化工学报 ›› 2022, Vol. 73 ›› Issue (11): 4791-4813.DOI: 10.11949/0438-1157.20220868
侯旺君1,2( ), 闫翎鹏2,3, 曹哲勇1, 郑静霞1,2(
), 闫翎鹏2,3, 曹哲勇1, 郑静霞1,2( ), 杨永珍1,2(
), 杨永珍1,2( )
)
收稿日期:2022-06-21
修回日期:2022-09-05
出版日期:2022-11-05
发布日期:2022-12-06
通讯作者:
郑静霞,杨永珍
作者简介:侯旺君(1998—),男,硕士研究生, hwj980307@163.com
基金资助:
Wangjun HOU1,2( ), Lingpeng YAN2,3, Zheyong CAO1, Jingxia ZHENG1,2(
), Lingpeng YAN2,3, Zheyong CAO1, Jingxia ZHENG1,2( ), Yongzhen YANG1,2(
), Yongzhen YANG1,2( )
)
Received:2022-06-21
Revised:2022-09-05
Online:2022-11-05
Published:2022-12-06
Contact:
Jingxia ZHENG, Yongzhen YANG
摘要:
煤是自然界中分布最广、储量最丰富的含碳资源,其分子结构与纳米碳材料具有天然的相似性,是优质的纳米碳材料前体。多年来,以煤为前体制备的各种纳米碳材料已被广泛应用于能源、信息、环境和生物医学等领域。其中,煤基零维纳米碳材料如纳米金刚石、富勒烯、碳纳米洋葱、碳点等,因其具有小的纳米尺寸、大的比表面积、独特的球形结构等,表现出优异的荧光特性、电化学性能以及催化性能等,在能源转化和存储等领域展现出极大的应用潜力。本文综述了基于煤炭及其衍生物为前驱体的各类零维纳米碳材料的制备方法和性能,并对其在照明显示、电化学储能、光/电催化等方面的应用进展进行总结,指出目前存在的问题与挑战及其解决策略,最后对其未来发展进行了展望。这为促进煤炭的高附加值转化和利用以及大规模制备煤基零维纳米碳材料提供理论和实践支持。
中图分类号:
侯旺君, 闫翎鹏, 曹哲勇, 郑静霞, 杨永珍. 煤基零维纳米碳材料的合成、性能及其在能源转换和存储应用中的研究进展[J]. 化工学报, 2022, 73(11): 4791-4813.
Wangjun HOU, Lingpeng YAN, Zheyong CAO, Jingxia ZHENG, Yongzhen YANG. Research progress of synthesis and properties of coal-based zero-dimensional nanocarbon materials and their applications in energy conversion and storage[J]. CIESC Journal, 2022, 73(11): 4791-4813.
| 产物 | 前体 | 合成方法 | 粒径 | 产率/荧光量子产率(QY) | 性能 | 应用领域 | 文献 |
|---|---|---|---|---|---|---|---|
| NDs | 煤 | 超声法 | 4~15 nm | — | 紫外光下激发为明亮蓝色荧光 | 生物成像,光伏工程 | [ |
| 无烟煤 | 激光烧蚀法 | 3~5 nm | 产率6.2% | 在420 nm激发下在乙醇溶液中为绿色荧光,在水溶液中为蓝色荧光 | 生物成像,光伏,光电子学 | [ | |
| 焦炭 | 激光烧蚀法 | 3.2 nm | 产率6% | ||||
| 煤 | 化学氧化法 | 2.1~6.6 nm | 产率4%~6% | — | 生物传感,药物运输 | [ | |
| C60 | 褐煤 | 电弧放电法 | — | 产率4.5% | — | — | [ |
| 烟煤 | 电弧放电法 | — | 产率0.67%~2.38% | — | — | [ | |
| 无烟煤 | 电弧放电法 | — | 产率5.96% | — | 电化学储能 | ||
| CNOs | 煤 | 化学氧化法 | 5~20 nm | 产率76.25% | 自然光下,作为催化剂对2-硝基苯酚降解效率很高 | 光催化降解 | [ |
| 煤层气 | CVD法 | 5~200 nm | 产率70% | 内嵌催化剂CNOs具有铁磁性;比容量达到142.31 F/g;电化学性能良好 | 超级电容器,气敏传感器 | [ | |
| 煤 | 射频等离子法 | 10~35 nm | — | CNOs具有空心多面体或准球形形态,化学性能稳定 | — | [ | |
| CDs | 煤 | 激光烧蚀法 | 9.75~30.25 nm | — | 紫外光下激发为绿色荧光;优异的光稳定性、低毒性和生物相容性 | 生物成像 | [ |
| 焦煤 | 激光烧蚀法 | 约35 nm | QY 34% | 蓝色荧光,激发依赖性;随着粒径减小,最强发射峰蓝移 | — | [ | |
| 焦炭 | 超声法 | 5.0~6.0 nm | QY 9.2% | 蓝色荧光,最佳发射波长为410 nm | 照明显示,LED | [ | |
| 无烟煤 | 溶剂热法 | 3.0~6.5 nm | 产率25.6%,QY 47% | 在紫外激发下显示蓝色荧光 | 光动力治疗,生物成像 | [ | |
| 煤焦油 | 溶剂热法 | 1.5~4.5 nm | QY 29.7% | 激发依赖;495~575 nm激发波长下发出橙色荧光,发射波长红移为598~612 nm | 生物成像 | [ | |
| 煤焦油沥青 | 溶剂热法 | 1.9~5.8 nm | 产率18%~23% | 结晶度高,分散性好;粒径可控,光吸附能力强 | 光催化制氢 | [ | |
| 煤焦油中喹啉不溶物 | 化学氧化法 | 1.0~14.0 nm | QY 8.5% | 紫外光激发下发出绿色荧光,最佳发射波长为578 nm | 光学照明,生物成像 | [ | |
| 褐煤 | 化学氧化/超声法 | 35 nm | QY 7% | 激发依赖,分别在435和403 nm处观察到最佳发射峰;室温磷光 | 传感 | [ |
表1 煤基NDs、C60、CNOs和CDs的合成、性能及应用研究进展
Table 1 Research progress on synthesis, properties and applications of coal-based NDs, C60, CNOs and CDs
| 产物 | 前体 | 合成方法 | 粒径 | 产率/荧光量子产率(QY) | 性能 | 应用领域 | 文献 |
|---|---|---|---|---|---|---|---|
| NDs | 煤 | 超声法 | 4~15 nm | — | 紫外光下激发为明亮蓝色荧光 | 生物成像,光伏工程 | [ |
| 无烟煤 | 激光烧蚀法 | 3~5 nm | 产率6.2% | 在420 nm激发下在乙醇溶液中为绿色荧光,在水溶液中为蓝色荧光 | 生物成像,光伏,光电子学 | [ | |
| 焦炭 | 激光烧蚀法 | 3.2 nm | 产率6% | ||||
| 煤 | 化学氧化法 | 2.1~6.6 nm | 产率4%~6% | — | 生物传感,药物运输 | [ | |
| C60 | 褐煤 | 电弧放电法 | — | 产率4.5% | — | — | [ |
| 烟煤 | 电弧放电法 | — | 产率0.67%~2.38% | — | — | [ | |
| 无烟煤 | 电弧放电法 | — | 产率5.96% | — | 电化学储能 | ||
| CNOs | 煤 | 化学氧化法 | 5~20 nm | 产率76.25% | 自然光下,作为催化剂对2-硝基苯酚降解效率很高 | 光催化降解 | [ |
| 煤层气 | CVD法 | 5~200 nm | 产率70% | 内嵌催化剂CNOs具有铁磁性;比容量达到142.31 F/g;电化学性能良好 | 超级电容器,气敏传感器 | [ | |
| 煤 | 射频等离子法 | 10~35 nm | — | CNOs具有空心多面体或准球形形态,化学性能稳定 | — | [ | |
| CDs | 煤 | 激光烧蚀法 | 9.75~30.25 nm | — | 紫外光下激发为绿色荧光;优异的光稳定性、低毒性和生物相容性 | 生物成像 | [ |
| 焦煤 | 激光烧蚀法 | 约35 nm | QY 34% | 蓝色荧光,激发依赖性;随着粒径减小,最强发射峰蓝移 | — | [ | |
| 焦炭 | 超声法 | 5.0~6.0 nm | QY 9.2% | 蓝色荧光,最佳发射波长为410 nm | 照明显示,LED | [ | |
| 无烟煤 | 溶剂热法 | 3.0~6.5 nm | 产率25.6%,QY 47% | 在紫外激发下显示蓝色荧光 | 光动力治疗,生物成像 | [ | |
| 煤焦油 | 溶剂热法 | 1.5~4.5 nm | QY 29.7% | 激发依赖;495~575 nm激发波长下发出橙色荧光,发射波长红移为598~612 nm | 生物成像 | [ | |
| 煤焦油沥青 | 溶剂热法 | 1.9~5.8 nm | 产率18%~23% | 结晶度高,分散性好;粒径可控,光吸附能力强 | 光催化制氢 | [ | |
| 煤焦油中喹啉不溶物 | 化学氧化法 | 1.0~14.0 nm | QY 8.5% | 紫外光激发下发出绿色荧光,最佳发射波长为578 nm | 光学照明,生物成像 | [ | |
| 褐煤 | 化学氧化/超声法 | 35 nm | QY 7% | 激发依赖,分别在435和403 nm处观察到最佳发射峰;室温磷光 | 传感 | [ |

图2 CNOs 的制备、纯化及活化技术路线图(a) [29]; CVD法制备碳包覆催化剂颗粒和空心CNOs的生长过程示意图(b) [30]
Fig.2 Technology road-mapping of the synthesis, purification and functionalization of CNOs (a) [29]; Schematic diagram of the growth process of carbon coated catalyst particles and hollow CNOs prepared by CVD (b) [30]

图3 烟煤为碳源合成GQDs的示意图(a) [14]; H2O2作氧化剂合成煤基CDs的示意图(b) [45]
Fig.3 Schematic representation of the synthesis of GQDs from bituminous coal (a) [14]; Schematic diagram of the synthesis of coal-based CDs with H2O2 as oxidant (b) [45]

图4 DMF作溶剂合成煤基CDs的示意图(a) [39]; 制备煤基FCNPs的路线示意图(b) [43]
Fig.4 Schematic diagram of the synthesis of coal-based CDs using DMF as solvent (a) [39]; Schematic diagram of the route to prepare coal-based FCNPs (b) [43]

图5 低品质煤在H2O2溶剂中超声法合成NDs示意图(a)[12]; 煤在DMF溶剂中超声法合成GQDs示意图(b) [47]
Fig.5 Schematic diagram of NDs synthesized by ultrasonication of low quality coal in H2O2 solvent (a) [12]; Schematic diagram of GQDs synthesized by ultrasonication of coal in DMF solvent (b) [47]
| 合成方法 | 主要前体 | 主要产物 | 优点 | 缺点 |
|---|---|---|---|---|
| CVD法 | 煤层气(甲烷),乙炔,煤沥青 | C60,CDs,CNOs | 操作简单,成本低,易于大批量生产 | 伴随有杂质相(无定形碳、石墨、催化剂),纯化困难 |
| 化学氧化法 | 褐煤,烟煤,无烟煤,焦炭 | NDs,GQDs,CDs | 设备简单,能耗低,操作简便 | 含有杂质,纯化困难 |
| 溶剂热法 | 煤焦油,烟煤,无烟煤 | GQDs,CDs | 操作简单,能耗低,不需要特殊的反应条件和设备 | 产量低,伴随着有毒气体的释放 |
| 超声法 | 烟煤,无烟煤 | NDs,CQDs | 反应速率快,操作简便 | 加热效率低,能耗高 |
| 电化学氧化法 | 焦炭或无烟煤混合煤焦油 | C60,CNOs,CDs | 成本低,效率高,产量高 | 含有杂质相(金属杂质、无定形碳),设备复杂,原料需力学性能好 |
| 电弧放电法 | 焦炭,无烟煤,石墨 | C60,CNOs,CDs | 产物结晶性好,能够大量制备,易于收集 | 含有大量含碳杂质(无定形碳、碳纳米管及金属杂质等),设备复杂 |
| 等离子体法 | 烟煤,无烟煤 | CNOs,CDs | 成本低,能大量制备 | 含有金属杂质及无定形碳 |
| 热解法 | 炭黑,纳米金刚石 | C60,CNOs | 可大量制备,操作简便 | 产物纯净度低,纯化困难 |
| 微波法 | 褐煤,烟煤,无烟煤 | NDs,CDs | 反应速率快,高效 | 产量不高,纯化过程复杂 |
| 热处理法 | 烟煤,无烟煤,石墨 | CNOs,CDs | 可大量制备,操作简单 | 能耗高,产物纯净度低,纯化困难 |
表2 目前制备煤基NDs、C60、CNOs和CDs各种方法的优缺点
Table 2 The advantages and disadvantages of various preparation methods for coal-based NDs, C60, CNOs and CDs
| 合成方法 | 主要前体 | 主要产物 | 优点 | 缺点 |
|---|---|---|---|---|
| CVD法 | 煤层气(甲烷),乙炔,煤沥青 | C60,CDs,CNOs | 操作简单,成本低,易于大批量生产 | 伴随有杂质相(无定形碳、石墨、催化剂),纯化困难 |
| 化学氧化法 | 褐煤,烟煤,无烟煤,焦炭 | NDs,GQDs,CDs | 设备简单,能耗低,操作简便 | 含有杂质,纯化困难 |
| 溶剂热法 | 煤焦油,烟煤,无烟煤 | GQDs,CDs | 操作简单,能耗低,不需要特殊的反应条件和设备 | 产量低,伴随着有毒气体的释放 |
| 超声法 | 烟煤,无烟煤 | NDs,CQDs | 反应速率快,操作简便 | 加热效率低,能耗高 |
| 电化学氧化法 | 焦炭或无烟煤混合煤焦油 | C60,CNOs,CDs | 成本低,效率高,产量高 | 含有杂质相(金属杂质、无定形碳),设备复杂,原料需力学性能好 |
| 电弧放电法 | 焦炭,无烟煤,石墨 | C60,CNOs,CDs | 产物结晶性好,能够大量制备,易于收集 | 含有大量含碳杂质(无定形碳、碳纳米管及金属杂质等),设备复杂 |
| 等离子体法 | 烟煤,无烟煤 | CNOs,CDs | 成本低,能大量制备 | 含有金属杂质及无定形碳 |
| 热解法 | 炭黑,纳米金刚石 | C60,CNOs | 可大量制备,操作简便 | 产物纯净度低,纯化困难 |
| 微波法 | 褐煤,烟煤,无烟煤 | NDs,CDs | 反应速率快,高效 | 产量不高,纯化过程复杂 |
| 热处理法 | 烟煤,无烟煤,石墨 | CNOs,CDs | 可大量制备,操作简单 | 能耗高,产物纯净度低,纯化困难 |

图8 PVA和PVA/GQDs薄膜在紫外灯(波长为365 nm)下的照片(a) [79];CQDs基LED器件的横截面视图(b);在3.2 V下工作的LED及其发射光谱图(c);白光LED的CIE色度图(d) [38]
Fig.8 Photographs of PVA and PVA/GQDs films under UV lamp (wavelength of 365 nm) (a) [79]; Cross-sectional view of CQDs-based LED devices (b); LEDs operating at 3.2 V and their emission spectra plots (c); CIE chromaticity plots of white LEDs (d) [38]
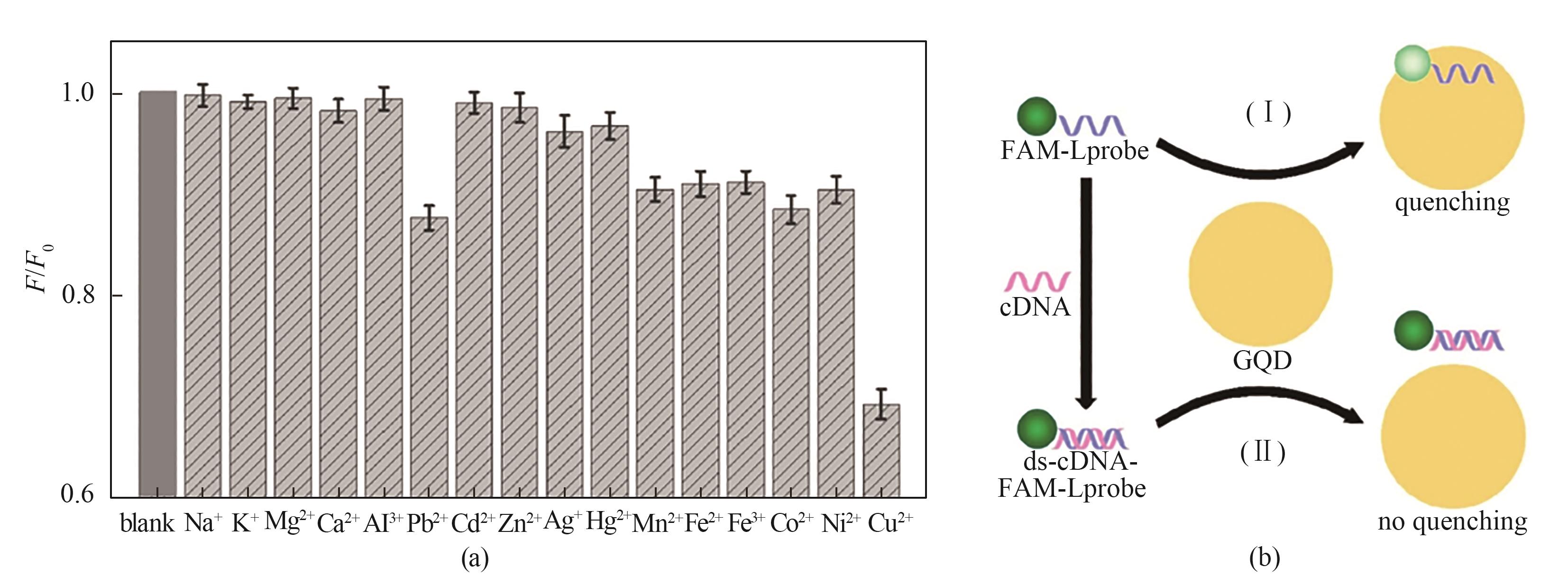
图9 在PBS缓冲液中CDs作为金属离子探针的选择性(a) [80]; 基于GQDs作为纳米猝灭器的DNA传感策略示意图(b) [81]
Fig.9 Selectivity of CDs as metal ion probes in PBS buffer (a) [80]; Schematic of DNA sensing strategy based on GQDs as nano-quenchers (b) [81]

图11 CoDC-0.5在三电极系统中的CV曲线(a)和恒流充放电曲线(b); CoDC-0.5在10 A/g时的循环性能(c); CoDC-0.5、活性炭和还原氧化石墨烯在不同质量负载下的电容(d); CoDC-0.5在4~25 mg/cm2不同负载下的Nyquist图(e); CoDC-0.5、活性炭和还原氧化石墨烯在20 mg/cm2负荷下的Bode图(f) [90]
Fig.11 CV curves (a) and galvanostatic charge-discharge curves (b) of the CoDC-0.5 in three-electrode system; Cycle performance of the CoDC-0.5 at 10 A/g (c); Areal capacitances of the CoDC-0.5, activated carbon, and reduced graphene oxide with different mass loadings (d); Nyquist plots of the CoDC-0.5 with various mass loadings from 4 to 25 mg/cm (e); Bode plots of the CoDC-0.5, activated carbon, and reduced graphene oxide under a mass loading of 20 mg/cm (f) [90]

图12 BN-GQD/G复合材料的制备示意图(a);样品的电催化活性(b) [101]
Fig.12 Schematic illustration of the preparation of the BN-GQD/G nanocomposite (a); Electrocatalytic activity of samples (b) [101]
| 96 | Khan K, Tareen A K, Aslam M, et al. Facile synthesis of mayenite electride nanoparticles encapsulated in graphitic shells like carbon nano onions: non-noble-metal electrocatalysts for oxygen reduction reaction (ORR)[J]. Frontiers in Chemistry, 2020, 7: 934. |
| 97 | Choi E Y, Kim C K. Fabrication of nitrogen-doped nano-onions and their electrocatalytic activity toward the oxygen reduction reaction[J]. Scientific Reports, 2017, 7: 4178. |
| 98 | Choi E Y, Lee D, Kim J, et al. Enhanced electrocatalytic activity of N-doped nano-onion/gold nanorod nanocomposites for the oxygen reduction reaction[J]. Electrochimica Acta, 2022, 405: 139816. |
| 99 | Cheng R Q, Jiang M, Li K Q, et al. Dimensional engineering of carbon dots derived sulfur and nitrogen co-doped carbon as efficient oxygen reduction reaction electrocatalysts for aluminum-air batteries[J]. Chemical Engineering Journal, 2021, 425: 130603. |
| 100 | Yang M, Lian Z, Si C W, et al. Revealing the intrinsic relation between heteroatom dopants and graphene quantum dots as a bi-functional ORR/OER catalyst[J]. Molecular Catalysis, 2022, 518: 112109. |
| 101 | Fei H L, Ye R Q, Ye G L, et al. Boron-and nitrogen-doped graphene quantum dots/graphene hybrid nanoplatelets as efficient electrocatalysts for oxygen reduction[J]. ACS Nano, 2014, 8(10): 10837-10843. |
| 1 | Li H Q, He X J, Wu T T, et al. Synthesis, modification strategies and applications of coal-based carbon materials[J]. Fuel Processing Technology, 2022, 230: 107203. |
| 2 | Raj A M, Balachandran M. Coal-based fluorescent zero-dimensional carbon nanomaterials: a short review[J]. Energy & Fuels, 2020, 34(11): 13291-13306.. |
| 3 | Williams O, Ure A, Stevens L, et al. Formation of metallurgical coke within minutes through coal densification and microwave energy[J]. Energy & Fuels, 2019, 33(7): 6817-6828. |
| 4 | 张睿哲, 李可可, 张凯博, 等. 煤基碳量子点/氮化碳复合材料制备及其光催化还原CO2性能[J]. 化工学报, 2020, 71(6): 2788-2794. |
| Zhang R Z, Li K K, Zhang K B, et al. Coal-based carbon quantum dots/carbon nitride composites for photocatalytic CO2 reduction[J]. CIESC Journal, 2020, 71(6): 2788-2794. | |
| 5 | Li K, Liu G, Zheng L, et al. Coal-derived carbon nanomaterials for sustainable energy storage applications[J]. New Carbon Materials, 2021, 36(1): 133-154. |
| 6 | Hassan M, Gomes V G. Coal derived carbon nanomaterials-recent advances in synthesis and applications[J]. Applied Materials Today, 2018, 12: 342-358. |
| 7 | Xia C L, Zhu S J, Feng T L, et al. Evolution and synthesis of carbon dots: from carbon dots to carbonized polymer dots[J]. Advanced Science, 2019, 6(23): 1901316. |
| 8 | Zhang T, Liu J L, Wang C, et al. Synthesis of graphene and related two-dimensional materials for bioelectronics devices[J]. Biosensors & Bioelectronics, 2017, 89: 28-42. |
| 9 | 康孟孟, 赵翰庆, 宋玮, 等. 不同维度的煤基纳米碳材料的制备及储能应用[J]. 现代化工, 2019, 39(8): 49-53. |
| Kang M M, Zhao H Q, Song W, et al. Preparation and energy storage application of coal-based carbon materials with various dimensions[J]. Modern Chemical Industry, 2019, 39(8): 49-53. | |
| 10 | Kumar P, Dua S, Kaur R, et al. A review on advancements in carbon quantum dots and their application in photovoltaics[J]. RSC Advances, 2022, 12(8): 4714-4759. |
| 11 | Zhai Y P, Zhang B W, Shi R, et al. Carbon dots as new building blocks for electrochemical energy storage and electrocatalysis[J]. Advanced Energy Materials, 2022, 12(6): 2103426. |
| 12 | Das T, Saikia B K. Nanodiamonds produced from low-grade Indian coals[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(11): 9619-9624. |
| 13 | Tiwari A J, Ashraf-Khorassani M, Marr L C. C60 fullerenes from combustion of common fuels[J]. Science of the Total Environment, 2016, 547: 254-260. |
| 14 | Ye R Q, Xiang C S, Lin J, et al. Coal as an abundant source of graphene quantum dots[J]. Nature Communications, 2013, 4(1):1-7. |
| 15 | Du A B, Liu X G, Fu D J, et al. Onion-like fullerenes synthesis from coal[J]. Fuel, 2007, 86(1/2): 294-298. |
| 16 | Fu D J, Liu X G, Lin X, et al. Synthesis of encapsulating and hollow onion-like fullerenes from coal[J]. Journal of Materials Science, 2007, 42(11): 3805-3809. |
| 17 | Ōsawa E. Recent progress and perspectives in single-digit nanodiamond[J]. Diamond and Related Materials, 2007, 16(12): 2018-2022. |
| 18 | Manoj B, Raj A M, Chirayil G T. Facile synthesis of preformed mixed nano-carbon structure from low rank coal[J]. Materials Science Poland, 2018, 36(1): 14-20. |
| 19 | Balasubramanian G, Chan I Y, Kolesov R, et al. Nanoscale imaging magnetometry with diamond spins under ambient conditions[J]. Nature, 2008, 455(7213): 648-651. |
| 20 | Kroto H W, Heath J R, O’Brien S C, et al. C60: buckminsterfullerene[J]. Nature, 1985, 318(6042): 162-163. |
| 21 | Ramazani A, Moghaddasi M A, Mashhadi Malekzadeh A, et al. Industrial oriented approach on fullerene preparation methods[J]. Inorganic Chemistry Communications, 2021, 125: 108442. |
| 22 | Pérez-Ojeda M E, Castro E, Kröckel C, et al. Carbon nano-onions: potassium intercalation and reductive covalent functionalization[J]. Journal of the American Chemical Society, 2021, 143(45): 18997-19007. |
| 23 | Zeiger M, Jäckel N, Mochalin V N, et al. Review: carbon onions for electrochemical energy storage[J]. Journal of Materials Chemistry A, 2016, 4(9): 3172-3196. |
| 24 | Wang C X, Strauss V, Kaner R B. Carbon nanodots for capacitor electrodes[J]. Trends in Chemistry, 2019, 1(9): 858-868. |
| 25 | Jiang K, Wang Y H, Gao X L, et al. Facile, quick, and gram-scale synthesis of ultralong-lifetime room-temperature-phosphorescent carbon dots by microwave irradiation[J]. Angewandte Chemie International Edition, 2018, 57(21): 6216-6220. |
| 26 | Xu X Y, Ray R, Gu Y L, et al. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments[J]. Journal of the American Chemical Society, 2004, 126(40): 12736-12737. |
| 27 | Kundu N, Sadhukhan D, Sarkar S. Fluorescent carbon nano-materials from coal-based precursors: unveiling structure-function relationship between coal and nano-materials[J]. Carbon Letters, 2022, 32(3): 671-702. |
| 28 | El-Shabasy R M, Farouk Elsadek M, Mohamed Ahmed B, et al. Recent developments in carbon quantum dots: properties, fabrication techniques, and bio-applications[J]. Processes, 2021, 9(2): 388. |
| 29 | 张敏. 煤层气催化裂解制备纳米洋葱碳及其应用研究[D]. 太原: 太原理工大学, 2017. |
| Zhang M. Catalytic cracking of coal bed methane for carbon nano onions and their application research[D]. Taiyuan: Taiyuan University of Technology, 2017. | |
| 30 | He C N, Shi C S, Du X W, et al. TEM investigation on the initial stage growth of carbon onions synthesized by CVD[J]. Journal of Alloys and Compounds, 2008, 452(2): 258-262. |
| 31 | Xiao J, Liu P, Yang G W. Nanodiamonds from coal under ambient conditions[J]. Nanoscale, 2015, 7(14): 6114-6125. |
| 32 | Raj A M, Manoj B. Cost-effective route to nanodiamonds from low-rank coal and their fluorescent & dielectric characteristics[J]. Ceramics International, 2022, 48(1): 887-895. |
| 33 | Pang L S K. Fullerenes from brown (lignite) coal[J]. Fuel Processing Technology, 1993, 34(2): 147-155. |
| 34 | Qiu J S, Zhou Y, Yang Z G, et al. Preparation of fullerenes using carbon rods manufactured from Chinese hard coals[J]. Fuel, 2000, 79(11): 1303-1308. |
| 35 | Das T, Boruah P K, Das M R, et al. Formation of onion-like fullerene and chemically converted graphene-like nanosheets from low-quality coals: application in photocatalytic degradation of 2-nitrophenol[J]. RSC Advances, 2016, 6(42): 35177-35190. |
| 36 | Kang S, Kim K M, Son Y, et al. Graphene oxide quantum dots derived from coal for bioimaging: facile and green approach[J]. Scientific Reports, 2019, 9(1): 1-7. |
| 37 | Thiyagarajan S K, Raghupathy S, Palanivel D, et al. Fluorescent carbon nano dots from lignite: unveiling the impeccable evidence for quantum confinement[J]. Physical Chemistry Chemical Physics: PCCP, 2016, 18(17): 12065-12073. |
| 38 | Feng X T, Zhang Y. A simple and green synthesis of carbon quantum dots from coke for white light-emitting devices[J]. RSC Advances, 2019, 9(58): 33789-33793. |
| 39 | Li M Y, Yu C, Hu C, et al. Solvothermal conversion of coal into nitrogen-doped carbon dots with singlet oxygen generation and high quantum yield[J]. Chemical Engineering Journal, 2017, 320: 570-575. |
| 40 | Geng B J, Yang D W, Zheng F F, et al. Facile conversion of coal tar to orange fluorescent carbon quantum dots and their composite encapsulated by liposomes for bioimaging[J]. New Journal of Chemistry, 2017, 41(23): 14444-14451. |
| 41 | Bai J P, Xiao N, Wang Y W, et al. Coal tar pitch derived nitrogen-doped carbon dots with adjustable particle size for photocatalytic hydrogen generation[J]. Carbon, 2021, 174: 750-756. |
| 42 | Kundu N, Bhunia P, Sarkar S, et al. Highly fluorescent carbon dots from quinoline insoluble residues in coal tar[J]. Optical Materials, 2020, 100: 109638. |
| 43 | Awati A, Maimaiti H, Xu B, et al. A comparative study on the preparation methods and properties of coal-based fluorescent carbon nanoparticles[J]. Surface and Interface Analysis, 2020, 52(3): 98-109. |
| 44 | Gong Z B, Bai C N, Qiang L, et al. Onion-like carbon films endow macro-scale superlubricity[J]. Diamond and Related Materials, 2018, 87: 172-176. |
| 45 | Hu S L, Wei Z J, Chang Q, et al. A facile and green method towards coal-based fluorescent carbon dots with photocatalytic activity[J]. Applied Surface Science, 2016, 378: 402-407. |
| 46 | Prazyan T L, Dyagilev D V. The Kuzbass Basin coals as a raw material for the preparation of carbon quantum dots[J]. Journal of Physics: Conference Series, 2021, 1749(1): 012046. |
| 47 | Zhang Y T, Li K K, Ren S Z, et al. Coal-derived graphene quantum dots produced by ultrasonic physical tailoring and their capacity for Cu(Ⅱ) detection[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(11): 9793-9799. |
| 48 | Saikia M, Hower J C, Das T, et al. Feasibility study of preparation of carbon quantum dots from Pennsylvania anthracite and Kentucky bituminous coals[J]. Fuel, 2019, 243: 433-440. |
| 49 | Houtmeyers S, Degrève J, Willems K, et al. Comparing the influence of low power ultrasonic and microwave pre-treatments on the solubilisation and semi-continuous anaerobic digestion of waste activated sludge[J]. Bioresource Technology, 2014, 171: 44-49. |
| 50 | He M Q, Guo X R, Huang J Z, et al. Mass production of tunable multicolor graphene quantum dots from an energy resource of coke by a one-step electrochemical exfoliation[J]. Carbon, 2018, 140: 508-520. |
| 51 | Hu C, Yu C, Li M, et al. Nitrogen-doped carbon dots decorated on graphene: a novel all-carbon hybrid electrocatalyst for enhanced oxygen reduction reaction[J]. Chemical Communications, 2015, 51(16): 3419-3422. |
| 52 | Kroto H W. The stability of the fullerenes C n, with n=24, 28, 32, 36, 50, 60 and 70[J]. Nature, 1987, 329(6139): 529–531. |
| 53 | 邱介山, 罗长齐, 周颖, 等. 由冶金焦制备富勒烯的研究[J]. 燃料化学学报, 1997, 25(2): 109-113. |
| Qiu J S, Luo C Q, Zhou Y, et al. Preparation and characterization of fullerenes from metallurgical cokes[J]. Journal of Fuel Chemistry and Technology, 1997, 25(2): 109-113. | |
| 54 | 周颖, 邱介山, 杨兆国, 等. 富勒烯C60的制备研究最佳制备条件的确定[J]. 新型炭材料, 1999, 14(4): 17-21. |
| Zhou Y, Qiu J S, Yang Z G, et al. Study on the preparation fullerenes C60 the optimal preparation condition[J]. New Carbon Materials, 1999, 14(4): 17-21. | |
| 55 | Qiu J S, Li Y F, Wang Y P, et al. Preparation of carbon-coated magnetic iron nanoparticles from composite rods made from coal and iron powders[J]. Fuel Processing Technology, 2004, 86(3): 267-274. |
| 56 | 王海英, 王晓敏, 章海霞, 等. 电弧放电制备内包金属纳米洋葱状富勒烯的研究[J]. 材料热处理学报, 2003, 24(4):41-42. |
| Wang H Y, Wang X M, Zhang H X, et al. Studies on the preparation of metal encapsulated nanosized onion-like fullerenes by arc discharge[J]. Transactions of Materials and Heat Treatment, 2003, 24(4):41-42. | |
| 57 | Guo J J, Wang X M, Yao Y L, et al. Structure of nanocarbons prepared by arc discharge in water[J]. Materials Chemistry and Physics, 2007, 105(2/3): 175-178. |
| 58 | Ghorai S, Roy I, De S, et al. Exploration of the potential efficacy of natural resource-derived blue-emitting graphene quantum dots in cancer therapeutic applications[J]. New Journal of Chemistry, 2020, 44(14): 5366-5376. |
| 59 | Lian W T, Song H H, Chen X H, et al. The transformation of acetylene black into onion-like hollow carbon nanoparticles at 1000℃ using an iron catalyst[J]. Carbon, 2008, 46(3): 525-530. |
| 60 | Shenderova O A, Shames A I, Nunn N A, et al. Synthesis, properties, and applications of fluorescent diamond particles[J]. Journal of Vacuum Science & Technology B, 2019, 37(3): 030802. |
| 61 | Torelli M D, Nunn N A, Shenderova O A. A perspective on fluorescent nanodiamond bioimaging[J]. Small, 2019, 15(48): 1902151. |
| 62 | Ma B, Sun Y P. Fluorescence spectra and quantum yields of [60] fullerene and [70] fullerene under different solvent conditions. A quantitative examination using a near-infrared-sensitive emission spectrometer[J]. Journal of the Chemical Society, Perkin Transactions 2, 1996(10): 2157. |
| 63 | Hutchison K, Gao J, Schick G, et al. Bucky light bulbs: white light electroluminescence from a fluorescent C60 adduct-single layer organic LED[J]. Journal of the American Chemical Society, 1999, 121(23): 5611-5612. |
| 64 | Cheng J X, Fang Y, Huang Q J, et al. Blue-green photoluminescence from pyridine-C60 adduct[J]. Chemical Physics Letters, 2000, 330(3/4): 262-266. |
| 65 | Bradley S J, Kroon R, Laufersky G, et al. Heterogeneity in the fluorescence of graphene and graphene oxide quantum dots[J]. Microchimica Acta, 2017, 184(3): 871-878. |
| 66 | Semeniuk M, Yi Z, Poursorkhabi V, et al. Future perspectives and review on organic carbon dots in electronic applications[J]. ACS Nano, 2019, 13(6): 6224-6255. |
| 67 | Zang J B, Wang Y H, Zhao S Z, et al. Electrochemical properties of nanodiamond powder electrodes[J]. Diamond and Related Materials, 2007, 16(1): 16-20. |
| 68 | 孙明晓, 邝普兴, 解增旗, 等. 电化学聚合制备含富勒烯交联聚合物薄膜及超级电容器应用[J]. 高分子学报, 2018(2): 231-238. |
| Sun M X, Kuang P X, Xie Z Q, et al. Synthesis of cross-linked thin films containing fullerene units and their performance of supercapacitor[J]. Acta Polymerica Sinica, 2018(2): 231-238. | |
| 69 | Yue T, Shen B X, Gao P. Carbon material/MnO2 as conductive skeleton for supercapacitor electrode material: a review[J]. Renewable and Sustainable Energy Reviews, 2022, 158: 112131. |
| 70 | Tian L, Li Z, Wang P, et al. Carbon quantum dots for advanced electrocatalysis[J]. Journal of Energy Chemistry, 2021, 55: 279-294. |
| 71 | He Z G, Liu S J, Zhang C, et al. Coal based carbon dots: recent advances in synthesis, properties, and applications[J]. Nano Select, 2021, 2(9): 1589-1604. |
| 72 | Yu J, Zhang C X, Yang Y L, et al. Lignite-derived carbon quantum dot/TiO2 heterostructure nanocomposites: photoinduced charge transfer properties and enhanced visible light photocatalytic activity[J]. New Journal of Chemistry, 2019, 43(46): 18355-18368. |
| 73 | Bharadwaj N, Nair A S, Das S, et al. Size-dependent effects in fullerene-based catalysts for nonaqueous Li-air battery applications[J]. ACS Applied Energy Materials, 2022, 5(3): 3380-3391. |
| 74 | Camisasca A, Sacco A, Brescia R, et al. Boron/nitrogen-codoped carbon nano-onion electrocatalysts for the oxygen reduction reaction[J]. ACS Applied Nano Materials, 2018, 1(10): 5763-5773. |
| 75 | Han M, Lu S Y, Qi F, et al. Carbon dots-implanted graphitic carbon nitride nanosheets for photocatalysis: simultaneously manipulating carrier transport in inter-and intralayers[J]. Solar RRL, 2020, 4(4): 1900517. |
| 76 | Sarma D, Majumdar B, Sarma T K. Visible-light induced enhancement in the multi-catalytic activity of sulfated carbon dots for aerobic carbon-carbon bond formation[J]. Green Chemistry, 2019, 21(24): 6717-6726. |
| 77 | 陈克龙, 黄建花, g-C 3N4-CdS-NiS2复合纳米管的制备及可见光催化分解水制氢[J]. 化工学报, 2020, 71(1): 397-408. |
| Chen K L, Huang J H. g-C3N4-CdS-NiS2 composite nanotube: synthesis and its photocatalytic activity for H2 generation under visible light [J]. CIESC Journal, 2020, 71(1): 397-408. | |
| 78 | Yu H J, Shi R, Zhao Y F, et al. Smart utilization of carbon dots in semiconductor photocatalysis[J]. Advanced Materials, 2016, 28(43): 9454-9477. |
| 79 | Kovalchuk A, Huang K W, Xiang C S, et al. Luminescent polymer composite films containing coal-derived graphene quantum dots[J]. ACS Applied Materials & Interfaces, 2015, 7(47): 26063-26068. |
| 80 | Hu C, Yu C, Li M Y, et al. Chemically tailoring coal to fluorescent carbon dots with tuned size and their capacity for Cu(Ⅱ) detection[J]. Small, 2014, 10(23): 4926-4933. |
| 81 | Yew Y T, Loo A H, Sofer Z, et al. Coke-derived graphene quantum dots as fluorescence nanoquencher in DNA detection[J]. Applied Materials Today, 2017, 7: 138-143. |
| 82 | Campos-Cuerva C, Zieba M, Sebastian V, et al. Screen-printed nanoparticles as anti-counterfeiting tags[J]. Nanotechnology, 2016, 27(9): 095702. |
| 83 | Leifgen M, Schröder T, Gädeke F, et al. Evaluation of nitrogen- and silicon-vacancy defect centres as single photon sources in quantum key distribution[J]. New Journal of Physics, 2014, 16(2): 023021. |
| 84 | Meng Y S, Wang G R, Xiao M J, et al. Ionic liquid-derived Co3O4/carbon nano-onions composite and its enhanced performance as anode for lithium-ion batteries[J]. Journal of Materials Science, 2017, 52(22): 13192-13202. |
| 85 | Zhang E J, Jia X X, Wang B, et al. Carbon dots@rGO paper as freestanding and flexible potassium-ion batteries anode[J]. Advanced Science, 2020, 7(15): 2000470. |
| 86 | Zhang Y T, Zhang K B, Jia K L, et al. Preparation of coal-based graphene quantum dots/α-Fe2O3 nanocomposites and their lithium-ion storage properties[J]. Fuel, 2019, 241: 646-652. |
| 87 | Zheng S, Ma J, Wu Z S, et al. All-solid-state flexible planar lithium ion micro-capacitors[J]. Energy & Environmental Science, 2018, 11(8): 2001-2009. |
| 88 | Yu S Y, Xu J, Kato H, et al. Phosphorus-doped nanocrystalline diamond for supercapacitor application[J]. ChemElectroChem, 2019, 6(4): 1088-1093. |
| 89 | Gao Y, Zhou Y S, Qian M, et al. Chemical activation of carbon nano-onions for high-rate supercapacitor electrodes[J]. Carbon, 2013, 51: 52-58. |
| 90 | Zhang S, Zhu J Y, Qing Y, et al. Ultramicroporous carbons puzzled by graphene quantum dots: integrated high gravimetric, volumetric, and areal capacitances for supercapacitors[J]. Advanced Functional Materials, 2018, 28(52): 1805898. |
| 91 | Zhang S, Zhu J Y, Qing Y, et al. Construction of hierarchical porous carbon nanosheets from template-assisted assembly of coal-based graphene quantum dots for high performance supercapacitor electrodes[J]. Materials Today Energy, 2017, 6: 36-45. |
| 92 | Ni M, Leung M K H, Leung D Y C, et al. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production[J]. Renewable and Sustainable Energy Reviews, 2007, 11(3): 401-425. |
| 93 | Regulska E, Olejnik P, Zubyk H, et al. Nanostructural catalyst: metallophthalocyanine and carbon nano-onion with enhanced visible-light photocatalytic activity towards organic pollutants[J]. RSC Advances, 2020, 10(18): 10910-10920. |
| 94 | Fu Y Y, Liu Y, Li H. Onion-like carbon-modified TiO2 coating by suspension plasma spray with enhanced photocatalytic performances[J]. Journal of Nanoparticle Research, 2019, 21(8): 1-12. |
| 95 | Qu Y M, Chen T, Wang G Y. Hydrogenation of nitrobenzene catalyzed by Pd promoted Ni supported on C60 derivative[J]. Applied Surface Science, 2019, 465: 888-894. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [3] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [4] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [5] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [6] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [7] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [8] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [9] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [10] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [11] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [12] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [13] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [14] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [15] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号