 |
| 钛(Ti)/(μg/kg) | ≤100 | ≤50 |
| 锌(Zn)/(μg/kg) | ≤100 | ≤50 |
表1 《电子级磷酸》质量标准(GB/T 28159—2011)
Table1 Quality standard for Electronic Grade Phosphoric Acid (GB/T 28159—2011)
| 项目 | 指标 | |
|---|---|---|
| E1 | E2 | |
磷酸(H3PO4)质量分数 (85%)/(%) | 85~87 | 85~87 |
易氧化物(以H3PO4计) 质量分数/% | ≤0.005 | ≤0.001 |
| 硝酸盐( | ≤5 | ≤0.5 |
| 硫酸盐( | ≤10 | ≤5 |
| 氯化物(Cl-)质量分数/(mg/kg) | ≤1 | ≤0.5 |
| 铝(Al)/(μg/kg) | ≤200 | ≤50 |
| 硼(B)/(μg/kg) | — | ≤50 |
| 锑(Sb)/(μg/kg) | ≤3000 | ≤300 |
| 砷(As)/(μg/kg) | ≤100 | ≤20 |
| 钡(Ba)/(μg/kg) | ≤100 | ≤20 |
| 镉(Cd)/(μg/kg) | ≤100 | ≤20 |
| 钙(Ca)/(μg/kg) | ≤1000 | ≤50 |
| 铬(Cr)/(μg/kg) | ≤100 | ≤20 |
| 钴(Co)/(μg/kg) | ≤100 | ≤20 |
| 铜(Cu)/(μg/kg) | ≤50 | ≤20 |
| 镓(Ga)/(μg/kg) | ≤100 | ≤10 |
| 金(Au)/(μg/kg) | ≤100 | ≤10 |
| 铁(Fe)/(μg/kg) | ≤300 | ≤50 |
| 铅(Pb)/(μg/kg) | ≤100 | ≤20 |
| 锂(Li)/(μg/kg) | ≤100 | ≤10 |
| 镁(Mg)/(μg/kg) | ≤100 | ≤20 |
| 锰(Mn)/(μg/kg) | ≤100 | ≤20 |
| 镍(Ni)/(μg/kg) | ≤100 | ≤20 |
| 钾(K)/(μg/kg) | ≤100 | ≤20 |
| 银(Ag)/(μg/kg) | ≤100 | ≤20 |
| 钠(Na)/(μg/kg) | ≤500 | ≤50 |
| 锡(Sn)/(μg/kg) | — | ≤10 |
| 锶(Sr)/(μg/kg) | ≤100 | ≤20 |
| 钛(Ti)/(μg/kg) | ≤100 | ≤50 |
| 锌(Zn)/(μg/kg) | ≤100 | ≤50 |
| 级别 | 单项金属杂质 | 控制微粒粒径 | 颗粒数 | IC集成度 |
|---|---|---|---|---|
| Grade1 | ≤100 ppb | ≥1 μm | ≤25/ml | 64 K |
| Grade2 | ≤10 ppb | ≥0.5 μm | ≤25/ml | 4 M |
| Grade3 | ≤1 ppb | ≥0.5 μm | ≤5/ml | 256 M |
| Grade4 | ≤0.1 ppb | ≥0.2 μm | 16 G | |
| Grade5 | ≤0.01 ppb |
表2 SEMI对于湿电子化学品的纯度要求[20]
Table 2 The purity of wet electronic chemicals in Semiconductor Equipment Materials International[20]
| 级别 | 单项金属杂质 | 控制微粒粒径 | 颗粒数 | IC集成度 |
|---|---|---|---|---|
| Grade1 | ≤100 ppb | ≥1 μm | ≤25/ml | 64 K |
| Grade2 | ≤10 ppb | ≥0.5 μm | ≤25/ml | 4 M |
| Grade3 | ≤1 ppb | ≥0.5 μm | ≤5/ml | 256 M |
| Grade4 | ≤0.1 ppb | ≥0.2 μm | 16 G | |
| Grade5 | ≤0.01 ppb |
| Molar ratio | Al3+ removal efficiency/% | Mg2+ removal efficiency/% | F- removal efficiency/% | Phenomenon |
|---|---|---|---|---|
| Na+∶Al3+∶Mg2+∶F-= 1∶1∶1∶1 | 0 | 0 | 0 | no precipitation |
| Na+∶Al3+∶Mg2+∶F-= 2∶1∶1∶2 | 0 | 0 | 0 | no precipitation |
| Na+∶Al3+∶Mg2+∶F-= 3∶1∶1∶3 | 0 | 0 | 0 | no precipitation |
| Na+∶Al3+∶Mg2+∶F-= 4∶1∶1∶4 | 48.9 | 44.5 | 38.4 | precipitation |
| Na+∶Al3+∶Mg2+∶F-= 5∶1∶1∶5 | 65.4 | 55.4 | 58.1 | precipitation |
| Na+∶Al3+∶Mg2+∶F-= 6∶1∶1∶6 | 99.4 | 83.8 | 88.7 | precipitation |
| Na+∶Al3+∶Mg2+∶F-= 7∶1∶1∶6 | 98.1 | 83.4 | 88.2 | precipitation |
| Na+∶Al3+∶Mg2+∶F-= 5∶1∶1∶6 | 99.6 | 80.1 | 82.6 | precipitation |
| Na+∶Al3+∶Mg2+∶F-= 5∶1∶1∶7 | 99.5 | 96.7 | 84.5 | precipitation |
| Na+∶Al3+∶Mg2+∶F-= 5∶1.5∶1∶6 | 96.98 | 93.68 | 95.17 | precipitation |
| Na+∶Al3+∶Mg2+∶F-= 6∶1.5∶1∶7 | 96.94 | 93.55 | 85.23 | precipitation |
表3 水溶液中不同比例Na+、Al3+、Mg2+、F-混合后的沉淀情况与去除效果[32]
Table 3 Precipitation situations and removal efficiencies of Al3+, Mg2+ and F- for the Na x Al y Mg z F w in aqueous solution[32]
| Molar ratio | Al3+ removal efficiency/% | Mg2+ removal efficiency/% | F- removal efficiency/% | Phenomenon |
|---|---|---|---|---|
| Na+∶Al3+∶Mg2+∶F-= 1∶1∶1∶1 | 0 | 0 | 0 | no precipitation |
| Na+∶Al3+∶Mg2+∶F-= 2∶1∶1∶2 | 0 | 0 | 0 | no precipitation |
| Na+∶Al3+∶Mg2+∶F-= 3∶1∶1∶3 | 0 | 0 | 0 | no precipitation |
| Na+∶Al3+∶Mg2+∶F-= 4∶1∶1∶4 | 48.9 | 44.5 | 38.4 | precipitation |
| Na+∶Al3+∶Mg2+∶F-= 5∶1∶1∶5 | 65.4 | 55.4 | 58.1 | precipitation |
| Na+∶Al3+∶Mg2+∶F-= 6∶1∶1∶6 | 99.4 | 83.8 | 88.7 | precipitation |
| Na+∶Al3+∶Mg2+∶F-= 7∶1∶1∶6 | 98.1 | 83.4 | 88.2 | precipitation |
| Na+∶Al3+∶Mg2+∶F-= 5∶1∶1∶6 | 99.6 | 80.1 | 82.6 | precipitation |
| Na+∶Al3+∶Mg2+∶F-= 5∶1∶1∶7 | 99.5 | 96.7 | 84.5 | precipitation |
| Na+∶Al3+∶Mg2+∶F-= 5∶1.5∶1∶6 | 96.98 | 93.68 | 95.17 | precipitation |
| Na+∶Al3+∶Mg2+∶F-= 6∶1.5∶1∶7 | 96.94 | 93.55 | 85.23 | precipitation |
| 项目 | 层熔融结晶 | 悬浮熔融结晶 |
|---|---|---|
| 熔融体温度 | 高于或低于关键组分 熔点附近 | 低于关键组分熔点附近 |
| 结晶热转移 | 通过晶层 | 通过熔液 |
| 晶体生长速率 | 快,10-7~10-5 m/s | 慢,10-8~10-7 m/s |
| 晶体熔液相界面积 | 小,10~102 m2/m3 | 大,约104 m2/m3 |
| 转动装置 | 无 | 有 |
| 传质速率 | 大 | 小 |
| 结垢现象 | 无 | 有 |
| 流体输送 | 易,均为液体 | 难,固液混合物 |
| 固液分离 | 易,液体单独排出 | 难 |
| 装置放大 | 容易 | 较难 |
表4 层熔融结晶技术与悬浮熔融结晶技术的比较[50]
Table 4 Comparison of layer melt crystallization technology and suspension melt crystallization technology[50]
| 项目 | 层熔融结晶 | 悬浮熔融结晶 |
|---|---|---|
| 熔融体温度 | 高于或低于关键组分 熔点附近 | 低于关键组分熔点附近 |
| 结晶热转移 | 通过晶层 | 通过熔液 |
| 晶体生长速率 | 快,10-7~10-5 m/s | 慢,10-8~10-7 m/s |
| 晶体熔液相界面积 | 小,10~102 m2/m3 | 大,约104 m2/m3 |
| 转动装置 | 无 | 有 |
| 传质速率 | 大 | 小 |
| 结垢现象 | 无 | 有 |
| 流体输送 | 易,均为液体 | 难,固液混合物 |
| 固液分离 | 易,液体单独排出 | 难 |
| 装置放大 | 容易 | 较难 |
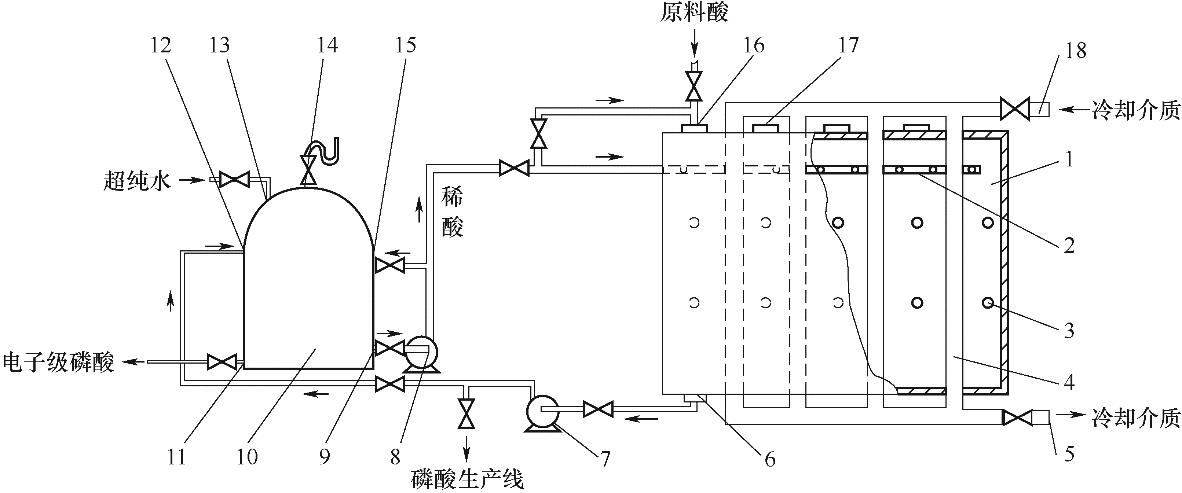
图12 列管结晶生产电子级磷酸的装置结构示意图[42]1—结晶槽;2—喷淋器;3—红外灯管;4—立式列管;5—冷却介质出口;6—出酸口;7—出酸泵;8—循环泵;9—循环酸出口;10—调酸罐;11—产品出口;12—进料口;13—进水口;14—排气口;15—循环酸进口;16—进酸口;17—晶种投加口;18—冷却介质入口
Fig.12 Schematic diagram of a tube crystallization device for producing electronic grade phosphoric acid[42]1—crystallization tank; 2—spray thrower; 3—infrared lamp; 4—vertical tube; 5—cooling medium outlet; 6—acid outlet; 7—acid pump; 8—circulation pump; 9—recycle acid export; 10—acid tank; 11—product export; 12—feed port; 13—water inlet; 14—exhaust port; 15—circulating acid inlet; 16—acid inlet; 17—seed feeding port; 18—cooling medium inlet
| 1 | 冉孟杰, 杨润德, 任国兴. 电子级磷酸的制备工艺及研究进展[J]. 化肥设计, 2024, 62(2): 1-3, 16. |
| Ran M J, Yang R D, Reng G X. Preparation process and research progress of electronic grade phosphoric acid[J]. Chemical Fertilizer Design, 2024, 62(2): 1-3, 16. | |
| 2 | 肖睿, 李韬, 任美洁, 等. 湿法磷酸除砷制备电子级磷酸研究进展[J]. 湿法冶金, 2024, 43(5): 504-512. |
| Xiao R, Li T, Ren M J, et al. Research progress on preparation of electronic grade phosphoric acid by removing arsenic from wet-process phosphoric acid[J]. Hydrometallurgy of China, 2024, 43(5): 504-512. | |
| 3 | 齐亚兵, 张思敬. 湿法磷酸净化技术研究新进展及应用现状[J]. 应用化工, 2022, 51(9): 2798-2804. |
| Qi Y B, Zhang S J. New research progress and present application situation of purification technology for wet-process phosphoric acid[J]. Applied Chemical Industry, 2022, 51(9): 2798-2804. | |
| 4 | 黄欣雨, 甘晨, 张名扬, 等. 湿法磷酸萃取技术发展现状与研究进展[J]. 工程科学学报, 2024, 46(11): 1948-1959. |
| Huang X Y, Gan C, Zhang M Y, et al. Development status and research progress of wet phosphoric acid extraction technology[J]. Chinese Journal of Engineering, 2024, 46(11): 1948-1959. | |
| 5 | 陶隆海, 王永杰, 段家堂, 等. 湿法磷酸副产渣酸和萃余酸研究进展与展望[J]. 无机盐工业, 2024, 56(3): 12-18, 79. |
| Tao L H, Wang Y J, Duan J T, et al. Research progress and prospect of sludge acid and raffinate acid by-product of wet-process phosphoric acid[J]. Inorganic Chemicals Industry, 2024, 56(3): 12-18, 79. | |
| 6 | 杨书丽. 电子工业清洗混合废酸分离及资源化工艺研究[D]. 绵阳: 绵阳师范学院, 2023. |
| Yang S L. Research on the separation and resource utilization process of mixed waste acid from electronic industry cleaning[D]. Mianyang: Mianyang Teachers' College, 2023. | |
| 7 | 倪双林, 马航, 曾波, 等. 湿法磷酸生产新工艺开发研究[J]. 云南化工, 2024, 51(S1): 1-10. |
| Ni S L, Ma H, Zeng B, et al. Development on new process of wet process phosphoric acid production[J]. Yunnan Chemical Technology, 2024, 51(S1): 1-10. | |
| 8 | 杨着, 姜飞. 电子级磷酸在电子工业中的应用及发展[J]. 当代化工研究, 2021(15): 101-102. |
| Yang Z, Jiang F. Application and development of electronic grade phosphoric acid in electronic industry[J]. Modern Chemical Research, 2021(15): 101-102. | |
| 9 | 林军, 吴小海, 洪春美, 等. 电子级磷酸的制备工艺与应用研究进展[J]. 化工技术与开发, 2012, 41(2): 32-34, 4. |
| Lin J, Wu X H, Hong C M, et al. Progress on preparation technology and application study of electronic grade phosphoric acid[J]. Technology & Development of Chemical Industry, 2012, 41(2): 32-34, 4. | |
| 10 | Kranz C, Wyczanowski S, Baumann U, et al. Industrial cleaning sequences for Al2O3-passivated PERC solar cells[J]. Energy Procedia, 2014, 55: 211-218. |
| 11 | Seo D, Bae J S, Oh E, et al. Selective wet etching of Si3N4/SiO2 in phosphoric acid with the addition of fluoride and silicic compounds[J]. Microelectronic Engineering, 2014, 118: 66-71. |
| 12 | 冯凯, 张庭, 王书萍, 等. 磷酸中金属离子的种类与含量对多晶硅蚀刻速率的影响[J]. 中国标准化, 2024(S1): 314-319. |
| Feng K, Zhang T, Wang S P, et al. Effect of the type and content of metal ions in phosphoric acid on the etching rate of polysilicon[J]. China Standardization, 2024(S1): 314-319. | |
| 13 | Benrabah S, Legallais M, Besson P, et al. H3PO4-based wet chemical etching for recovery of dry-etched GaN surfaces[J]. Applied Surface Science, 2022, 582: 152309. |
| 14 | Sreejith K P, Sharma A K, Basu P K, et al. Etching methods for texturing industrial multi-crystalline silicon wafers: a comprehensive review[J]. Solar Energy Materials and Solar Cells, 2022, 238: 111531. |
| 15 | Azaroual M, Kervevan C, Lassin A, et al. Thermo-kinetic and physico-chemical modeling of processes generating scaling problems in phosphoric acid and fertilizers production industries[J]. Procedia Engineering, 2012, 46: 68-75. |
| 16 | Zan C, Shi L, Song Y Z, et al. Evaluation method for thermal processing of phosphoric acid with waste heat recovery[J]. Energy, 2006, 31(14): 2791-2804. |
| 17 | Liu Q, Liu W Z, Lv L, et al. Study on reactions of gaseous P2O5 with Ca3(PO4)2 and SiO2 during a rotary kiln process for phosphoric acid production[J]. Chinese Journal of Chemical Engineering, 2018, 26(4): 795-805. |
| 18 | El-Shall H, Abdel-Aal E A, Moudgil B M. Effect of surfactants on phosphogypsum crystallization and filtration during wet-process phosphoric acid production[J]. Separation Science and Technology, 2000, 35(3): 395-410. |
| 19 | Ye C W, Li J. Wet process phosphoric acid purification by solvent extraction using N-octanol and tributylphosphate mixtures[J]. Journal of Chemical Technology & Biotechnology, 2013, 88(9): 1715-1720. |
| 20 | 张大洲, 龙辉, 卢文新, 等. 电子级磷酸研究现状及发展趋势分析[J]. 化肥设计, 2022, 60(4): 1-4, 20. |
| Zhang D Z, Long H, Lu W X, et al. Analysis of existing researches and development trends of electronic-grade phosphoric acid[J]. Chemical Fertilizer Design, 2022, 60(4): 1-4, 20. | |
| 21 | Reyes L H, Medina I S, Mendoza R N, et al. Extraction of cadmium from phosphoric acid using resins impregnated with organophosphorus extractants[J]. Industrial & Engineering Chemistry Research, 2001, 40(5): 1422-1433. |
| 22 | Jin Y, Ma Y J, Weng Y L, et al. Solvent extraction of Fe3+ from the hydrochloric acid route phosphoric acid by D2EHPA in kerosene[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(5): 3446-3452. |
| 23 | Forouzesh M, Fatehifar E, Khoshbouy R, et al. Experimental investigation of iron removal from wet phosphoric acid through chemical precipitation process[J]. Chemical Engineering Research and Design, 2023, 189: 308-318. |
| 24 | Xu S S, He R R, Dong C J, et al. Acid stable layer-by-layer nanofiltration membranes for phosphoric acid purification[J]. Journal of Membrane Science, 2022, 644: 120090. |
| 25 | 林军, 吴小海, 苏杰文, 等. 一种搅拌结晶生产电子级磷酸的方法: 103771367B[P]. 2016-01-27. |
| Lin J, Wu X H, Su J W, et al. A method for producing electronic-grade phosphoric acid by stirring and crystallizing: 103771367B[P]. 2016-01-27. | |
| 26 | 余留洋, 刘书博, 贾晟哲, 等. 电子级磷酸的纯化精制技术发展现状与研究进展[J]. 化工学报, 2024, 75(1): 1-19. |
| Yu L Y, Liu S B, Jia S Z, et al. Current status and research progress of purification technology of electronic grade phosphoric acid[J]. CIESC Journal, 2024, 75(1): 1-19. | |
| 27 | Azimi A, Azari A, Rezakazemi M, et al. Removal of heavy metals from industrial wastewaters: a review[J]. ChemBioEng Reviews, 2017, 4(1): 37-59. |
| 28 | Meng C, Du M Y, Zhang Z B, et al. Open-framework vanadate as efficient ion exchanger for uranyl removal[J]. Environmental Science & Technology, 2024, 58(21): 9456-9465. |
| 29 | Hu Z J, Zhang T, Lv L, et al. Extraction performance and mechanism of TBP in the separation of Fe3+ from wet-processing phosphoric acid[J]. Separation and Purification Technology, 2021, 272: 118822. |
| 30 | Shinwari K J. Emerging technologies for the recovery of bioactive compounds from saffron species[M]. Amsterdam: Elsevier, 2021: 143-182. |
| 31 | 李军, 钟本和, 何浩明, 等. 用湿法磷酸制备工业级磷酸和食品级磷酸的方法: 1483666[P]. 2004-03-24. |
| Li J, Zhong B H, He H M, et al. Method for preparing industrial and food grade phosphoric acid from wet process phosphoric acid: 1483666[P]. 2004-03-24. | |
| 32 | He B B, Fu Y, Zu Y, et al. Simultaneous removal of Al3+, Mg2+ and F- from wet-process phosphoric acid achieved by a co-precipitation strategy[J]. Separation and Purification Technology, 2024, 341: 126855. |
| 33 | Crini G, Lichtfouse E. Advantages and disadvantages of techniques used for wastewater treatment[J]. Environmental Chemistry Letters, 2019, 17(1): 145-155. |
| 34 | Obotey Ezugbe E, Rathilal S. Membrane technologies in wastewater treatment: a review[J]. Membranes, 2020, 10(5): 89. |
| 35 | Tsushima I, Maeda K, Yamamoto T, et al. Continuous crystallization of phosphoric acid using suspension crystallizer: effect of operating conditions on purity of crystals[J]. Crystal Research and Technology, 2022, 57(1): 2100102. |
| 36 | Ma Y, Hu Z P, Wang M, et al. A simple method to study hemihydrate phosphoric acid crystals growth process[J]. Crystal Research and Technology, 2016, 51(5): 337-343. |
| 37 | Tang H. Purification of phosphoric acid by melt crystallization[D]. Saxony-Anhalt: Martin Luther University, 2016. |
| 38 | 王静康, 姜晓滨, 侯宝红, 等. 液膜结晶制备电子级磷酸的方法: 101774555B[P]. 2011-08-31. |
| Wang J K, Jiang X B, Hou B H, et al. A method for preparing electronic grade phosphoric acid through liquid membrane crystallization: 101774555B[P]. 2011-08-31. | |
| 39 | 刘海岛, 尹秋响. 熔融结晶及其耦合技术研究的进展[J]. 化学工业与工程, 2004, 21(5): 367-371. |
| Liu H D, Yin Q X. Progress in melt crystallization and its hybrid technique[J]. Chemical Industry and Engineering, 2004, 21(5): 367-371. | |
| 40 | Jiang X B, Xiao W, He G H. Falling film melt crystallization (Ⅲ): Model development, separation effect compared to static melt crystallization and process optimization[J]. Chemical Engineering Science, 2014, 117: 198-209. |
| 41 | 朱健. 熔融结晶法制备电子级磷酸的方法: 1843900[P]. 2006-10-11. |
| Zhu J. A process for preparaing electronic phosphoric acid with melt crystallization method: 1843900[P]. 2006-10-11. | |
| 42 | 林军, 吴小海, 苏杰文, 等. 一种列管结晶生产电子级磷酸的装置: 103896231B[P]. 2016-03-02. |
| Lin J, Wu X H, Su J W, et al. A device for producing electronic grade phosphoric acid through tube crystallization: 103896231B[P]. 2016-03-02. | |
| 43 | 林军, 吴小海, 苏杰文, 等. 一种电子级磷酸隔板结晶装置: 103771374B[P]. 2016-04-20. |
| Lin J, Wu X H, Su J W, et al. A kind of electron-level phosphoric acid baffle crystallization device: 103771374B[P]. 2016-04-20. | |
| 44 | 林军, 吴小海, 苏杰文, 等. 一种电子级磷酸热风加热结晶装置: 103771373B[P]. 2015-10-28. |
| Lin J, Wu X H, Su J W, et al. A kind of electron-level phosphoric acid hot-blast heating crystallization apparatus: 103771373B[P]. 2015-10-28. | |
| 45 | 程景才, 张碧玉, 杨超, 等. 一种制备电子级磷酸的熔融结晶器及其方法与电子级磷酸: 113842663A[P]. 2021-12-28. |
| Cheng J C, Zhang B Y, Yang C, et al. A melt crystallizer for preparing electronic-grade phosphoric acid, a method thereof and the electronic-grade phosphoric acid: 113842663A[P]. 2021-12-28. | |
| 46 | Jin Z X, Gao Z T, Gao Z Y, et al. Purification method of phosphoric acid through layercrystallization including washing operation: KR1020060111282A[P]. 2005-04-22. |
| 47 | 肖立华. 冷却结晶法制电子级磷酸的研究[D]. 昆明: 昆明理工大学, 2008. |
| Xiao L H. Study on preparation of electronic grade phosphoric acid by cooling crystallization[D]. Kunming: Kunming University of Science and Technology, 2008. | |
| 48 | Chen A M, Zhu J W, Chen K, et al. Melt suspension crystallization for purification of phosphoric acid[J]. Asia-Pacific Journal of Chemical Engineering, 2013, 8(3): 354-361. |
| 49 | Wang B M, Li J, Qi Y B, et al. Phosphoric acid purification by suspension melt crystallization: parametric study of the crystallization and sweating steps[J]. Crystal Research and Technology, 2012, 47(10): 1113-1120. |
| 50 | 姜晓滨. 熔融结晶法制备高纯磷酸过程研究[D]. 天津: 天津大学, 2012. |
| Jiang X B. Research on melt crystallization preparation process of hyperpure phosphate acid[D]. Tianjin: Tianjin University, 2012. | |
| 51 | 许史杰, 李嘉琦, 王彦飞, 等. 超声波辅助耦合熔融结晶制备超高纯邻氯对氨基甲苯的方法: 118439960A[P]. 2024-08-06. |
| Xu S J, Li J Q, Wang Y F, et al. A method for preparing ultra-high purity o-chloro-p-aminotoluene by ultrasonic-assisted coupling melt crystallization: 118439960A[P]. 2024-08-06. | |
| 52 | 王彦飞, 马明佳, 许史杰. 一种耦合熔融结晶制备高纯三烯丙基异氰脲酸酯的方法: 117800927A[P]. 2024-04-02. |
| Wang Y F, Ma M J, Xu S J. A method for preparing high-purity triallyl isocyanurate by coupling melt crystallization: 117800927A[P]. 2024-04-02. | |
| 53 | Beierling T, Gorny R, Sadowski G. Modeling growth rates in static layer melt crystallization[J]. Crystal Growth & Design, 2013, 13(12): 5229-5240. |
| 54 | Jia S Z, Yu L Y, Gao Z G, et al. Performance and analysis of key factors on the design of melt crystallization-based separation process[J]. AIChE Journal, 2023, 69(8): e18117. |
| 55 | Myasnikov S K. Melt entrapment and the effective distribution coefficient during the growth of a crystal layer[J]. Theoretical Foundations of Chemical Engineering, 2003, 37(1): 45-50. |
| 56 | Myasnikov S K. Transport of impurities out of a two-phase crystal layer into a melt under the effect of a temperature gradient: mechanisms and kinetics[J]. Theoretical Foundations of Chemical Engineering, 2003, 37(2): 137-143. |
| 57 | Taran A L, Nosov G A, Myasnikov S K, et al. Kinetics of crystallization of binary melts of eutectic-forming substances[J]. Theoretical Foundations of Chemical Engineering, 2004, 38(2): 164-168. |
| 58 | Ding S P, Huang X, Yin Q X, et al. Static layer melt crystallization: effects of impurities on the growth behaviors of crystal layers[J]. Separation and Purification Technology, 2021, 279: 119764. |
| 59 | Bai Y H, Zhu Z X, Yin Q X, et al. Facile model for predicting sweat mass and concentration in layer melt crystallization[J]. Industrial & Engineering Chemistry Research, 2022, 61(10): 3704-3712. |
| 60 | Guardani R, Neiro S M S, Bülau H, et al. Experimental comparison and simulation of static and dynamic solid layer melt crystallization[J]. Chemical Engineering Science, 2001, 56(7): 2371-2379. |
| 61 | Zheng R, Kennedy P K. A model for post-flow induced crystallization: general equations and predictions[J]. Journal of Rheology, 2004, 48(4): 823-842. |
| 62 | Urwin S J, Levilain G, Marziano I, et al. A structured approach to cope with impurities during industrial crystallization development[J]. Organic Process Research & Development, 2020, 24(8): 1443-1456. |
| 63 | Pietsch W. Agglomeration in Industry[M]. Weinheim: John Wiley & Sons, 2004. |
| 64 | Tsushima I, Maeda K, Arafune K, et al. Industrial crystallization of potassium sulfate using a suspension crystallizer: inclusion of mother liquor and an impurity distribution model[J]. Journal of Chemical Engineering of Japan, 2022, 55(4): 188-192. |
| 65 | 陈爱梅, 朱家文, 武斌, 等. 熔融悬浮结晶法提纯湿法磷酸[J]. 化学工程, 2012, 40(8): 52-56. |
| Chen A M, Zhu J W, Wu B, et al. Purification of wet-process phosphoric acid by melt suspension crystallization[J]. Chemical Engineering (China), 2012, 40(8): 52-56. | |
| 66 | Qin F G F, Chen X D, Free K. Freezing on subcooled surfaces, phenomena, modeling and applications[J]. International Journal of Heat and Mass Transfer, 2009, 52(5/6): 1245-1253. |
| 67 | Zipp G L, Randolph A D. Selective fines destruction in batch crystallization[J]. Industrial & Engineering Chemistry Research, 1989, 28(9): 1446-1448. |
| 68 | 林军, 吴小海, 苏杰文, 等. U型管静态多级熔融结晶法制备电子级磷酸: 103754848B[P]. 2016-06-08. |
| Lin J, Wu X H, Su J W, et al. A method for preparing an electronic-grade phosphoric acid by U-shaped pipe static multistage melt crystallization: 103754848B[P]. 2016-06-08. | |
| 69 | 钟本和, 陈亮, 李军, 等. 溶剂萃取法净化湿法磷酸的新进展[J]. 化工进展, 2005, 24(6): 596-602. |
| Zhong B H, Chen L, Li J, et al. New progress in purification of wet process phosphoric acid by solvent extraction[J]. Chemical Industry and Engineering Progress, 2005, 24(6): 596-602. | |
| 70 | 李翠莲, 杨云碧. 湿法磷酸净化技术及发展[J]. 资源节约与环保, 2019(3): 4. |
| Li C L, Yang Y B. Purification technology of wet phosphoric acid and its development[J]. Resources Economization & Environmental Protection, 2019(3): 4. | |
| 71 | 权晓威. 一种提纯肥料磷酸制电子级磷酸系统: 118079435A[P]. 2024-05-28. |
| Quan X W. An electronic grade phosphoric acid production system for purifying fertilizer phosphoric acid: 118079435A[P]. 2024-05-28. | |
| 72 | Hassene M, Drouglazet G. The attractions of melt station crystallization[J]. Chemical Engineering, 1995, 102(9): 108. |
| 73 | Myasnikov S K, Uteshinsky A D, Kulov N N. Hybrid of pervaporation and condensation-distillation crystallization: a new combined separation technology[J]. Theoretical Foundations of Chemical Engineering, 2003, 37(6): 527-532. |
| 74 | Myasnikov S K, Uteshinsky A D, Kulov N N. Kinetics of the washing of granules in the melt[J]. Theoretical Foundations of Chemical Engineering, 2001, 35(2): 107-118. |
| 75 | 孙虹. 焦油中精蒽/咔唑提取工艺的评述[J]. 煤炭转化, 1998, 21(2): 29-32. |
| Sun H. A review of abstraction pure anthracene and carbozle from tar[J]. Coal Conversion, 1998, 21(2): 29-32. | |
| 76 | 何洋江, 徐委岭, 戴朝云, 等. 一种亚氨基二苄提纯工艺: 118561754A[P]. 2024-08-30. |
| He Y J, Xu W L, Dai C Y, et al. An iminodibenzyl purification process: 118561754A[P]. 2024-08-30. | |
| 77 | Ban H, Cheng Y W, Wang L J, et al. Preparation of high-purity 2,6-naphthalenedicarboxylic acid from coal tar distillate[J]. Chemical Engineering & Technology, 2019, 42(6): 1188-1198. |
| 78 | 叶青, 王车礼, 裘兆蓉. 减压精馏-熔融结晶耦合装置提纯人造麝香的研究[J]. 现代化工, 2001, 21(1): 32-35. |
| Ye Q, Wang C L, Qiu Z R. Purification of artificial musk DDHI by combined vacuum distillation melt crystallization technology[J]. Modern Chemical Industry, 2001, 21(1): 32-35. | |
| 79 | Kontos S S, Katrivesis F K, Constantinou T C, et al. Implementation of membrane filtration and melt crystallization for the effective treatment and valorization of olive mill wastewaters[J]. Separation and Purification Technology, 2018, 193: 103-111. |
| [1] | 孟祥军, 杨林睿, 彭立培, 杨献奎, 花莹曦, 张人仁, 郑凯天, 许春建. 三氟化氮精馏分离流程的设计与控制[J]. 化工学报, 2025, 76(2): 707-717. |
| [2] | 郭恭涵, 丁晖殿, 李强, 贾胜坤, 钱行, 苑杨, 陈海胜, 罗祎青. 间歇精馏操作过程的动态贝叶斯优化方法[J]. 化工学报, 2025, 76(2): 755-768. |
| [3] | 杨林睿, 刘鉴漪, 李玲, 何永超, 郑凯天, 任建坡, 许春建. 苯/环己烷/环己烯萃取精馏过程的流程设计与节能[J]. 化工学报, 2025, 76(2): 731-743. |
| [4] | 齐琪, 郭利平, 石李明, 郑映, 潘鹏举. 山梨醇类成核剂改性聚丙烯及其共聚物的结晶行为与性能[J]. 化工学报, 2024, 75(7): 2688-2699. |
| [5] | 薛潇, 商敏静, 苏远海. 微反应器内药物连续流合成的研究进展[J]. 化工学报, 2024, 75(4): 1439-1454. |
| [6] | 张文惠, 唐茹意, 崔希利, 邢华斌. 羧酸端基Y型全氟聚醚的氟谱解析及结构表征[J]. 化工学报, 2024, 75(4): 1718-1723. |
| [7] | 刘静, 杨文博, 吕英迪, 陶胜洋. 喷雾-反溶剂结晶法制备掺杂铝粉的复合微球[J]. 化工学报, 2024, 75(4): 1724-1734. |
| [8] | 成文凯, 颜金钰, 王嘉骏, 冯连芳. 卧式捏合反应器及其在聚合工业中的研究进展[J]. 化工学报, 2024, 75(3): 768-781. |
| [9] | 陈饶, 赵鑫, 陈戴欣, 姜圣坤, 廉应江, 王金波, 杨梅, 陈光文. 微反应器内甲苯连续二硝化制备二硝基甲苯[J]. 化工学报, 2024, 75(3): 867-876. |
| [10] | 马韶阳, 徐涵卓, 张亮亮, 孙宝昌, 邹海魁, 罗勇, 初广文. 液-液非均相反应器研究进展[J]. 化工学报, 2024, 75(3): 727-742. |
| [11] | 刘昌会, 肖桐, 刘庆祎, 耿龙, 赵佳腾. 多孔二氧化钛强化的相变材料储热机理研究[J]. 化工学报, 2024, 75(2): 706-714. |
| [12] | 连斌, 龙妍, 徐啟蕾, 单宝明, 王学重, 张方坤. 间歇冷却结晶过程模型参数及操作敏感性分析[J]. 化工学报, 2024, 75(12): 4587-4595. |
| [13] | 郭磊磊, 吴震, 杨福胜, 张早校. 基于流通式金属氢化物反应器的氢高效分离提纯实验研究[J]. 化工学报, 2024, 75(12): 4576-4586. |
| [14] | 张德旺, 赵乾坤, 郭笑妮, 尧超群, 陈光文. 微反应器内牛顿/非牛顿流体液-液两相流流动和传质研究[J]. 化工学报, 2024, 75(11): 4162-4169. |
| [15] | 顾天宇, 陈献富, 王思琪, 徐鹏, 邱鸣慧, 范益群. 膜技术在杜仲有效成分分离纯化中的应用研究进展[J]. 化工学报, 2024, 75(11): 4005-4019. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号