化工学报 ›› 2021, Vol. 72 ›› Issue (2): 748-760.DOI: 10.11949/0438-1157.20201063
收稿日期:2020-07-30
修回日期:2020-09-22
出版日期:2021-02-05
发布日期:2021-02-05
通讯作者:
王运东
作者简介:于涛(1997—),男,博士研究生,基金资助:
YU Tao1( ),WANG Yundong1(
),WANG Yundong1( ),LIU Zuohua2,MA Jianxiu3,JING Yu3
),LIU Zuohua2,MA Jianxiu3,JING Yu3
Received:2020-07-30
Revised:2020-09-22
Online:2021-02-05
Published:2021-02-05
Contact:
WANG Yundong
摘要:
目前硫化氢(H2S)日益成为制约工业化发展的一个重要因素,即使在较低的浓度下仍会破坏环境、腐蚀设备。如何能将含硫气体清洁、高效、深度脱除已成为国内外学者的研究重点。吸附法脱硫以其脱硫程度高、高效环保、操作简单和吸附剂可重复利用等优点具有良好的发展前景。研制一种选择性好、吸附容量大、性能稳定、再生性好的吸附剂是吸附法脱硫的关键。本文以深度脱除H2S为目标,综述了碳基材料、多孔金属氧化物、沸石分子筛、金属有机框架(MOFs)等几种不同吸附材料的研究进展,总结深度脱硫今后的发展方向,旨在为后续的研究提供借鉴。
中图分类号:
于涛, 王运东, 刘作华, 马建修, 靖宇. 硫化氢深度吸附材料的研究进展[J]. 化工学报, 2021, 72(2): 748-760.
YU Tao, WANG Yundong, LIU Zuohua, MA Jianxiu, JING Yu. Research progress of hydrogen sulfide deep adsorption materials[J]. CIESC Journal, 2021, 72(2): 748-760.
| 材料 | 比表面积/(m2/g) | 孔体积/ (cm3/g) | 平均孔径/nm | 气体组成 | 操作 温度/℃ | 穿透容量①/(mg/g) | 文献 |
|---|---|---|---|---|---|---|---|
| WSC | 860 | 0.40 | 0.7 | 50% H2, 15% CO2, 9% CO, 2% N2, 24% H2O, 10-3 H2S 50% H2, 15% CO2, 9% CO, 2% N2, 24% H2O, 10-3 H2S | 150 150 | 71(0.2) | [ |
| WSCU | 945 | 0.43 | 0.8 | 133(0.2) | [ | ||
| S208C | 898 | 0.48 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 27.2(ND) | [ |
| S-A | 905 | 0.48 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 26.4(ND) | [ |
| S-AU | 640 | 0.34 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 34.0(ND) | [ |
| S-AM | 37 | 0.02 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 0.4(-) | [ |
| S-B | 882 | 0.47 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 17.6(ND) | [ |
| S-BU | 808 | 0.43 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 54.1(ND) | [ |
| S-BM | 732 | 0.39 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 71.9(ND) | [ |
| AC-KOH/KI | 1042 | 0.42 | — | 2% O2, 10-4 H2S, 90%相对湿度, N2平衡 | 45 45 | 65.6(0.2) | [ |
| 10-4 H2S, 90%相对湿度, N2平衡 | 34.9(0.2) | [ | |||||
| RB2-NaOH50 | 758 | 0.25 | 1.56 | 2.5×10-4 H2S, N2平衡 | 25 | 43.5(ND) | [ |
| AC-NaOH | — | — | — | 3×10-3 H2S, He平衡 | 30 | 0.4(10) | [ |
| AC-KI | — | — | — | 3×10-3 H2S, He平衡 | 30 | 1.56(<10) | [ |
| AC-Na2CO3 | — | — | — | 3×10-3 H2S, He平衡 | 30 | 1.58(<10) | [ |
| AC-KOH | — | — | — | 3×10-3 H2S, He平衡 | 30 | 1.58(<10) | [ |
| GAC | 1678±41 | 0.75±0.03 | 0.89±0.16 | 80% H2O, 1% H2S, 空气 80% H2O, 1% H2S, 空气 | 25 25 | 53(N) | [ |
| MgO-GAC | 1358±39 | 0.60±0.01 | 0.88±0.23 | 275(N) | [ | ||
| Cu0.5Zn0.5/AC | 570 | 0.76 | — | 10-4 H2S, 2.5×10-3 O2, 50%相对湿度, N2平衡 | 30 | 25.0(ND) | [ |
| NORIT | 978 | 0.30 | — | 5×10-5 H2S, N2平衡 | — | - (<0.5) | [ |
表1 不同活性炭的结构参数及H2S吸附性能
Table 1 Parameters and adsorption ability of active carbon materials for H2S removal
| 材料 | 比表面积/(m2/g) | 孔体积/ (cm3/g) | 平均孔径/nm | 气体组成 | 操作 温度/℃ | 穿透容量①/(mg/g) | 文献 |
|---|---|---|---|---|---|---|---|
| WSC | 860 | 0.40 | 0.7 | 50% H2, 15% CO2, 9% CO, 2% N2, 24% H2O, 10-3 H2S 50% H2, 15% CO2, 9% CO, 2% N2, 24% H2O, 10-3 H2S | 150 150 | 71(0.2) | [ |
| WSCU | 945 | 0.43 | 0.8 | 133(0.2) | [ | ||
| S208C | 898 | 0.48 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 27.2(ND) | [ |
| S-A | 905 | 0.48 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 26.4(ND) | [ |
| S-AU | 640 | 0.34 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 34.0(ND) | [ |
| S-AM | 37 | 0.02 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 0.4(-) | [ |
| S-B | 882 | 0.47 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 17.6(ND) | [ |
| S-BU | 808 | 0.43 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 54.1(ND) | [ |
| S-BM | 732 | 0.39 | — | 60% CH4, 40% CO2, 10-3 H2S, 70% H2O预加湿 | — | 71.9(ND) | [ |
| AC-KOH/KI | 1042 | 0.42 | — | 2% O2, 10-4 H2S, 90%相对湿度, N2平衡 | 45 45 | 65.6(0.2) | [ |
| 10-4 H2S, 90%相对湿度, N2平衡 | 34.9(0.2) | [ | |||||
| RB2-NaOH50 | 758 | 0.25 | 1.56 | 2.5×10-4 H2S, N2平衡 | 25 | 43.5(ND) | [ |
| AC-NaOH | — | — | — | 3×10-3 H2S, He平衡 | 30 | 0.4(10) | [ |
| AC-KI | — | — | — | 3×10-3 H2S, He平衡 | 30 | 1.56(<10) | [ |
| AC-Na2CO3 | — | — | — | 3×10-3 H2S, He平衡 | 30 | 1.58(<10) | [ |
| AC-KOH | — | — | — | 3×10-3 H2S, He平衡 | 30 | 1.58(<10) | [ |
| GAC | 1678±41 | 0.75±0.03 | 0.89±0.16 | 80% H2O, 1% H2S, 空气 80% H2O, 1% H2S, 空气 | 25 25 | 53(N) | [ |
| MgO-GAC | 1358±39 | 0.60±0.01 | 0.88±0.23 | 275(N) | [ | ||
| Cu0.5Zn0.5/AC | 570 | 0.76 | — | 10-4 H2S, 2.5×10-3 O2, 50%相对湿度, N2平衡 | 30 | 25.0(ND) | [ |
| NORIT | 978 | 0.30 | — | 5×10-5 H2S, N2平衡 | — | - (<0.5) | [ |
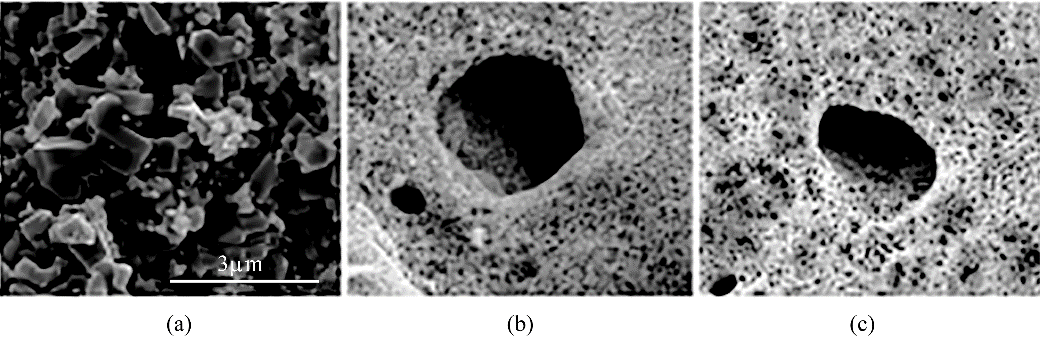
图3 市售ZnO (a)、多孔ZnO (b)与Ni掺杂多孔ZnO (c)的SEM图(图中标尺相同)[34]
Fig.3 SEM images of commercial ZnO (a), porous ZnO (b) and Ni-doped ZnO (c) (The scale bar is the same for all images)[34]
| 材料 | 比表面积/(m2/g) | 孔体积/(cm3/g) | 平均孔径/nm | 气体组成 | 操作温度/℃ | 饱和容量①/(mg/g) | 穿透容量①/(mg/g) | 文献 |
|---|---|---|---|---|---|---|---|---|
| 3DOM-Fe2O3 | 16~44 | — | 20~33 | 5% H2, 3×10-4 H2S, N2平衡 5% H2, 3×10-4 H2S, N2平衡 | 350 350 | — | 37.18(<0.2) | [ |
| 3DOM-Fe2O3/SiO2 | 80~220 | — | 6~17 | — | 38.92(<0.2) | [ | ||
| MFT | — | — | — | 39.8% H2, 32.6% CO2, 2.5×10-3 H2S, N2平衡 | 400 | 216(N) | — | [ |
| Engelhard ZnO | 53.13 | 0.28 | — | 8×10-6 H2S, 34.4% H2, 20% H2O, N2平衡 | 300 | — | 28.1(0.02) | [ |
| ZSCo-0 | 151.3 | 0.42 | 7.40 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 174.6 | 117.8(ND) | [ |
| ZSCo-0.05 | 188.7 | 0.58 | 7.82 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 195.2 | 146.4(ND) | [ |
| ZSCo-0.15 | 206.4 | 0.63 | 9.58 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 208.4 | 169.8(ND) | [ |
| ZSCo-0.3 | 192.1 | 0.55 | 9.24 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 230.5 | 188.4(ND) | [ |
| ZSCo-0.50 | 161.5 | 0.46 | 7.53 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 194.1 | 143.4(ND) | [ |
| Extruded-Fe2O3 | 60.6 | 13.90 | 99.0 | 6×10-4 H2S, 混合气体 | 30 ± 2 | 22.4 | 16.0(N) | [ |
| Fe-Cu-Al-O | 96.7 | — | — | 10-3 H2S, CO2 | 40 | — | 113.9(ND) | [ |
| ZnO | 10.2 | 0.029 | 介-大孔 | 51% H2, 30% He, 10% H2O, 4×10-4 H2S | 400 | 457.3(2) | — | [ |
| Ni-ZnO | 6.8 | 0.025 | 介-大孔 | 51% H2, 30% He, 10% H2O, 4×10-4 H2S | 400 | 730.0(2) | — | [ |
| HZn20SC | 272.59 | 0.17 | — | 33% CO, 39% H2, 5% H2O,3×10-4 H2S, N2平衡 | 450 | — | 44.6(ND) | [ |
| Z20M4C6SC | 207.33 | 0.03 | — | 33% CO, 39% H2, 5×10-4 H2S, N2平衡 | 500 | — | 138.4(ND) | [ |
| ZnO | — | — | — | 54% H2, 21%CO2, 2.5×10-5 H2S, He 平衡 | 150 | — | 131(<0.1) | [ |
| — | — | — | 54% H2, 21%CO2, 2.5×10-5 H2S, He 平衡 | 200 | — | 110(<0.1) | [ | |
| 3DOM-ZnFe2O4/SiO2 | 213.10 | 0.39 | 7.40 | 5% H2, 3% H2O, 0.1% H2S, N2平衡 | 500 | — | 92(<0.5) | [ |
| 3DOM-ZnO/SiO2 | 336 | 0.244 | 1.436 | 3% H2O, 500 mg/m3 H2S, N2平衡 | 室温 | — | 135(<0.72) | [ |
表2 不同多孔金属氧化物的结构参数及H2S吸附性能
Table 2 Parameters and adsorption ability of porous metal oxide materials for H2S removal
| 材料 | 比表面积/(m2/g) | 孔体积/(cm3/g) | 平均孔径/nm | 气体组成 | 操作温度/℃ | 饱和容量①/(mg/g) | 穿透容量①/(mg/g) | 文献 |
|---|---|---|---|---|---|---|---|---|
| 3DOM-Fe2O3 | 16~44 | — | 20~33 | 5% H2, 3×10-4 H2S, N2平衡 5% H2, 3×10-4 H2S, N2平衡 | 350 350 | — | 37.18(<0.2) | [ |
| 3DOM-Fe2O3/SiO2 | 80~220 | — | 6~17 | — | 38.92(<0.2) | [ | ||
| MFT | — | — | — | 39.8% H2, 32.6% CO2, 2.5×10-3 H2S, N2平衡 | 400 | 216(N) | — | [ |
| Engelhard ZnO | 53.13 | 0.28 | — | 8×10-6 H2S, 34.4% H2, 20% H2O, N2平衡 | 300 | — | 28.1(0.02) | [ |
| ZSCo-0 | 151.3 | 0.42 | 7.40 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 174.6 | 117.8(ND) | [ |
| ZSCo-0.05 | 188.7 | 0.58 | 7.82 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 195.2 | 146.4(ND) | [ |
| ZSCo-0.15 | 206.4 | 0.63 | 9.58 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 208.4 | 169.8(ND) | [ |
| ZSCo-0.3 | 192.1 | 0.55 | 9.24 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 230.5 | 188.4(ND) | [ |
| ZSCo-0.50 | 161.5 | 0.46 | 7.53 | 8×10-4 H2S, 混合气体(N2, H2O) | 30 | 194.1 | 143.4(ND) | [ |
| Extruded-Fe2O3 | 60.6 | 13.90 | 99.0 | 6×10-4 H2S, 混合气体 | 30 ± 2 | 22.4 | 16.0(N) | [ |
| Fe-Cu-Al-O | 96.7 | — | — | 10-3 H2S, CO2 | 40 | — | 113.9(ND) | [ |
| ZnO | 10.2 | 0.029 | 介-大孔 | 51% H2, 30% He, 10% H2O, 4×10-4 H2S | 400 | 457.3(2) | — | [ |
| Ni-ZnO | 6.8 | 0.025 | 介-大孔 | 51% H2, 30% He, 10% H2O, 4×10-4 H2S | 400 | 730.0(2) | — | [ |
| HZn20SC | 272.59 | 0.17 | — | 33% CO, 39% H2, 5% H2O,3×10-4 H2S, N2平衡 | 450 | — | 44.6(ND) | [ |
| Z20M4C6SC | 207.33 | 0.03 | — | 33% CO, 39% H2, 5×10-4 H2S, N2平衡 | 500 | — | 138.4(ND) | [ |
| ZnO | — | — | — | 54% H2, 21%CO2, 2.5×10-5 H2S, He 平衡 | 150 | — | 131(<0.1) | [ |
| — | — | — | 54% H2, 21%CO2, 2.5×10-5 H2S, He 平衡 | 200 | — | 110(<0.1) | [ | |
| 3DOM-ZnFe2O4/SiO2 | 213.10 | 0.39 | 7.40 | 5% H2, 3% H2O, 0.1% H2S, N2平衡 | 500 | — | 92(<0.5) | [ |
| 3DOM-ZnO/SiO2 | 336 | 0.244 | 1.436 | 3% H2O, 500 mg/m3 H2S, N2平衡 | 室温 | — | 135(<0.72) | [ |
| 材料 | 比表面积/(m2/g) | 孔体积/(cm3/g) | 平均孔径/nm | 气体组成 | 操作温度/℃ | 饱和容量/(mg/g) | 穿透容量①/ (mg/g) | 文献 |
|---|---|---|---|---|---|---|---|---|
| 天然斜发沸石 | 34.2 | 0.12 | 17.9 | 59.95% CH4, 39.95% CO2, 0.1% H2S | 25 | — | 1.4(<3) | [ |
| 4A | 49.5 | — | — | 10-3 H2S, N2平衡 | 50 | — | 8.36(~0) | [ |
| 13X | 567 | 0.34 | — | 2×10-2 H2S, 10-2 SO2, 15% H2O, N2平衡 | 150 | — | 179.7(ND) | [ |
| NaX | 503 | 0.255 | — | 63% N2, 35% CO2, 2% H2S, 2×10-4 COS | 25 | 24.96 | 21.44(ND) | [ |
| ZnX | 538 | 0.307 | — | 63% N2, 35% CO2, 2% H2S, 2×10-4 COS | 25 | 48.96 | 45.44(ND) | [ |
| CoX | 511 | 0.288 | — | 63% N2, 35% CO2, 2% H2S, 2×10-4 COS | 25 | 44.16 | 41.28(ND) | [ |
| AgX | 333 | 0.169 | — | 63% N2, 35% CO2, 2% H2S, 2×10-4 COS | 25 | 51.84 | 48.96(ND) | [ |
| 13X Ex-Cu | 239 | — | — | 2×10-4 H2S, N2平衡 | 120 | — | 40.12±0.6(ND) | [ |
| 13X-Ex-Cu (2.3mmol/g Cu) | 370 | — | — | 8×10-6 H2S, He平衡 | 40 | — | 39.8(0) | [ |
| 5-TiO2/zeolite | 93.42±0.06 | 0.59±0.06 | — | 65% CH4, 35% N2, 0.1% H2S | 25 | — | 4.43(ND) | [ |
| 20Zn/NaA zeolite | 7.99 | — | 2.43 | 2×10-4 H2S, N2平衡 | 28 | — | 15.75(0) | [ |
表3 不同沸石分子筛的结构参数及H2S吸附性能
Table 3 Parameters and adsorption ability of zeolite materials for H2S removal
| 材料 | 比表面积/(m2/g) | 孔体积/(cm3/g) | 平均孔径/nm | 气体组成 | 操作温度/℃ | 饱和容量/(mg/g) | 穿透容量①/ (mg/g) | 文献 |
|---|---|---|---|---|---|---|---|---|
| 天然斜发沸石 | 34.2 | 0.12 | 17.9 | 59.95% CH4, 39.95% CO2, 0.1% H2S | 25 | — | 1.4(<3) | [ |
| 4A | 49.5 | — | — | 10-3 H2S, N2平衡 | 50 | — | 8.36(~0) | [ |
| 13X | 567 | 0.34 | — | 2×10-2 H2S, 10-2 SO2, 15% H2O, N2平衡 | 150 | — | 179.7(ND) | [ |
| NaX | 503 | 0.255 | — | 63% N2, 35% CO2, 2% H2S, 2×10-4 COS | 25 | 24.96 | 21.44(ND) | [ |
| ZnX | 538 | 0.307 | — | 63% N2, 35% CO2, 2% H2S, 2×10-4 COS | 25 | 48.96 | 45.44(ND) | [ |
| CoX | 511 | 0.288 | — | 63% N2, 35% CO2, 2% H2S, 2×10-4 COS | 25 | 44.16 | 41.28(ND) | [ |
| AgX | 333 | 0.169 | — | 63% N2, 35% CO2, 2% H2S, 2×10-4 COS | 25 | 51.84 | 48.96(ND) | [ |
| 13X Ex-Cu | 239 | — | — | 2×10-4 H2S, N2平衡 | 120 | — | 40.12±0.6(ND) | [ |
| 13X-Ex-Cu (2.3mmol/g Cu) | 370 | — | — | 8×10-6 H2S, He平衡 | 40 | — | 39.8(0) | [ |
| 5-TiO2/zeolite | 93.42±0.06 | 0.59±0.06 | — | 65% CH4, 35% N2, 0.1% H2S | 25 | — | 4.43(ND) | [ |
| 20Zn/NaA zeolite | 7.99 | — | 2.43 | 2×10-4 H2S, N2平衡 | 28 | — | 15.75(0) | [ |
| 材料 | 比表面积/(m2/g) | 孔体积/(cm3/g) | 平均孔径/nm | 气体组成 | 操作温度/℃ | 饱和容量①/(mg/g) | 穿透容量①/(mg/g) | 文献 |
|---|---|---|---|---|---|---|---|---|
| MIL-53(Al) | — | — | — | H2S 1.6 MPa | 30 | 376.6(N) | — | [ |
| MIL-53(Cr) | — | — | — | H2S 1.6 MPa | 30 | 419.8(N) | — | [ |
| MIL-53(Fe) | — | — | — | H2S 1.6 MPa | 30 | 273.0(N) | — | [ |
| MIL-47(V) | — | — | — | H2S 1.6 MPa | 30 | 467.2(N) | — | [ |
| MIL-100(Cr) | 1900 | — | — | H2S 2 MPa | 30 | 534.4(N) | — | [ |
| MIL-101(Cr) | 2600 | — | — | H2S 2 MPa | 30 | 1228.8(N) | — | [ |
| MIL-101-NH2(Cr) | 2096 | — | — | 89% CH4, 10% CO2, 1% H2S | 20 | 14.6(ND) | — | [ |
| MIL-125-NH2(Ti) | 1612 | — | — | 89% CH4, 10% CO2, 1% H2S | 20 | 12.3(ND) | — | [ |
| MOF-199 | — | — | — | 400 mg/m3 H2S, 600 mg/m3 CH3CH2SH, 600 mg/m3 CH3SCH3 | 80 | — | 57.2(ND) | [ |
| MOF-199 | 1459 | 0.73 | 0.52 | 600 mg/m3 H2S, N2平衡 | 室温 | — | 53.44(ND) | [ |
| TEA/MOF-199-2 | 187 | 0.15 | 0.43 | 600 mg/m3 H2S, N2平衡 | 室温 | — | 87.68(ND) | [ |
| MEA/MOF-199-2 | 18 | 0.12 | 1.28 | 600 mg/m3 H2S, N2平衡 | 室温 | — | 26.65(ND) | [ |
| Cu(NO3)2@UiO-67(bipy) | 549 | — | — | 10-3 H2S,空气, 70% 相对湿度 | 20 | — | 78(N) | [ |
| CuMGO(10%(mass)) | 1002 | 0.53 | — | 10-3 H2S,空气 | 室温 | 120(N) | — | [ |
| MOF-GOPSN | 1722 | 0.68 | — | 10-3 H2S, 空气 | — | — | 109±4(ND) | [ |
| MOF-GOSA | 1419 | 0.58 | — | 10-3 H2S, 空气 | — | — | 133±5(ND) | [ |
| MOF-GOPSN | 1722 | 0.68 | — | 10-3 H2S, 空气, 71% 相对湿度 | — | — | 125±2(ND) | [ |
| MOF-GOSA | 1419 | 0.58 | — | 10-3 H2S, 空气, 71% 相对湿度 | — | — | 241±6(ND) | [ |
| Sep/Cu-BTC | 270.5 | 0.32 | 4.74 | 9.58×10-6 H2S, 50.76% 湿度 | 15 | — | 55.13(<1) | [ |
| MAC-2 | 1415 | 0.66 | — | 500 mg/m3 H2S, 600 mg/m3 CH3SCH3, 湿空气 | — | — | 84.6(ND) | [ |
表4 不同MOF的结构参数及H2S吸附性能
Table 4 Parameters and adsorption ability of metal organic frames for H2S removal
| 材料 | 比表面积/(m2/g) | 孔体积/(cm3/g) | 平均孔径/nm | 气体组成 | 操作温度/℃ | 饱和容量①/(mg/g) | 穿透容量①/(mg/g) | 文献 |
|---|---|---|---|---|---|---|---|---|
| MIL-53(Al) | — | — | — | H2S 1.6 MPa | 30 | 376.6(N) | — | [ |
| MIL-53(Cr) | — | — | — | H2S 1.6 MPa | 30 | 419.8(N) | — | [ |
| MIL-53(Fe) | — | — | — | H2S 1.6 MPa | 30 | 273.0(N) | — | [ |
| MIL-47(V) | — | — | — | H2S 1.6 MPa | 30 | 467.2(N) | — | [ |
| MIL-100(Cr) | 1900 | — | — | H2S 2 MPa | 30 | 534.4(N) | — | [ |
| MIL-101(Cr) | 2600 | — | — | H2S 2 MPa | 30 | 1228.8(N) | — | [ |
| MIL-101-NH2(Cr) | 2096 | — | — | 89% CH4, 10% CO2, 1% H2S | 20 | 14.6(ND) | — | [ |
| MIL-125-NH2(Ti) | 1612 | — | — | 89% CH4, 10% CO2, 1% H2S | 20 | 12.3(ND) | — | [ |
| MOF-199 | — | — | — | 400 mg/m3 H2S, 600 mg/m3 CH3CH2SH, 600 mg/m3 CH3SCH3 | 80 | — | 57.2(ND) | [ |
| MOF-199 | 1459 | 0.73 | 0.52 | 600 mg/m3 H2S, N2平衡 | 室温 | — | 53.44(ND) | [ |
| TEA/MOF-199-2 | 187 | 0.15 | 0.43 | 600 mg/m3 H2S, N2平衡 | 室温 | — | 87.68(ND) | [ |
| MEA/MOF-199-2 | 18 | 0.12 | 1.28 | 600 mg/m3 H2S, N2平衡 | 室温 | — | 26.65(ND) | [ |
| Cu(NO3)2@UiO-67(bipy) | 549 | — | — | 10-3 H2S,空气, 70% 相对湿度 | 20 | — | 78(N) | [ |
| CuMGO(10%(mass)) | 1002 | 0.53 | — | 10-3 H2S,空气 | 室温 | 120(N) | — | [ |
| MOF-GOPSN | 1722 | 0.68 | — | 10-3 H2S, 空气 | — | — | 109±4(ND) | [ |
| MOF-GOSA | 1419 | 0.58 | — | 10-3 H2S, 空气 | — | — | 133±5(ND) | [ |
| MOF-GOPSN | 1722 | 0.68 | — | 10-3 H2S, 空气, 71% 相对湿度 | — | — | 125±2(ND) | [ |
| MOF-GOSA | 1419 | 0.58 | — | 10-3 H2S, 空气, 71% 相对湿度 | — | — | 241±6(ND) | [ |
| Sep/Cu-BTC | 270.5 | 0.32 | 4.74 | 9.58×10-6 H2S, 50.76% 湿度 | 15 | — | 55.13(<1) | [ |
| MAC-2 | 1415 | 0.66 | — | 500 mg/m3 H2S, 600 mg/m3 CH3SCH3, 湿空气 | — | — | 84.6(ND) | [ |
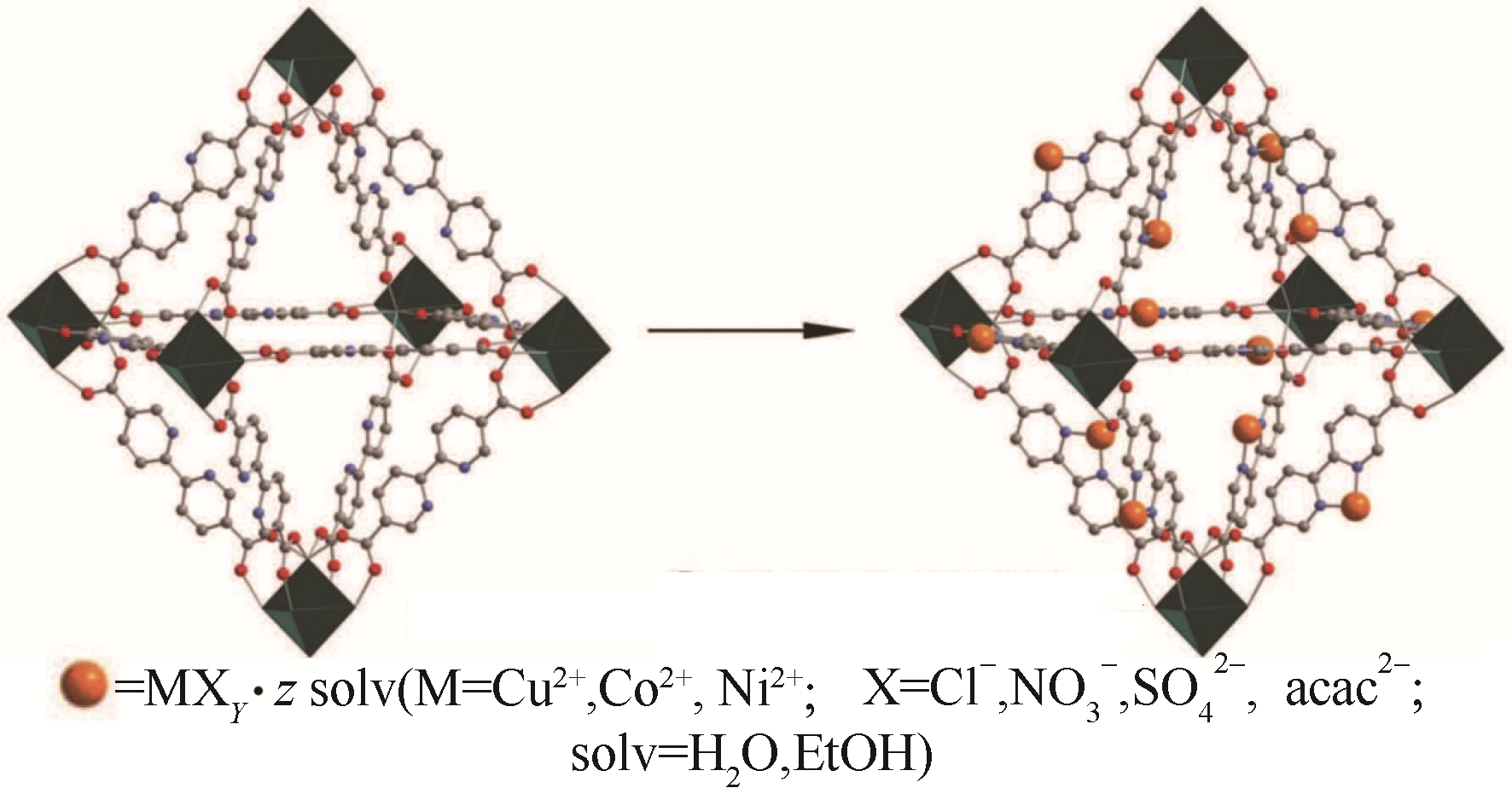
图6 将金属盐整合进入UiO-67(bipy)示意图[61](绿色八面体代表[Zr6O4(OH)4]12+团簇;灰色、红色和蓝色球体分别代表碳、氧和氮原子;橙色球体代表不同的金属复合体)
Fig.6 Postsynthetic insertion of metal salts into UiO-67(bipy)[61] (Green octahedra represents the [Zr6O4(OH)4]12+ cluster. Grey, red and blue spheres represent carbon, oxygen and nitrogen atoms, respectively. The orange spheres represent the respective metal complex)
| 65 | Shi R H, Zhang Z R, Fan H L, et al. Cu-based metal-organic framework/activated carbon composites for sulfur compounds removal[J]. Applied Surface Science, 2017, 394: 394-402. |
| 66 | Li Z J, Xiao Y L, Xue W J, et al. Ionic liquid/metal-organic framework composites for H2S removal from natural gas: a computational exploration[J]. The Journal of Physical Chemistry C, 2015, 119(7): 3674-3683. |
| 1 | 刘新鹏. 用于硫化氢脱除与硫资源回收的绿色脱硫新体系性能研究[D]. 济南: 山东大学, 2017. |
| Liu X P. Study on the performance of new green systems for hydrogen sulfide removal and sulfur recovery[D]. Jinan: Shandong University, 2017. | |
| 2 | Bao J E, Krishnan G N, Jayaweera P. Effect of various coal contaminants on the performance of solid oxide fuel cells (Ⅱ): ppm and sub-ppm level testing[J]. Journal of Power Sources, 2009, 193(2): 617-624. |
| 3 | International Standards Organization. Hydrogen fuel—product specification (2): Proton exchange membrane (PEM) fuel cell applications for road vehicles: [S]. 2012. |
| 4 | 高齐. 碳材料表面碱性官能团对于低浓度H2S的去除机制研究[D]. 上海: 上海大学, 2018. |
| Gao Q. Removal mechanism of low concentration H2S by surface basic functional groups on carbon materials[D]. Shanghai: Shanghai University, 2018. | |
| 5 | 王睿, 石冈, 魏伟胜, 等. 工业气体中H2S的脱除方法——发展现状与展望[J]. 天然气工业, 1999, 19(3): 84-90. |
| Wang R, Shi G, Wei W S, et al. Methods of removing hydrogen sulfide from industrial gas—present situation and prospects[J]. Natural Gas Industry, 1999, 19(3): 84-90. | |
| 6 | Westmoreland P R, Harrison D P. Evaluation of candidate solids for high-temperature desulfurization of low-Btu gases[J]. Environmental Science & Technology, 1976, 10(7): 659- 661. |
| 7 | Cazorla-Amorós D, Alcañiz-Monge J, Linares-Solano A. Characterization of activated carbon fibers by CO2 adsorption[J]. Langmuir, 1996, 12(11): 2820-2824. |
| 67 | Liu G P, Cadiau A, Liu Y, et al. Enabling fluorinated MOF-based membranes for simultaneous removal of H2S and CO2 from natural gas[J]. Angewandte Chemie-International Edition, 2018, 57(45): 14811-14816. |
| 8 | Peluso A, Gargiulo N, Aprea P, et al. Nanoporous materials as H2S adsorbents for biogas purification: a review[J]. Separation and Purification Reviews, 2019, 48(1): 78-89. |
| 9 | Wu X X, Schwartz V, Overbury S H, et al. Desulfurization of gaseous fuels using activated carbons as catalysts for the selective oxidation of hydrogen sulfide[J]. Energy & Fuels, 2005, 19(5): 1774-1782. |
| 10 | Shen F, Liu J, Zhang Z, et al. Density functional study of hydrogen sulfide adsorption mechanism on activated carbon[J]. Fuel Processing Technology, 2018, 171: 258-264. |
| 11 | Bashkova S, Baker F S, Wu X X, et al. Activated carbon catalyst for selective oxidation of hydrogen sulphide: on the influence of pore structure, surface characteristics, and catalytically-active nitrogen[J]. Carbon, 2007, 45(6): 1354-1363. |
| 12 | Quan W Y, Jiang X, Wang X X, et al. Influence of loading a tertiary amine on activated carbons and effect of CO2 on adsorptive H2S removal from biogas[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(27): 9998-10008. |
| 13 | Seredych M, Bandosz T J. Role of microporosity and nitrogen functionality on the surface of activated carbon in the process of desulfurization of digester gas[J]. The Journal of Physical Chemistry C, 2008, 112(12): 4704-4711. |
| 14 | Barelli L, Bidini G, de Arespacochaga N, et al. Biogas use in high temperature fuel cells: enhancement of KOH-KI activated carbon performance toward H2S removal[J]. International Journal of Hydrogen Energy, 2017, 42(15): 10341-10353. |
| 15 | Tsai J H, Jeng F T, Chiang H L. Removal of H2S from exhaust gas by use of alkaline activated carbon[J]. Adsorption, 2001, 7: 357-366. |
| 16 | Sitthikhankaew R, Predapitakkun S, Kiattikomol R W, et al. Comparative study of hydrogen sulfide adsorption by using alkaline impregnated activated carbons for hot fuel gas purification[J]. Energy Procedia, 2011, 9: 15-24. |
| 17 | Siriwardane I W, Udangawa R, de Silva R M, et al. Synthesis and characterization of nano magnesium oxide impregnated granular activated carbon composite for H2S removal applications[J]. Materials & Design, 2017, 136: 127-136. |
| 18 | Cimino S, Lisi L, Erto A, et al. Role of H2O and O2 during the reactive adsorption of H2S on CuO-ZnO/activated carbon at low temperature[J]. Microporous and Mesoporous Materials, 2020, 295: 109949. |
| 19 | Monteleone G, de Francesco M, Stefano G, et al. Deep H2S removal from biogas for molten carbonate fuel cell (MCFC) systems[J]. Chemical Engineering Journal, 2011, 173(3): 407-414. |
| 20 | Sun M H, Wang X Z, Pan X, et al. Nitrogen-rich hierarchical porous carbon nanofibers for selective oxidation of hydrogen sulfide[J]. Fuel Processing Technology, 2019, 191: 121-128. |
| 21 | Qi J, Wei G, Li Y, et al. Porous carbon spheres for simultaneous removal of benzene and H2S[J]. Chemical Engineering Journal, 2018, 339: 499-508. |
| 22 | Zhang Z, Wang J, Li W, et al. Millimeter-sized mesoporous carbon spheres for highly efficient catalytic oxidation of hydrogen sulfide at room temperature[J]. Carbon, 2016, 96: 608-615. |
| 23 | Chen Q J, Wang J T, Liu X J, et al. Alkaline carbon nanotubes as effective catalysts for H2S oxidation[J]. Carbon, 2011, 49(12): 3773-3780. |
| 24 | Khabazipour M, Anbia M. Removal of hydrogen sulfide from gas streams using porous materials: a review[J]. Industrial & Engineering Chemistry Research, 2019, 58(49): 22133-22164. |
| 25 | Fan H L, Sun T, Zhao Y P, et al. Three-dimensionally ordered macroporous iron oxide for removal of H2S at medium temperatures[J]. Environmental Science & Technology, 2013, 47(9): 4859-4865. |
| 26 | Bao W R, Zhang Z Y, Ren X R, et al. Desulfurization behavior of iron-based sorbent with MgO and TiO2 additive in hot coal gas[J]. Energy & Fuels, 2009, 23(7): 3600-3604. |
| 27 | Novochinskii I I, Song C S, Ma X L, et al. Low-temperature H2S removal from steam-containing gas mixtures with ZnO for fuel cell application(1): ZnO particles and extrudates[J]. Energy & Fuels, 2004, 18(2): 576-583. |
| 28 | Yang C, Yang S, Fan H L, et al. A sustainable design of ZnO-based adsorbent for robust H2S uptake and secondary utilization as hydrogenation catalyst[J]. Chemical Engineering Journal, 2020, 382: 122892. |
| 29 | Habeeb O A, Kanthasamy R, Ali G A M, et al. Hydrogen sulfide emission sources, regulations, and removal techniques: a review[J]. Reviews in Chemical Engineering, 2018, 34(6): 837-854. |
| 30 | Pahalagedara L R, Poyraz A S, Song W Q, et al. Low temperature desulfurization of H2S: high sorption capacities by mesoporous cobalt oxide via increased H2S diffusion[J]. Chemistry of Materials, 2014, 26(22): 6613-6621. |
| 31 | Liu X, Meng X, Zhao J. Synthesis of nanocrystalline iron oxides with mesostructure as desulfurizer[J]. Materials Letters, 2013, 92: 255-258. |
| 32 | Long N Q, Loc T X. Experimental and modeling study on room-temperature removal of hydrogen sulfide using a low-cost extruded Fe2O3-based adsorbent[J]. Adsorption, 2016, 22(3): 397-408. |
| 33 | Liu D, Chen S Y, Fei X Y, et al. Regenerable CuO-based adsorbents for low temperature desulfurization application[J]. Industrial & Engineering Chemistry Research, 2015, 54(14): 3556-3562. |
| 34 | Tran D T. Synthesis of porous ZnO based materials using an agarose gel template for H2S desulfurization[J]. RSC Advances, 2016, 6(2): 1339-1345. |
| 35 | Zheng X R, Bao, W R, Jin Q M, et al. Use of high-pressure impregnation in preparing Zn-based sorbents for deep desulfurization of hot coal gas[J]. Energy & Fuels, 2011, 25(7): 2997-3001. |
| 36 | Zheng X R, Bao W R, Chang L P, et al. Interaction among metal components of Zn-Mn-Cu-based sorbents prepared by high-pressure impregnation method and its effect on the removal of H2S from hot coal gas[J]. Energy & Fuels, 2012, 26(6): 3393-3398. |
| 37 | Li L, King D L. H2S removal with ZnO during fuel processing for PEM fuel cell applications[J]. Catalysis Today, 2006, 116(4): 537-541. |
| 38 | Li L, Zhang H B, Zhou P, et al. Three dimensional ordered macroporous zinc ferrite composited silica sorbents with promotional desulfurization and regeneration activity at mid-high temperature[J]. Applied Surface Science, 2019, 470: 177-186. |
| 39 | Wei Y, Liu J, Zhao Z, et al. The catalysts of three-dimensionally ordered macroporous Ce1-xZrxO2-supported gold nanoparticles for soot combustion: the metal-support interaction[J]. Journal of Catalysis, 2012, 287: 13-29. |
| 40 | Wang L J, Fan H L, Shangguan J, et al. Design of a sorbent to enhance reactive adsorption of hydrogen sulfide[J]. ACS Applied Materials & Interfaces, 2014, 6(23): 21167-21177. |
| 41 | 陈曦. 提高Y型和X型分子筛吸附功能深度净化天然气过程基础研究[D]. 上海: 华东理工大学, 2018. |
| Chen X. The fundamental study on enhancing adsorption performance of zeolite Y and X for deep purification of sour natural gas[D]. Shanghai: East China University of Science and Technology, 2018. | |
| 42 | 王洪国. 清洁燃料选择性吸附脱硫过程中硫化物吸附行为的研究[D]. 青岛: 中国石油大学, 2010. |
| Wang H G. Studies of adsorption behavior of sulfur compounds in selective adsorption desulfurization for clean fuel[D]. Qingdao: China University of Petroleum, 2010. | |
| 43 | Kristóf T. Selective removal of hydrogen sulphide from industrial gas mixtures using zeolite NaA[J]. Hungarian Journal of Industrial Chemistry, 2017, 45(1): 9-15. |
| 44 | Alonso-Vicario A, Ochoa-Gómez J R, Gil-Río S, et al. Purification and upgrading of biogas by pressure swing adsorption on synthetic and natural zeolites[J]. Microporous and Mesoporous Materials, 2010, 134(1/2/3): 100-107. |
| 45 | Liu X, Wang R. Effective removal of hydrogen sulfide using 4A molecular sieve zeolite synthesized from attapulgite[J]. Journal of Hazardous Materials, 2017, 326: 157-164. |
| 46 | Yang K, Su B, Shi L, et al. Adsorption mechanism and regeneration performance of 13X for H2S and SO2[J]. Energy & Fuels, 2018, 32(12): 12742-12749. |
| 47 | Chen X, Shen B, Sun H, et al. Ion-exchange modified zeolites X for selective adsorption desulfurization from Claus tail gas: experimental and computational investigations[J]. Microporous and Mesoporous Materials, 2018, 261: 227-236. |
| 48 | Barelli L, Bidini G, Micoli L, et al. 13X Ex-Cu zeolite performance characterization towards H2S removal for biogas use in molten carbonate fuel cells[J]. Energy, 2018, 160: 44-53. |
| 49 | Micoli L, Bagnasco G, Turco M. H2S removal from biogas for fuelling MCFCs: new adsorbing materials[J]. International Journal of Hydrogen Energy, 2014, 39(4): 1783-1787. |
| 50 | Liu C, Zhang R, Wei S, et al. Selective removal of H2S from biogas using a regenerable hybrid TiO2/zeolite composite[J]. Fuel, 2015, 157: 183-190. |
| 51 | Abdullah A H, Mat R, Somderam S, et al. Hydrogen sulfide adsorption by zinc oxide-impregnated zeolite (synthesized from Malaysian kaolin) for biogas desulfurization[J]. Journal of Industrial and Engineering Chemistry, 2018, 65: 334-342. |
| 52 | Fellah M F. Adsorption of hydrogen sulfide as initial step of H2S removal: a DFT study on metal exchanged ZSM-12 clusters[J]. Fuel Processing Technology, 2016, 144: 191-196. |
| 53 | Yaghi O M, Li H L. Hydrothermal synthesis of a metal-organic framework containing large rectangular channels[J]. Journal of the American Chemical Society, 1995, 117: 10401-10402. |
| 54 | Furukawa H, Cordova K E, O'Keeffe M, et al. The chemistry and applications of metal-organic frameworks[J]. Science, 2013, 341(6149): 1230444. |
| 55 | Jiao L, Seow J Y R, Skinner W S, et al. Metal-organic frameworks: structures and functional applications[J]. Materials Today, 2019, 27: 43-68. |
| 56 | Hamon L, Serre C, Devic T. Comparative study of hydrogen sulfide adsorption in the MIL-53(Al, Cr, Fe), MIL-47(V), MIL-100(Cr), and MIL-101(Cr) metal-organic frameworks at room temperature[J]. Journal of the American Chemical Society, 2009, 131: 8775-8777. |
| 57 | Peluso A, Gargiulo N, Aprea P, et al. Modeling hydrogen sulfide adsorption on chromium-based MIL-101 metal organic framework[J]. Science of Advanced Materials, 2014, 6(1): 164-170. |
| 58 | Joshi J N, Zhu G, Lee J J. et al. Probing metal-organic framework design for adsorptive natural gas purification[J]. Langmuir, 2018, 34(29): 8443-8450. |
| 59 | Li Y, Wang L J, Fan H L, et al. Removal of sulfur compounds by a copper-based metal organic framework under ambient conditions[J]. Energy & Fuels, 2015, 29(1): 298-304. |
| 60 | Zhang H Y, Yang C, Geng Q, et al. Adsorption of hydrogen sulfide by amine-functionalized metal organic framework (MOF-199): an experimental and simulation study[J]. Applied Surface Science, 2019, 497: 143815. |
| 61 | Nickerl G, Leistner M, Helten S, et al. Integration of accessible secondary metal sites into MOFs for H2S removal[J]. Inorganic Chemistry Frontiers, 2014, 1(4): 325-330. |
| 62 | Petit C, Bandosz T J. Exploring the coordination chemistry of MOF-graphite oxide composites and their applications as adsorbents[J]. Dalton Transactions, 2012, 41(14): 4027-4035. |
| 63 | Ebrahim A M, Jagiello J, Bandosz T J. Enhanced reactive adsorption of H2S on Cu-BTC/S- and N-doped GO composites[J]. Journal of Materials Chemistry A, 2015, 3(15): 8194-8204. |
| 64 | Kakaei H, Beygzadeh M, Golbabaei F, et al. Preparation of a sepiolite/Cu-BDC nanocomposite and its application as an adsorbent in respirator cartridges for H2S removal[J]. New Journal of Chemistry, 2019, 43(29): 11575-11584. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [3] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [4] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [5] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [6] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [7] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [8] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [9] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [10] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [11] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [12] | 高金明, 郭玉娇, 鄂承林, 卢春喜. 一种封闭罩内顺流多旋臂气液分离器的分离特性研究[J]. 化工学报, 2023, 74(7): 2957-2966. |
| [13] | 文兆伦, 李沛睿, 张忠林, 杜晓, 侯起旺, 刘叶刚, 郝晓刚, 官国清. 基于自热再生的隔壁塔深冷空分工艺设计及优化[J]. 化工学报, 2023, 74(7): 2988-2998. |
| [14] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [15] | 韩奎奎, 谭湘龙, 李金芝, 杨婷, 张春, 张永汾, 刘洪全, 于中伟, 顾学红. 四通道中空纤维MFI分子筛膜用于二甲苯异构体分离[J]. 化工学报, 2023, 74(6): 2468-2476. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号