化工学报 ›› 2021, Vol. 72 ›› Issue (10): 5159-5171.DOI: 10.11949/0438-1157.20210575
收稿日期:2021-04-25
修回日期:2021-08-09
出版日期:2021-10-05
发布日期:2021-10-05
通讯作者:
周昊
作者简介:周昊(1973—),男,博士,教授,基金资助:
Hao ZHOU( ),Qiwei WU,Fangzheng CHENG
),Qiwei WU,Fangzheng CHENG
Received:2021-04-25
Revised:2021-08-09
Online:2021-10-05
Published:2021-10-05
Contact:
Hao ZHOU
摘要:
采用火焰喷雾合成法制备了Sr2+、Cu2+分别取代A、B位的La0.8Sr0.2Mn1-xCuxO3 (x=0,0.1,0.2,0.3,0.4)钙钛矿催化剂,并用于CO催化氧化实验,研究了水蒸气和CO2对催化剂CO氧化活性的影响。对不同取代量La0.8Sr0.2Mn1-xCuxO3 催化剂进行了XRD、SEM、EDS、BET、XPS、H2-TPR和O2-TPD等表征测试。结果表明,火焰喷雾合成法制备的钙钛矿催化剂具有良好的钙钛矿相、疏松多孔结构和催化氧化活性。其中,La0.8Sr0.2Mn0.9Cu0.1O3分别在119.4℃和133.3℃实现50%和90%的CO转化率。掺杂水蒸气和CO2会与CO在催化剂表面形成竞争吸附,导致5种催化剂性能衰减,但La0.8Sr0.2Mn0.9Cu0.1O3仍能在150.2℃实现90%的CO催化转化,在连续稳定性催化氧化测试中,5种催化剂性能衰减不超过10%。结合上述CO催化氧化实验,火焰喷雾合成法制备的催化剂具有良好的稳定性和催化活性,适合制备高CO催化氧化活性的钙钛矿催化剂。
中图分类号:
周昊,伍其威,程方正. 火焰喷雾合成法制备La0.8Sr0.2Mn1-xCuxO3催化氧化CO性能研究[J]. 化工学报, 2021, 72(10): 5159-5171.
Hao ZHOU,Qiwei WU,Fangzheng CHENG. Preparation of La0.8Sr0.2Mn1-xCuxO3 by flame spray synthesis method and catalytic performance for CO oxidation[J]. CIESC Journal, 2021, 72(10): 5159-5171.
| 工况 | 体积分数/% | ||||
|---|---|---|---|---|---|
| CO | O2 | Ar | CO2 | 水蒸气 | |
| 1 | 1 | 10 | 89 | 0 | 0 |
| 2 | 1 | 10 | 74 | 10 | 5 |
表1 La0.8Sr0.2Mn1-xCuxO3(x=0,0.1,0.2,0.3,0.4)催化剂实验工况
Table 1 Experimental conditions for catalyst performance test
| 工况 | 体积分数/% | ||||
|---|---|---|---|---|---|
| CO | O2 | Ar | CO2 | 水蒸气 | |
| 1 | 1 | 10 | 89 | 0 | 0 |
| 2 | 1 | 10 | 74 | 10 | 5 |
| 催化剂 | 元素含量/% | |||
|---|---|---|---|---|
| La | Sr | Mn | Cu | |
| La0.8Sr0.2MnO3 | 42.02 | 9.48 | 48.50 | 0.00 |
| La0.8Sr0.2Mn0.9Cu0.1O3 | 41.74 | 8.02 | 44.25 | 5.99 |
| La0.8Sr0.2Mn0.8Cu0.2O3 | 39.98 | 8.89 | 39.32 | 11.81 |
| La0.8Sr0.2Mn0.7Cu0.3O3 | 41.44 | 10.22 | 32.13 | 16.22 |
| La0.8Sr0.2Mn0.6Cu0.4O3 | 38.11 | 11.12 | 29.07 | 21.70 |
表2 La0.8Sr0.2Mn1-xCuxO3(x=0,0.1,0.2,0.3,0.4)EDS结果
Table 2 EDS results of La0.8Sr0.2Mn1-xCuxO3(x=0,0.1,0.2,0.3,0.4)
| 催化剂 | 元素含量/% | |||
|---|---|---|---|---|
| La | Sr | Mn | Cu | |
| La0.8Sr0.2MnO3 | 42.02 | 9.48 | 48.50 | 0.00 |
| La0.8Sr0.2Mn0.9Cu0.1O3 | 41.74 | 8.02 | 44.25 | 5.99 |
| La0.8Sr0.2Mn0.8Cu0.2O3 | 39.98 | 8.89 | 39.32 | 11.81 |
| La0.8Sr0.2Mn0.7Cu0.3O3 | 41.44 | 10.22 | 32.13 | 16.22 |
| La0.8Sr0.2Mn0.6Cu0.4O3 | 38.11 | 11.12 | 29.07 | 21.70 |
| 催化剂 | 平均比表面积/(m2/g) |
|---|---|
| La0.8Sr0.2MnO3 | 9.85 |
| La0.8Sr0.2Mn0.9Cu0.1O3 | 12.65 |
| La0.8Sr0.2Mn0.8Cu0.2O3 | 12.96 |
| La0.8Sr0.2Mn0.7Cu0.3O3 | 15.87 |
| La0.8Sr0.2Mn0.6Cu0.4O3 | 17.68 |
表3 BET法测定La0.8Sr0.2Mn1-xCuxO3(x=0,0.1,0.2,0.3,0.4)比表面积
Table 3 Specific surface area of La0.8Sr0.2Mn1-xCuxO3(x=0,0.1,0.2,0.3,0.4)tested by BET
| 催化剂 | 平均比表面积/(m2/g) |
|---|---|
| La0.8Sr0.2MnO3 | 9.85 |
| La0.8Sr0.2Mn0.9Cu0.1O3 | 12.65 |
| La0.8Sr0.2Mn0.8Cu0.2O3 | 12.96 |
| La0.8Sr0.2Mn0.7Cu0.3O3 | 15.87 |
| La0.8Sr0.2Mn0.6Cu0.4O3 | 17.68 |
| 催化剂 | 元素含量比 | |
|---|---|---|
| Cu+/(Cu++Cu2+) | Mn4+/(Mn4++Mn3+) | |
| La0.8Sr0.2MnO3 | — | 0.48 |
| La0.8Sr0.2Mn0.9Cu0.1O3 | 0.31 | 0.64 |
| La0.8Sr0.2Mn0.8Cu0.2O3 | 0.56 | 0.73 |
| La0.8Sr0.2Mn0.7Cu0.3O3 | 0.64 | 0.85 |
| La0.8Sr0.2Mn0.6Cu0.4O3 | 0.58 | 0.78 |
表4 La0.8Sr0.2Mn1-xCuxO3(x=0,0.1,0.2,0.3,0.4)催化剂表面不同价态元素含量比
Table 4 Element valence state and relative content results of La0.8Sr0.2Mn1-xCuxO3(x=0,0.1,0.2,0.3,0.4)
| 催化剂 | 元素含量比 | |
|---|---|---|
| Cu+/(Cu++Cu2+) | Mn4+/(Mn4++Mn3+) | |
| La0.8Sr0.2MnO3 | — | 0.48 |
| La0.8Sr0.2Mn0.9Cu0.1O3 | 0.31 | 0.64 |
| La0.8Sr0.2Mn0.8Cu0.2O3 | 0.56 | 0.73 |
| La0.8Sr0.2Mn0.7Cu0.3O3 | 0.64 | 0.85 |
| La0.8Sr0.2Mn0.6Cu0.4O3 | 0.58 | 0.78 |
| 催化剂 | H2 消耗量/ (mmol/g) | O2 脱附量/ (mmol/g) | ||||
|---|---|---|---|---|---|---|
| L.T.R | H.T.R | Total | w | α-O2 | β-O2 | |
| La0.8Sr0.2MnO3 | 0.95 | 1.52 | 2.47 | 0.93 | 0.193 | 0.270 |
| La0.8Sr0.2Mn0.9Cu0.1O3 | 1.32 | 1.18 | 2.50 | 1.00 | 0.220 | 0.359 |
| La0.8Sr0.2Mn0.8Cu0.2O3 | 1.33 | 1.08 | 2.41 | 1.00 | 0.203 | 0.328 |
| La0.8Sr0.2Mn0.7Cu0.3O3 | 1.48 | 0.82 | 2.30 | 0.91 | 0.251 | 0.433 |
| La0.8Sr0.2Mn0.6Cu0.4O3 | 1.68 | 0.76 | 2.44 | 0.93 | 0.307 | 0.477 |
表5 La0.8Sr0.2Mn1-xCuxO3(x=0,0.1,0.2,0.3,0.4)的H2消耗量和O2脱附量
Table 5 H2 consumption and O2 desorption of La0.8Sr0.2Mn1-xCuxO3(x=0,0.1,0.2,0.3,0.4)
| 催化剂 | H2 消耗量/ (mmol/g) | O2 脱附量/ (mmol/g) | ||||
|---|---|---|---|---|---|---|
| L.T.R | H.T.R | Total | w | α-O2 | β-O2 | |
| La0.8Sr0.2MnO3 | 0.95 | 1.52 | 2.47 | 0.93 | 0.193 | 0.270 |
| La0.8Sr0.2Mn0.9Cu0.1O3 | 1.32 | 1.18 | 2.50 | 1.00 | 0.220 | 0.359 |
| La0.8Sr0.2Mn0.8Cu0.2O3 | 1.33 | 1.08 | 2.41 | 1.00 | 0.203 | 0.328 |
| La0.8Sr0.2Mn0.7Cu0.3O3 | 1.48 | 0.82 | 2.30 | 0.91 | 0.251 | 0.433 |
| La0.8Sr0.2Mn0.6Cu0.4O3 | 1.68 | 0.76 | 2.44 | 0.93 | 0.307 | 0.477 |
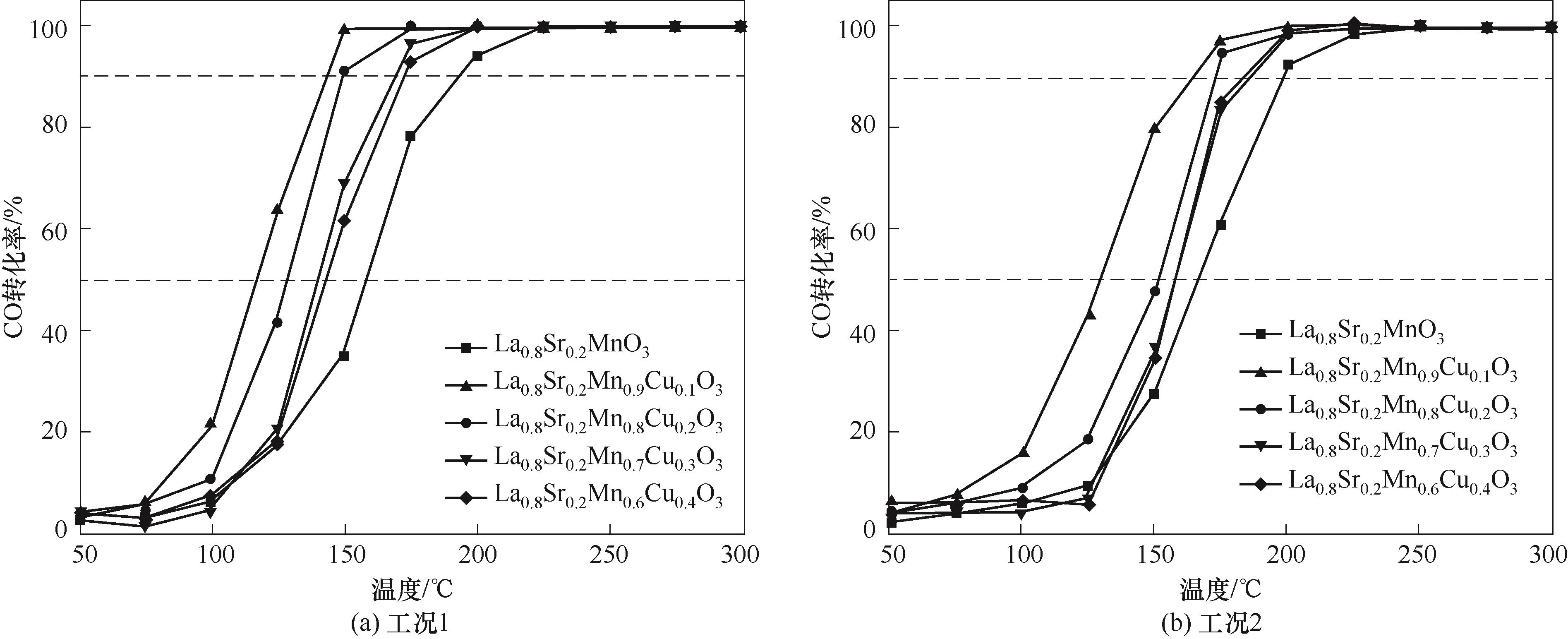
图9 La0.8Sr0.2Mn1-xCuxO3(x=0,0.1,0.2,0.3,0.4)在不同工况下的CO转化率
Fig.9 Catalytic performance for CO oxidation of La0.8Sr0.2Mn1-xCuxO3(x=0,0.1,0.2,0.3,0.4) under different conditions
工况1 催化剂循环 | La0.8Sr0.2MnO3 | La0.8Sr0.2Mn0.9Cu0.1O3 | La0.8Sr0.2Mn0.8Cu0.2O3 | La0.8Sr0.2Mn0.7Cu0.3O3 | La0.8Sr0.2Mn0.6Cu0.4O3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T50/℃ | T90/℃ | T50/℃ | T90/℃ | T50/℃ | T90/℃ | T50/℃ | T90/℃ | T50/℃ | T90/℃ | |
| 1st | 154.5 | 188.6 | 119.4 | 133.3 | 130.2 | 149.3 | 144.6 | 160.4 | 147.2 | 160.3 |
| 2nd | 157.2 | 188.3 | 120.4 | 135.3 | 132.6 | 150.2 | 147.8 | 163.6 | 146.3 | 161.7 |
| 3rd | 162.1 | 188.7 | 117.1 | 137.4 | 134.2 | 152.2 | 141.1 | 165.9 | 148.6 | 164.5 |
| 4th | 163.2 | 190.5 | 117.9 | 138.1 | 134.3 | 157.7 | 150.4 | 163.6 | 149.5 | 165.1 |
| 5th | 165.4 | 192.2 | 122.2 | 140.1 | 133.1 | 153.4 | 150.5 | 164.4 | 150.2 | 163.9 |
表6 工况1连续催化氧化实验中CO转化率为50%和90%时的对应温度
Table 6 Temperature of 50% and 90% conversion in consecutive CO catalytic oxidation of case 1
工况1 催化剂循环 | La0.8Sr0.2MnO3 | La0.8Sr0.2Mn0.9Cu0.1O3 | La0.8Sr0.2Mn0.8Cu0.2O3 | La0.8Sr0.2Mn0.7Cu0.3O3 | La0.8Sr0.2Mn0.6Cu0.4O3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T50/℃ | T90/℃ | T50/℃ | T90/℃ | T50/℃ | T90/℃ | T50/℃ | T90/℃ | T50/℃ | T90/℃ | |
| 1st | 154.5 | 188.6 | 119.4 | 133.3 | 130.2 | 149.3 | 144.6 | 160.4 | 147.2 | 160.3 |
| 2nd | 157.2 | 188.3 | 120.4 | 135.3 | 132.6 | 150.2 | 147.8 | 163.6 | 146.3 | 161.7 |
| 3rd | 162.1 | 188.7 | 117.1 | 137.4 | 134.2 | 152.2 | 141.1 | 165.9 | 148.6 | 164.5 |
| 4th | 163.2 | 190.5 | 117.9 | 138.1 | 134.3 | 157.7 | 150.4 | 163.6 | 149.5 | 165.1 |
| 5th | 165.4 | 192.2 | 122.2 | 140.1 | 133.1 | 153.4 | 150.5 | 164.4 | 150.2 | 163.9 |
工况2 催化剂循环 | La0.8Sr0.2MnO3 | La0.8Sr0.2Mn0.9Cu0.1O3 | La0.8Sr0.2Mn0.8Cu0.2O3 | La0.8Sr0.2Mn0.7Cu0.3O3 | La0.8Sr0.2Mn0.6Cu0.4O3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T50/℃ | T90/℃ | T50/℃ | T90/℃ | T50/℃ | T90/℃ | T50/℃ | T90/℃ | T50/℃ | T90/℃ | |
| 1st | 168.2 | 195.1 | 128.8 | 150.2 | 151.9 | 168.8 | 156.3 | 173.1 | 155.1 | 175.5 |
| 2nd | 174.9 | 198.2 | 133.9 | 152.1 | 152.1 | 169.3 | 157.8 | 177.2 | 158.7 | 178.3 |
| 3rd | 171.4 | 193.1 | 137.4 | 156.6 | 150.6 | 167.8 | 156.6 | 173.7 | 161.5 | 177.2 |
| 4th | 170.7 | 195.8 | 136.6 | 157.5 | 152.6 | 170.5 | 160.2 | 175.9 | 161.7 | 175.3 |
| 5th | 171.9 | 200.4 | 138.9 | 157.8 | 151.6 | 171.2 | 158.6 | 177.4 | 165.1 | 178.9 |
表7 工况2连续催化氧化实验中CO转化率为50%和90%时的对应温度
Table 7 Temperature of 50% and 90% conversion in consecutive CO catalytic oxidation of case 2
工况2 催化剂循环 | La0.8Sr0.2MnO3 | La0.8Sr0.2Mn0.9Cu0.1O3 | La0.8Sr0.2Mn0.8Cu0.2O3 | La0.8Sr0.2Mn0.7Cu0.3O3 | La0.8Sr0.2Mn0.6Cu0.4O3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T50/℃ | T90/℃ | T50/℃ | T90/℃ | T50/℃ | T90/℃ | T50/℃ | T90/℃ | T50/℃ | T90/℃ | |
| 1st | 168.2 | 195.1 | 128.8 | 150.2 | 151.9 | 168.8 | 156.3 | 173.1 | 155.1 | 175.5 |
| 2nd | 174.9 | 198.2 | 133.9 | 152.1 | 152.1 | 169.3 | 157.8 | 177.2 | 158.7 | 178.3 |
| 3rd | 171.4 | 193.1 | 137.4 | 156.6 | 150.6 | 167.8 | 156.6 | 173.7 | 161.5 | 177.2 |
| 4th | 170.7 | 195.8 | 136.6 | 157.5 | 152.6 | 170.5 | 160.2 | 175.9 | 161.7 | 175.3 |
| 5th | 171.9 | 200.4 | 138.9 | 157.8 | 151.6 | 171.2 | 158.6 | 177.4 | 165.1 | 178.9 |
| 催化剂 | 制备方法 | 反应条件 | 催化反应结果 | 文献 | |
|---|---|---|---|---|---|
| T50 | T90 | ||||
| LaMnO3 | 熔盐法 | 1% CO,1.25% O2,97.75% Ar | 212.0℃ | 250.0℃ | [ |
| LaMn0.8Fe0.2O3 | 187.0℃ | 199.0℃ | |||
| La0.8Sr0.2MnO3 | 溶胶-凝胶法 | 1.5% CO,98.5% Air | >200℃ | >200℃ | [ |
| Au/LaMnO3 | 溶胶-凝胶法 | 1% CO,20% O2,79%Ar | 150℃ | 175℃ | [ |
| LaMnO3 | Pechini法 | 2% CO,20% O2,78% Ar | 223.5℃ | 390.8℃ | [ |
| La0.8Sr0.2MnO3 | 火焰喷雾合成法 | 1% CO,10% O2,89% Ar | 154.5℃ | 188.6℃ | 本文 |
| La0.8Sr0.2Mn0.9Cu0.1O3 | 119.4℃ | 133.3℃ | |||
表8 文献中具有代表性钙钛矿催化剂与本文La0.8Sr0.2Mn1-xCuxO3 (0 ≤ x < 1)催化剂对CO氧化的催化性能比较
Table 8 Comparison of La0.8Sr0.2Mn1-xCuxO3 (0≤x<1) catalyst for CO oxidation between references and this work
| 催化剂 | 制备方法 | 反应条件 | 催化反应结果 | 文献 | |
|---|---|---|---|---|---|
| T50 | T90 | ||||
| LaMnO3 | 熔盐法 | 1% CO,1.25% O2,97.75% Ar | 212.0℃ | 250.0℃ | [ |
| LaMn0.8Fe0.2O3 | 187.0℃ | 199.0℃ | |||
| La0.8Sr0.2MnO3 | 溶胶-凝胶法 | 1.5% CO,98.5% Air | >200℃ | >200℃ | [ |
| Au/LaMnO3 | 溶胶-凝胶法 | 1% CO,20% O2,79%Ar | 150℃ | 175℃ | [ |
| LaMnO3 | Pechini法 | 2% CO,20% O2,78% Ar | 223.5℃ | 390.8℃ | [ |
| La0.8Sr0.2MnO3 | 火焰喷雾合成法 | 1% CO,10% O2,89% Ar | 154.5℃ | 188.6℃ | 本文 |
| La0.8Sr0.2Mn0.9Cu0.1O3 | 119.4℃ | 133.3℃ | |||
| 1 | 邢献军, 邢勇强, 虞浸, 等. 生活垃圾燃烧烟气中CO和NO的排放特性[J]. 过程工程学报, 2017, 17(4): 866-872. |
| Xing X J, Xing Y Q, Yu J, et al. Characteristics of CO and NO emission from municipal solid waste combustion[J]. The Chinese Journal of Process Engineering, 2017, 17(4): 866-872. | |
| 2 | 廖继勇,郑浩翔,甘敏, 等. 烧结烟气CO的产生及治理途径——生成机理及排放规律[J]. 烧结球团, 2021, 46(2): 1-7. |
| Liao J Y, Zheng H X, Gan M, et al. Generation and governance way of CO in sintering flue gas—formation mechanism and emission law[J]. Sintering and Pelletizing, 2021, 46(2): 1-7. | |
| 3 | Liu C, Yin P, Chen R J, et al. Ambient carbon monoxide and cardiovascular mortality: a nationwide time-series analysis in 272 cities in China[J]. The Lancet Planetary Health, 2018, 2(1): e12-e18. |
| 4 | 彭雪, 芦琛璘, 卢滇楠. 氧气和一氧化碳在人血红蛋白迁移过程研究[J]. 化工学报, 2020, 71(2): 724-735. |
| Peng X, Lu C L, Lu D N. Investigation on migration process of oxygen and carbon monoxide in human hemoglobin[J]. CIESC Journal, 2020, 71(2): 724-735. | |
| 5 | Liu Y X, Dai H X, Deng J G, et al. Mesoporous Co3O4-supported gold nanocatalysts: highly active for the oxidation of carbon monoxide, benzene, toluene, and o-xylene[J]. Journal of Catalysis, 2014, 309: 408-418. |
| 6 | 刘朋飞, 张所瀛, 杨祝红, 等. MOF作模板制备多孔Au/CuxO催化剂及其CO氧化性能[J]. 化工学报, 2016, 67(6): 2325-2331. |
| Liu P F, Zhang S Y, Yang Z H, et al. MOF-templated preparation of porous Au/Cux0 catalysts with octahedral structures for Co oxidation[J]. CIESC Journal, 2016, 67(6): 2325-2331. | |
| 7 | 朱丽, 邓芸, 宋宇鹏, 等. MnOx对Pt/CeZrO2催化剂在水汽和CO2环境下CO氧化的影响[J]. 化工学报, 2018, 69(4): 1484-1492. |
| Zhu L, Deng Y, Song Y P, et al. Effect of MnOx on Pt/CeZrO2 catalyzed CO oxidation under water vapor and CO2 conditions[J]. CIESC Journal, 2018, 69(4): 1484-1492. | |
| 8 | Li C P, Li T, Wang B, et al. Synthesis of La1-xSrxMnO3 cubic crystals with adjustable doping levels[J]. Journal of Crystal Growth, 2006, 295(2): 137-140. |
| 9 | 刘晓刚, 魏波, 史芸菲, 等. La1-xLixMnO3钙钛矿催化剂同时消除NO和碳烟催化性能[J]. 化工学报, 2020, 71(3): 1053-1059. |
| Liu X G, Wei B, Shi Y F, et al. Simultaneous removal of NO and soot over La1-xLixMnO3 perovskite[J]. CIESC Journal, 2020, 71(3): 1053-1059. | |
| 10 | 苏迎辉, 郑浩, 张磊, 等. LaMn1-x-yFexCoyO3-δ钙钛矿载氧体用于化学链部分氧化[J]. 化工学报, 2020, 71(11): 5265-5277. |
| Su Y H, Zheng H, Zhang L, et al. LaMn1-x-yFexCoyO3-δ perovskite based oxygen carriers for chemical looping partial oxidation structures for CO oxidation[J]. CIESC Journal, 2020, 71(11): 5265-5277. | |
| 11 | Bartoň J, Pour V. Oxidation of carbon monoxide by oxygen over pure and platinum-doped LaMnO3 perovskites[J]. Collection of Czechoslovak Chemical Communications, 1990, 55(8): 1928-1934. |
| 12 | Frozandeh-Mehr E, Malekzadeh A, Ghiasi M, et al. Effect of partial substitution of lanthanum by strontium or bismuth on structural features of the lanthanum manganite nanoparticles as a catalyst for carbon monoxide oxidation[J]. Catalysis Communications, 2012, 28: 32-37. |
| 13 | Teng F, Han W, Liang S H, et al. Catalytic behavior of hydrothermally synthesized La0.5Sr0.5MnO3 single-crystal cubes in the oxidation of CO and CH4[J]. Journal of Catalysis, 2007, 250(1): 1-11. |
| 14 | Kucharczyk B. Catalytic oxidation of carbon monoxide on Pd-containing LaMnO3 perovskites[J]. Catalysis Letters, 2015, 145(6): 1237-1245. |
| 15 | Gao Z M, Wang H S, Ma H W, et al. Preparation and characterization of the non-stoichiometric La-Mn perovskites[J]. Journal of Alloys and Compounds, 2015, 646: 73-79. |
| 16 | Wu Y H, Li L L, Chu B X, et al. Catalytic reduction of NO by CO over B-site partially substituted LaM0.25Co0.75O3 (M=Cu, Mn, Fe) perovskite oxide catalysts: the correlation between physicochemical properties and catalytic performance[J]. Applied Catalysis A: General, 2018, 568: 43-53. |
| 17 | Tarjomannejad A, Niaei A, Farzi A, et al. Catalytic oxidation of CO over LaMn1-xBxO3 (B= Cu, Fe) perovskite-type oxides[J]. Catalysis Letters, 2016, 146(8): 1544-1551. |
| 18 | Huang X H, Pan H Y, Chen K, et al. Facile synthesis of porous spherical La0.8Sr0.2Mn1–xCuxO3(0≤x≤0.4) and nanocubic La0.8Sr0.2MnO3 with high catalytic activity for CO[J]. CrystEngComm, 2018, 20(43): 7020-7029. |
| 19 | Xu G, Bai H W, Huang X Q, et al. Self-assembled 3D flower-like perovskite PbTiO3 nanostructures and their application in the catalytic oxidation of CO[J]. Journal of Materials Chemistry A, 2015, 3(2): 547-554. |
| 20 | 宗毅晨. 钛基纳米功能材料的火焰合成与反应特性研究[D]. 北京: 清华大学, 2016. |
| Zong Y C. Titanium-based functional nanomaterials: flame aerosol synthesis and reactions[D]. Beijing: Tsinghua University, 2016. | |
| 21 | 卫吉丽, 任翊华, 宗毅晨, 等. 高旋流数管状火焰纳米颗粒合成实验研究[J]. 工程热物理学报, 2018, 39(3): 670-674. |
| Wei J L, Ren Y H, Zong Y C, et al. Synthesis of nanoparticles in high swirl number tubular flames[J]. Journal of Engineering Thermophysics, 2018, 39(3): 670-674. | |
| 22 | Zhou H, Qi B Y. Investigation of flame spray synthesized La1-xSrxCoO3 perovskites with promotional catalytic performances on CO oxidation[J]. Journal of the Energy Institute, 2020, 93(6): 2381-2387. |
| 23 | Abe Y, Laine R M. Photocatalytic plate-like La2Ti2O7 nanoparticles synthesized via liquid-feed flame spray pyrolysis (LF-FSP) of metallo-organic precursors[J]. Journal of the American Ceramic Society, 2020, 103(9): 4832-4839. |
| 24 | Kim B J, Fabbri E, Abbott D F, et al. Functional role of Fe-doping in co-based perovskite oxide catalysts for oxygen evolution reaction[J]. Journal of the American Chemical Society, 2019, 141(13): 5231-5240. |
| 25 | Kim M S, Yang J B, Medvedeva J, et al. Electronic structure of La0.7Sr0.3Mn1–xCuxO3(0.0≤x≤0.30)[J]. Journal of Physics: Condensed Matter, 2008, 20(25): 255228. |
| 26 | Jia L S, Gao J, Fang W P, et al. Influence of copper content on structural features and performance of pre-reduced LaMn1-xCuxO3 (0≤x<1) catalysts for methanol synthesis from CO2/H2[J]. Journal of Rare Earths, 2010, 28(5): 747-751. |
| 27 | 蒯龙. 硝酸盐喷雾热解法制备介孔金属氧化物及其应用研究[D]. 芜湖: 安徽师范大学, 2016. |
| Kuai L. A study of mesoporous metal oxides' preparation and applications by the nitrates-spray-pyrolysis method[D]. Wuhu: Anhui Normal University, 2016. | |
| 28 | Da Y M, Zeng L R, Wang C Y, et al. Catalytic oxidation of diesel soot particulates over Pt substituted LaMn1-xPtxO3 perovskite oxides[J]. Catalysis Today, 2019, 327: 73-80. |
| 29 | 余文冲. 钙钛矿型锰基催化剂结构调控及其催化碳烟燃烧性能研究[D]. 重庆: 重庆大学, 2017. |
| Yu W C. Study on the structure regulation of perovskite type Mn-based catalysts and their catalytic performance on carbon soot combustion[D]. Chongqing: Chongqing University, 2017. | |
| 30 | 吴耀辉. 复合调变锰基钙钛矿的制备及其催化性能的研究[D]. 南宁: 广西大学, 2019. |
| Wu Y H. Synthesis and catalytic behaviors of muitiply optimized manganese-based perovskites[D]. Nanning: Guangxi University, 2019. | |
| 31 | Zhang C H, Zeng K, Wang C, et al. LaMnO3 perovskites via a facile nickel substitution strategy for boosting propane combustion performance[J]. Ceramics International, 2020, 46(5): 6652-6662. |
| 32 | Patcas F, Buciuman F C, Zsako J. Oxygen non-stoichiometry and reducibility of B-site substituted lanthanum manganites[J]. Thermochimica Acta, 2000, 360(1): 71-76. |
| 33 | Tien-Thao N, Alamdari H, Zahedi-Niaki M H, et al. LaCo1-xCuxO3-δ perovskite catalysts for higher alcohol synthesis[J]. Applied Catalysis A: General, 2006, 311: 204-212. |
| 34 | Rossetti I, Biffi C, Forni L. Oxygen non-stoichiometry in perovskitic catalysts: impact on activity for the flameless combustion of methane[J]. Chemical Engineering Journal, 2010, 162(2): 768-775. |
| 35 | Arandiyan H, Scott J, Wang Y, et al. Meso-molding three-dimensional macroporous perovskites: a new approach to generate high-performance nanohybrid catalysts[J]. ACS Applied Materials & Interfaces, 2016, 8(4): 2457-2463. |
| 36 | Liu T K, Yao Y Y, Wei L Q, et al. Preparation and evaluation of copper-manganese oxide as a high-efficiency catalyst for CO oxidation and NO reduction by CO[J]. The Journal of Physical Chemistry C, 2017, 121(23): 12757-12770. |
| 37 | Li X, Wang L J, Xia Q B, et al. Catalytic oxidation of toluene over copper and manganese based catalysts: effect of water vapor[J]. Catalysis Communications, 2011, 14(1): 15-19. |
| 38 | Yan X K, Huang Q, Li B, et al. Catalytic performance of LaCo0.5M0.5O3 (M=Mn, Cr, Fe, Ni, Cu) perovskite-type oxides and LaCo0.5Mn0.5O3 supported on cordierite for CO oxidation[J]. Journal of Industrial and Engineering Chemistry, 2013, 19(2): 561-565. |
| 39 | Deng Y, Wang T, Zhu L, et al. Enhanced performance of CO oxidation over Pt/CuCrOx catalyst in the presence of CO2 and H2O[J]. Applied Surface Science, 2018, 442: 613-621. |
| 40 | Zeng L P, Li K Z, Huang F, et al. Effects of Co3O4 nanocatalyst morphology on CO oxidation: synthesis process map and catalytic activity[J]. Chinese Journal of Catalysis, 2016, 37(6): 908-922. |
| 41 | Huang X H, Niu P J, Pan H Y, et al. Micromorphological control of porous LaMnO3 and LaMn0.8Fe0.2O3 and its catalytic oxidation performance for CO[J]. Journal of Solid State Chemistry, 2018, 265: 218-226. |
| 42 | Peng P Y, Tsai Y C, Yeh J T, et al. Influence of Sr substitution on catalytic performance of LaMnO3/Ni metal foam composite for CO oxidation[J]. Aerosol and Air Quality Research, 2015, 15(4): 1662-1671. |
| 43 | 何成欢, 郭杨龙, 郭耘, 等. 不同组成和结构LaMnO3钙钛矿负载Au催化剂的CO氧化活性[J]. 物理化学学报, 2019, 35(4): 422-430. |
| He C H, Guo Y L, Guo Y, et al. Catalytic activity of Au nanoparticles supported on LaMnO3 perovskite with different composition and structure[J]. Acta Physico-Chimica Sinica, 2019, 35(4): 422-430. | |
| 44 | Banisharif F, Dehghani M R, Mazloom G, Hojatpanah Y. Oxidation of greenhouse gases, CH4 and CO, over LaMnxNi1-xO3±δ mixed oxide[J]. Iranian Journal of Catalysis, 2019, 9(4): 357-368. |
| [1] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [2] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [3] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [4] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [5] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [6] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [7] | 韩晨, 司徒友珉, 朱斌, 许建良, 郭晓镭, 刘海峰. 协同处理废液的多喷嘴粉煤气化炉内反应流动研究[J]. 化工学报, 2023, 74(8): 3266-3278. |
| [8] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [9] | 张曼铮, 肖猛, 闫沛伟, 苗政, 徐进良, 纪献兵. 危废焚烧处理耦合有机朗肯循环系统工质筛选与热力学优化[J]. 化工学报, 2023, 74(8): 3502-3512. |
| [10] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [11] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [12] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [13] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [14] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [15] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号