化工学报 ›› 2019, Vol. 70 ›› Issue (4): 1429-1435.DOI: 10.11949/j.issn.0438-1157.20181396
收稿日期:2018-11-22
修回日期:2019-01-08
出版日期:2019-04-05
发布日期:2019-04-05
通讯作者:
左志军
作者简介:<named-content content-type="corresp-name">梁文胜</named-content>(1993—),男,硕士研究生,<email>13663434400@163.com</email>|左志军(1981—),男,博士,教授,<email>zuozhijun@tyut.edu.cn</email>
基金资助:
Wensheng LIANG( ),Jiangtao LIU,Yue ZHAO,Wei HUANG,Zhijun ZUO(
),Jiangtao LIU,Yue ZHAO,Wei HUANG,Zhijun ZUO( )
)
Received:2018-11-22
Revised:2019-01-08
Online:2019-04-05
Published:2019-04-05
Contact:
Zhijun ZUO
摘要:
在煤热解过程中加入特定的催化剂可以改变煤结构中相关化学键的结合能,使热解在相对温和的条件下进行,促使更多的小分子从煤结构上解离成为产物释放,并调节产物的产率和组成,提高转化率及产物的品质。由于煤化学结构的复杂性,从分子水平研究煤的催化热解行为非常困难。基于此,以煤的催化热解为背景,采用煤模型化合物,借助密度泛函理论(DFT),选取苯甲酸(C6H5COOH)为煤基模型,以NiO和Ni为催化剂,研究催化热解过程中催化剂价态改变对煤催化剂热解的作用。DFT结果显示,苯甲酸热解的主要路径为:C6H5COOH
中图分类号:
梁文胜, 刘江涛, 赵月, 黄伟, 左志军. NiO和Ni催化剂对苯甲酸热解机理的理论计算[J]. 化工学报, 2019, 70(4): 1429-1435.
Wensheng LIANG, Jiangtao LIU, Yue ZHAO, Wei HUANG, Zhijun ZUO. Theoretical calculation of effect of NiO and Ni catalysts for benzoic acid pyrolysis[J]. CIESC Journal, 2019, 70(4): 1429-1435.
| Catalyst | Specie | Site | E ads/eV | Bond length/nm |
|---|---|---|---|---|
| NiO(100) | C6H5COO | Nibri | -1.80 | d O—Ni =0.1980 |
| C6H5 | Otop | -1.10 | d C—O =0.1386 | |
| H | Otop | -1.20 | d H—O =0.0981 | |
| C6H6 | no bond | -0.01 | ||
| CO2 | Nitop,Otop | -0.05 | d C—O=0.1458,d O—Ni=0.2162 | |
| Ni (111) | C6H5COOH | top | -0.18 | d O—Ni =0.1987 |
| C6H6COO | bridge | -2.11 | d O—Ni =0.1956 | |
| C6H5COO | bridge | -3.08 | d O—Ni=0.1949 | |
| C6H5 | top | -2.88 | d O—Ni =0.1871 | |
| H | fcc | -2.35 | d H—Ni =0.1709 | |
| C6H6 | no bond | -0.05 | ||
| CO2 | no bond | -0.02 |
表1 NiO(100)和Ni(111)面上各中间体吸附能及其到吸附位点的键长
Table 1 Adsorption energies and geometrical parameters for relevant species on NiO(100) and Ni(111) surfaces
| Catalyst | Specie | Site | E ads/eV | Bond length/nm |
|---|---|---|---|---|
| NiO(100) | C6H5COO | Nibri | -1.80 | d O—Ni =0.1980 |
| C6H5 | Otop | -1.10 | d C—O =0.1386 | |
| H | Otop | -1.20 | d H—O =0.0981 | |
| C6H6 | no bond | -0.01 | ||
| CO2 | Nitop,Otop | -0.05 | d C—O=0.1458,d O—Ni=0.2162 | |
| Ni (111) | C6H5COOH | top | -0.18 | d O—Ni =0.1987 |
| C6H6COO | bridge | -2.11 | d O—Ni =0.1956 | |
| C6H5COO | bridge | -3.08 | d O—Ni=0.1949 | |
| C6H5 | top | -2.88 | d O—Ni =0.1871 | |
| H | fcc | -2.35 | d H—Ni =0.1709 | |
| C6H6 | no bond | -0.05 | ||
| CO2 | no bond | -0.02 |

图7 C6H5COOH在NiO(100)面上热解过程中的各基元反应的初态、过渡态以及末态构型
Fig.7 Geometrical structures of initial states, transition states, and final states for C6H5COOH pyrolysis on NiO(100) surface
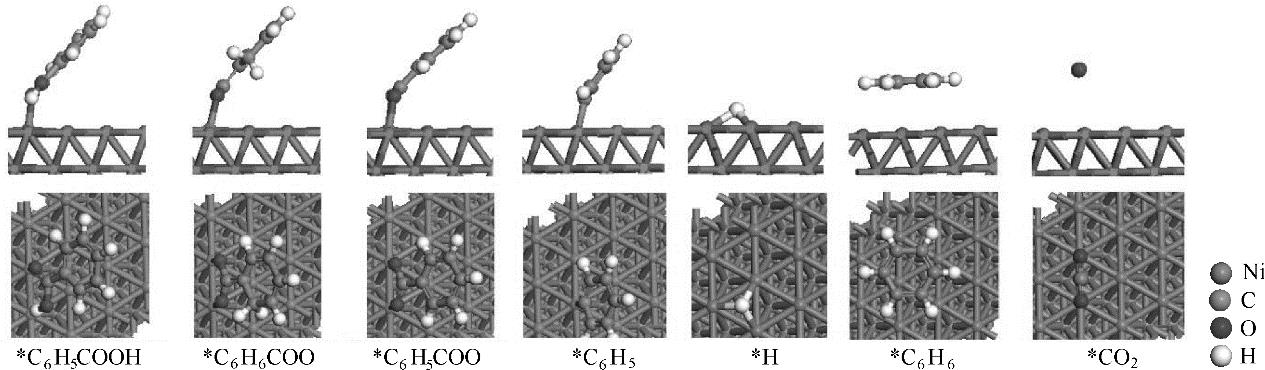
图8 Ni(111)面上C6H5COOH热解过程中各中间体最稳定吸附构型
Fig.8 The most stable adsorption structures of possible intermediates involved in pyrolysis of C6H5COOH on Ni(111) surface
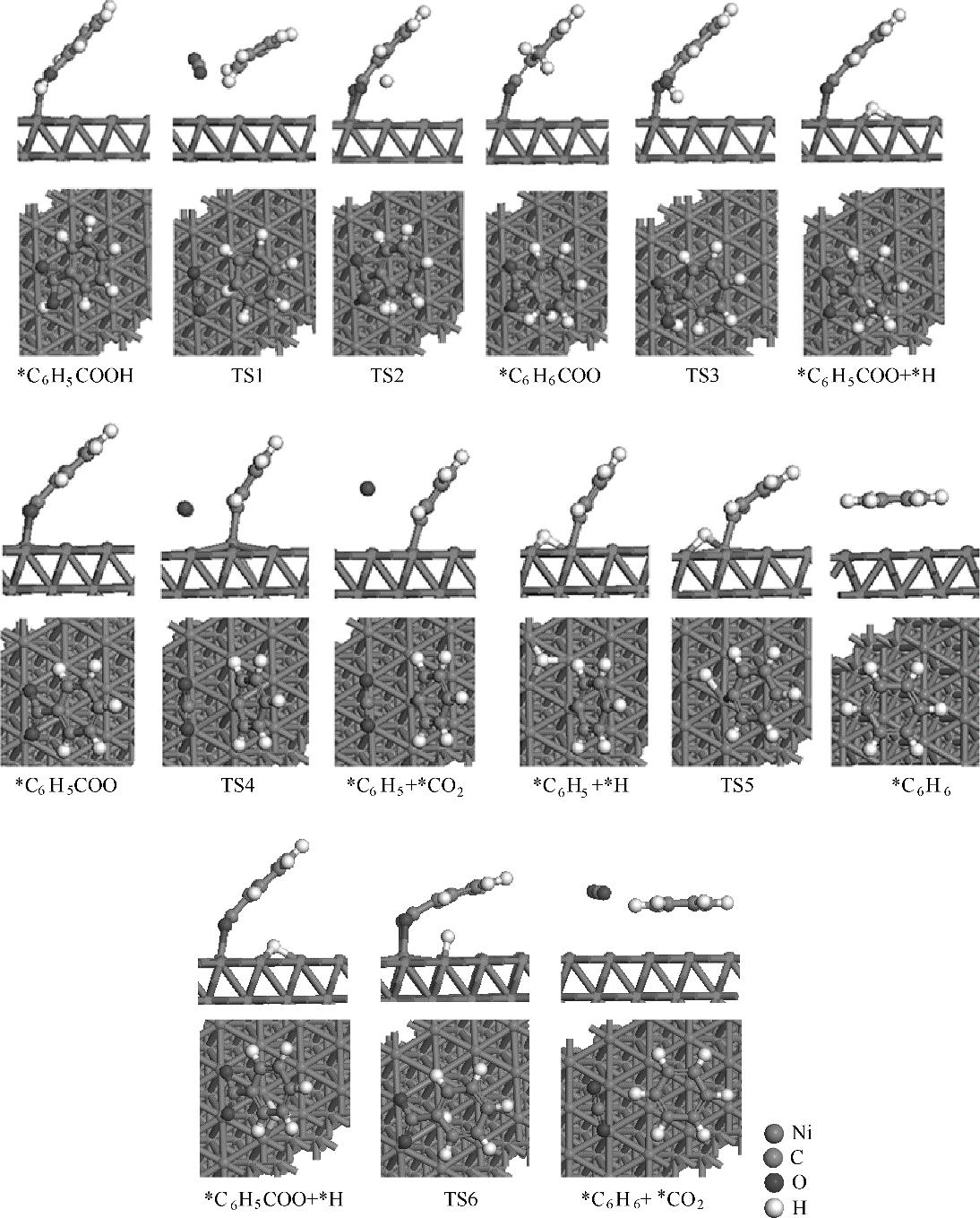
图10 C6H5COOH在Ni(111)面上热解过程中的各基元反应的初态、过渡态以及末态构型
Fig.10 Geometrical structures of initial states, transition states and final states for C6H5COOH pyrolysis on Ni(111) surface
| 1 | Han J , Wang X , Yue J , et al . Catalytic upgrading of coal pyrolysis tar over char-based catalysts[J]. Fuel Processing Technology, 2014, 122: 98-106. |
| 2 | Rombi E , Cutrufello M G , Atzori L , et al . CO methanation on Ni-Ce mixed oxides prepared by hard template method[J]. Applied Catalysis A: General, 2016, 515: 144-153. |
| 3 | Wang S G , Cao D B , Li Y W , et al . CO2 reforming of CH4 on Ni (111): a density functional theory calculation[J]. The Journal of Physical Chemistry B, 2006, 110(20): 9976-9983. |
| 4 | Wang S G , Liao X Y , Hu J , et al . Kinetic aspect of CO2 reforming of CH4 on Ni(111): a density functional theory calculation[J]. Surface Science, 2007, 601(5): 1271-1284. |
| 5 | Solomon P R , Serio M A , Carangelo R M , et al . Analysis of the Argonne premium coal samples by thermogravimetric Fourier transform infrared spectroscopy[J]. Energy & Fuels, 1990, 4(3): 319-333. |
| 6 | Choe S J , Kang H J , Park D H , et al . Adsorption and dissociation reaction of carbon dioxide on Ni (1 1 1) surface: molecular orbital study[J]. Applied Surface Science, 2001, 181(3/4): 265-276. |
| 7 | Kresse G , Furthmüller J . Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set[J]. Physical Review B, 1996, 54(16): 11169. |
| 8 | Kresse G , Furthmüller J . Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Computational Materials Science, 1996, 6(1): 15-50. |
| 9 | Blöchl P E . Projector augmented-wave method[J]. Physical Review B, 1994, 50(24): 17953-17979. |
| 10 | Perdew J P , Burke K , Ernzerhof M . Generalized gradient approximation made simple[J]. Physical Review Letters, 1996, 77(18): 3865. |
| 11 | Kresse G , Joubert D . From ultrasoft pseudopotentials to the projector augmented-wave method[J]. Physical Review B, 1999, 59(3): 1758. |
| 12 | Sheppard D , Xiao P , Chemelewski W , et al . A generalized solid-state nudged elastic band method[J]. J. Chem. Phys., 2012, 136(7): 074103. |
| 13 | Kong L , Li G , Jin L , et al . Pyrolysis behaviors of two coal-related model compounds on a fixed-bed reactor[J]. Fuel Processing Technology, 2015, 129: 113-119. |
| 14 | Li L , Fan H , Hu H . A theoretical study on bond dissociation enthalpies of coal based model compounds[J]. Fuel, 2015, 153: 70-77. |
| 15 | Wang M F , Zuo Z J , Ren R P , et al . Theoretical study on catalytic pyrolysis of benzoic acid as a coal-based model compound[J]. Energy & Fuels, 2016, 30(4): 2833-2840. |
| 16 | 凌丽霞, 赵俐娟, 章日光, 等 . 苯甲酸和苯甲醛热解机理的量子化学研究[J]. 化工学报, 2009, 60(5): 1224-1230. |
| Ling L X , Zhao L J , Zhang R G , et al . Pyrolysis mechanisms of benzoic acid and benzaldehyde based on quantum chemistry[J]. CIESC Journal, 2009, 60(5): 1224-1230. | |
| 17 | Xu B , Lu W , Sun Z , et al . High-quality oil and gas from pyrolysis of Powder River Basin coal catalyzed by an environmentally-friendly, inexpensive composite iron-sodium catalysts[J]. Fuel Processing Technology, 2017, 167: 334-344. |
| 18 | Rodriguez J A , Hanson J C , Frenkel A I , et al . Experimental and theoretical studies on the reaction of H2 with NiO: role of O vacancies and mechanism for oxide reduction[J]. Journal of the American Chemical Society, 2002, 124(2): 346-354. |
| 19 | Selcuk S , Selloni A . DFT+U study of the surface structure and stability of Co3O4(110): dependence on U[J]. The Journal of Physical Chemistry C, 2015, 119(18): 9973-9979. |
| 20 | Wang L , Maxisch T , Ceder G . Oxidation energies of transition metal oxides with in the GGA+U framework[J]. Physical Review B, 2006, 73(19): 195107. |
| 21 | Dudarev S L , Botton G A , Savrasov S Y , et al . Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+ U study[J]. Physical Review B, 1998, 57(3): 1505. |
| 22 | Hüfner S . Electronic structure of NiO and related 3d-transition-metal compounds[J]. Advances in Physics, 1994, 43(2): 183-356. |
| 23 | Yan M , Chen S P , Mitchell T E , et al . Atomistic studies of energies and structures of (hk0) surfaces in NiO[J]. Philosophical Magazine A, 1995, 72(1): 121-138. |
| 24 | Li L , Kanai Y . Antiferromagnetic structures and electronic energy levels at reconstructed NiO(111) surfaces: ADFT+U study[J]. Physical Review B, 2015, 91(23): 235304. |
| 25 | Zeng Y , Ma H , Zhang H , et al . Ni-Ce-Al composite oxide catalysts synthesized by solution combustion method: enhanced catalytic activity for CO methanation[J]. Fuel, 2015, 162: 16-22. |
| 26 | Kresse G , Hafner J . Ab initio molecular dynamics for liquid metals[J]. Physical Review B, 1993, 47(1): 558-561. |
| 27 | Rohrbach A , Hafner J , Kresse G . Molecular adsorption on the surface of strongly correlated transition-metal oxides: a case study for CO/NiO(100)[J]. Physical Review B, 2004, 69(7): 075413. |
| 28 | Wang B , Nisar J , Ahuja R . Molecular simulation for gas adsorption at NiO (100) surface[J]. ACS Applied Materials & Interfaces, 2012, 4(10): 5691-5697. |
| 29 | Eskay T P , Britt P F , Buchanan III A C . Pyrolysis of coal model compounds containing aromatic carboxylic acids: the role of carboxylic acids in cross-linking reactions in low-rank coal[R]. Oak Ridge National Lab., TN (United States), 1997. |
| 30 | Eskay T P , Britt P F , Buchanan A C . Does decarboxylation lead to cross-linking in low-rank coals?[J]. Energy & Fuels, 1996, 10(6): 1257-1261. |
| 31 | Manion J A , McMillen D F , Malhotra R . Decarboxylation and coupling reactions of aromatic acids under coal-liquefaction conditions[J]. Energy & Fuels, 1996, 10(3): 776-788. |
| [1] | 宋嘉豪, 王文. 斯特林发动机与高温热管耦合运行特性研究[J]. 化工学报, 2023, 74(S1): 287-294. |
| [2] | 连梦雅, 谈莹莹, 王林, 陈枫, 曹艺飞. 地下水预热新风一体化热泵空调系统制热性能研究[J]. 化工学报, 2023, 74(S1): 311-319. |
| [3] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [4] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [5] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [6] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [7] | 李科, 文键, 忻碧平. 耦合蒸气冷却屏的真空多层绝热结构对液氢储罐自增压过程的影响机制研究[J]. 化工学报, 2023, 74(9): 3786-3796. |
| [8] | 何松, 刘乔迈, 谢广烁, 王斯民, 肖娟. 高浓度水煤浆管道气膜减阻两相流模拟及代理辅助优化[J]. 化工学报, 2023, 74(9): 3766-3774. |
| [9] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [10] | 王浩, 王振雷. 基于自适应谱方法的裂解炉烧焦模型化简策略[J]. 化工学报, 2023, 74(9): 3855-3864. |
| [11] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [12] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [13] | 赵佳佳, 田世祥, 李鹏, 谢洪高. SiO2-H2O纳米流体强化煤尘润湿性的微观机理研究[J]. 化工学报, 2023, 74(9): 3931-3945. |
| [14] | 韩晨, 司徒友珉, 朱斌, 许建良, 郭晓镭, 刘海峰. 协同处理废液的多喷嘴粉煤气化炉内反应流动研究[J]. 化工学报, 2023, 74(8): 3266-3278. |
| [15] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号