化工学报 ›› 2023, Vol. 74 ›› Issue (1): 86-104.DOI: 10.11949/0438-1157.20221188
熊昊( ), 梁潇予, 张晨曦(
), 梁潇予, 张晨曦( ), 白浩隆, 范晓宇, 魏飞(
), 白浩隆, 范晓宇, 魏飞( )
)
收稿日期:2022-08-30
修回日期:2023-01-17
出版日期:2023-01-05
发布日期:2023-03-20
通讯作者:
张晨曦,魏飞
作者简介:熊昊(1997—),男,博士研究生,xiongh19@mails.tsinghua.edu.cn
基金资助:
Hao XIONG( ), Xiaoyu LIANG, Chenxi ZHANG(
), Xiaoyu LIANG, Chenxi ZHANG( ), Haolong BAI, Xiaoyu FAN, Fei WEI(
), Haolong BAI, Xiaoyu FAN, Fei WEI( )
)
Received:2022-08-30
Revised:2023-01-17
Online:2023-01-05
Published:2023-03-20
Contact:
Chenxi ZHANG, Fei WEI
摘要:
利用有限的石油资源生产高附加值化工原料(低碳烯烃与芳烃)是石油资源低碳高效利用的重要途径。基于我国的能源结构和在催化裂化领域的技术优势,重质原料油在分子筛限域催化下裂解直接制低碳烯烃与芳烃是我国炼油产业的重要发展方向。这对涉及分子筛内离散传递、吸附与反应等过程的调控和多相反应器的设计提出了挑战:(1)分子筛内大分子及芳烃的强吸附对低碳烯烃的传递造成较大的阻力,要求高剂油比及逆流接触,防止重质芳烃的优先吸附并实现低碳烯烃的深度裂解;(2)二次反应速度比一次反应快,要求毫秒级接触时间和近平推流的停留时间分布方可得到高的中间化学品收率。解决这些问题需要在催化剂及反应器两方面同时改进。在这个背景下,本文重点介绍了重质油催化裂化直接制化工品中的离散传递、反应与失活过程,以及气固多相反应工程等相关领域的进展,分析了小分子在分子筛中吸附扩散和相关主客体相互作用研究的最新工作,提出应使用具有晶面选择性的抗积炭高活性纳米分子筛和气固逆流接触的平推流反应器。最后介绍了清华大学自主研发的多级逆流下行催化裂解技术(multi-stage downer catalytic pyrolysis,MDCPTM)——通过毫秒级平推流的多级气固并流顺重力下行反应器,级间油气与催化剂逆流接触,大大提高了乙烯、丙烯收率,并减少柴油、油浆收率。1 kg/h的全流程实验结果表明MDCPTM单程双烯收率高达51.54%(质量分数,下同),且汽油中单环芳烃选择性可达80.78%。以MDCPTM为核心单元的重质油直接制化工品(heavy oil to chemicals, HOTC)工艺路线可以得到大于75%的综合化工品收率,与现有技术相比可以降低70%以上的碳排放。
中图分类号:
熊昊, 梁潇予, 张晨曦, 白浩隆, 范晓宇, 魏飞. 重质油直接制化工品:多级逆流下行催化裂解技术[J]. 化工学报, 2023, 74(1): 86-104.
Hao XIONG, Xiaoyu LIANG, Chenxi ZHANG, Haolong BAI, Xiaoyu FAN, Fei WEI. Heavy oil to chemicals: multi-stage downer catalytic pyrolysis[J]. CIESC Journal, 2023, 74(1): 86-104.
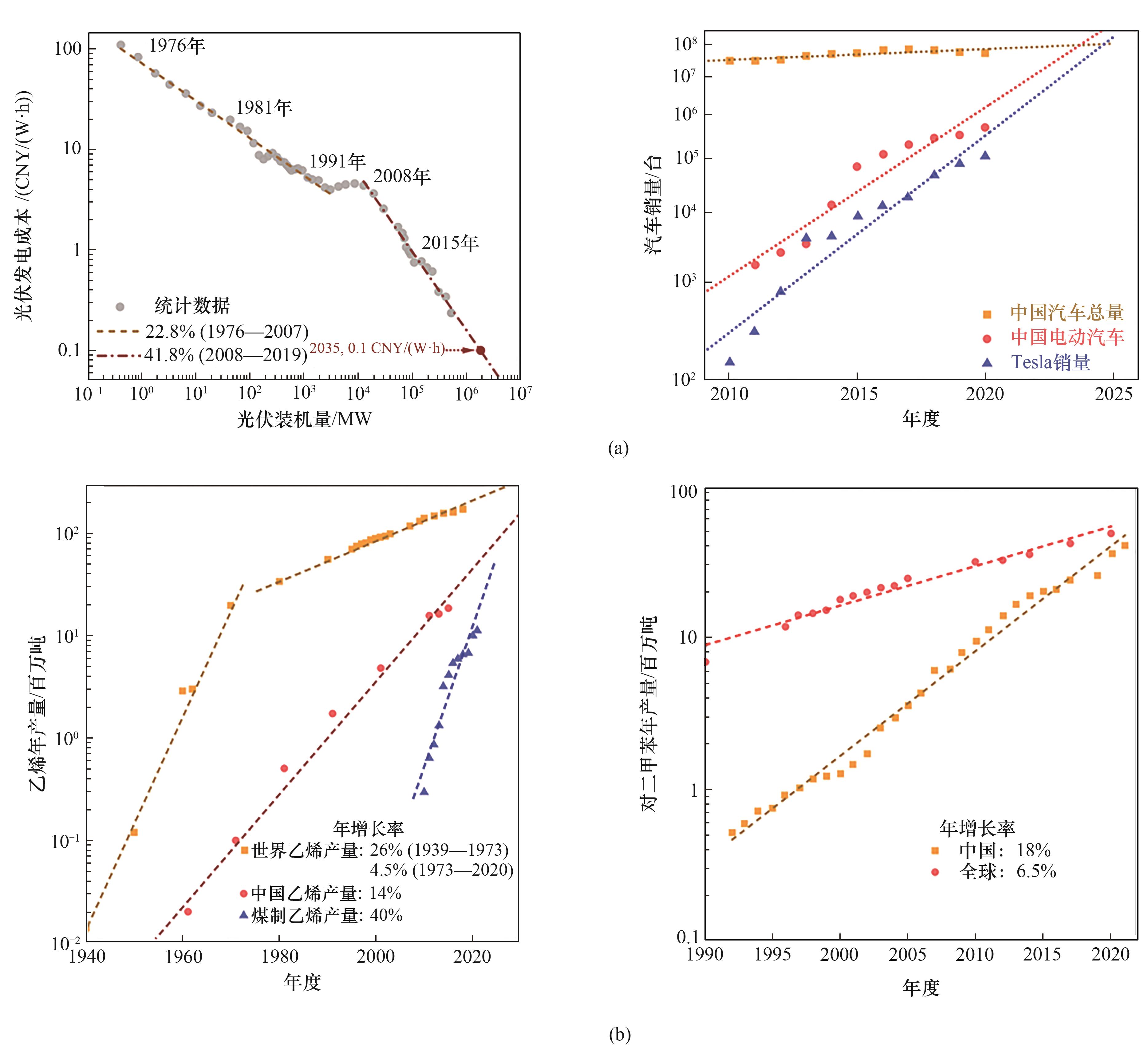
图1 (a)光伏产业的学习曲线与电动车增长趋势;(b)乙烯与对二甲苯产能的增长曲线
Fig.1 (a) Learning curve of the photovoltaic industry and growing trend of electric vehicles; (b) Growth curves of ethylene and para-xylene production capacity
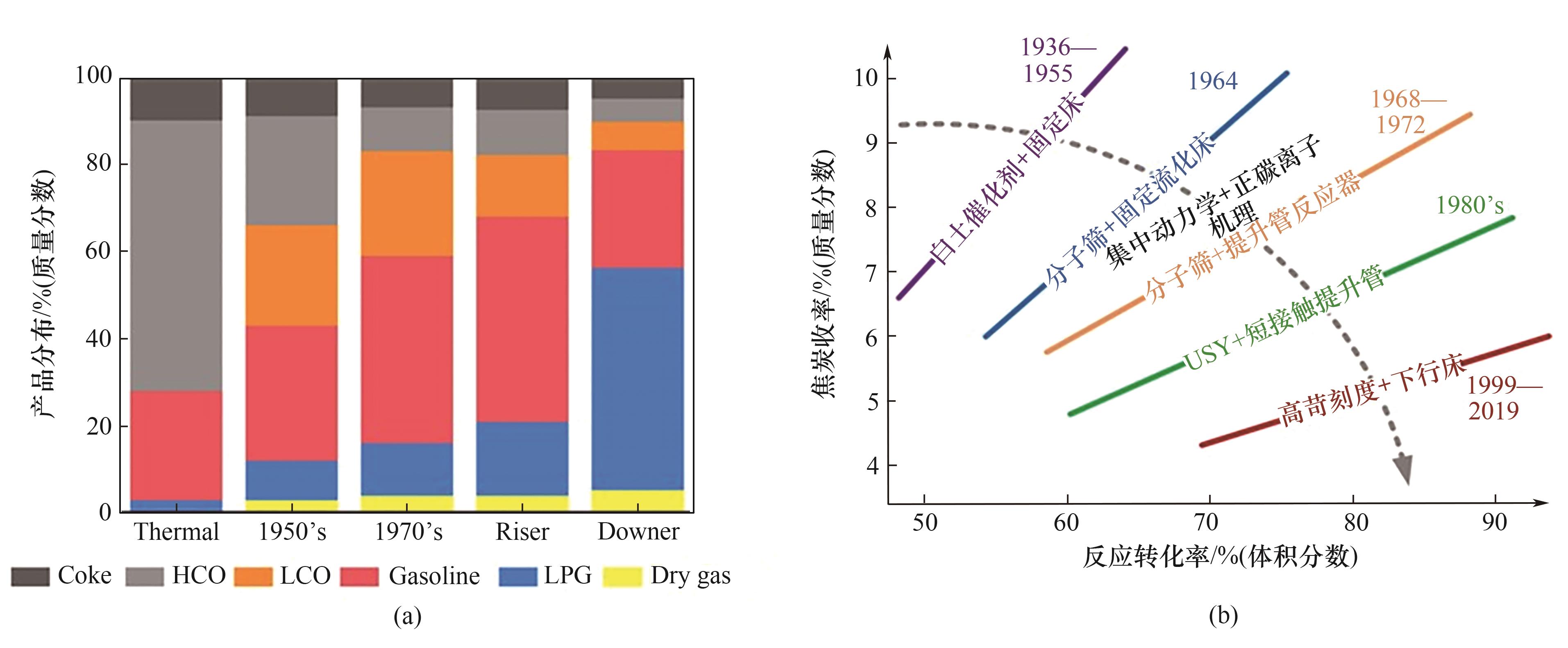
图3 (a)随着催化剂和反应器技术发展催化裂化产品分布的变化;(b)催化裂化的技术发展路径[5]
Fig.3 (a) Changes in the product distribution of catalytic cracking with the development of catalyst and reactor technology; (b) Technological development path of catalytic cracking[5]

图10 (a)提升管与下行床内的停留时间分布;(b)无分布器湍动入口形式[100];(c)气固快分结构[103]
Fig.10 (a) The residence time distribution in riser and downer; (b) The special inlet of downer without distributor[100]; (c)The fast separator of downer[103]
| 项目 | 数值 |
|---|---|
| 密度 | 0.904 g/cm3 |
| 黏度 | |
| 100℃ | 11.13 mm2/s |
| 80℃ | 19.27 mm2/s |
| 残炭 | 3.74% |
| 馏程 | |
| 初馏点 | 281.6℃ |
| 10% | 373.9℃ |
| 30% | 418.0℃ |
| 50% | 464.8℃ |
| 70% | 530.5℃ |
| 金属分析 | |
| Ni | 5.5 μg/g |
| V | 6.7 μg/g |
| Fe | 7.7 μg/g |
| Na | 1.3 μg/g |
| Cu | 0.2 μg/g |
| Ca | 3.2 μg/g |
| 四组分 | |
| 饱和分 | 66.4%(质量分数) |
| 芳香分 | 21.5%(质量分数) |
| 胶质 | 11.1%(质量分数) |
| 沥青质 | 1.0%(质量分数) |
| 元素分析 | |
| C | 86.36%(质量分数) |
| H | 12.81%(质量分数) |
| N | 0.19%(质量分数) |
| S | 0.45%(质量分数) |
表1 实验原料油性质
Table 1 The feed oil property for downer pilot plant
| 项目 | 数值 |
|---|---|
| 密度 | 0.904 g/cm3 |
| 黏度 | |
| 100℃ | 11.13 mm2/s |
| 80℃ | 19.27 mm2/s |
| 残炭 | 3.74% |
| 馏程 | |
| 初馏点 | 281.6℃ |
| 10% | 373.9℃ |
| 30% | 418.0℃ |
| 50% | 464.8℃ |
| 70% | 530.5℃ |
| 金属分析 | |
| Ni | 5.5 μg/g |
| V | 6.7 μg/g |
| Fe | 7.7 μg/g |
| Na | 1.3 μg/g |
| Cu | 0.2 μg/g |
| Ca | 3.2 μg/g |
| 四组分 | |
| 饱和分 | 66.4%(质量分数) |
| 芳香分 | 21.5%(质量分数) |
| 胶质 | 11.1%(质量分数) |
| 沥青质 | 1.0%(质量分数) |
| 元素分析 | |
| C | 86.36%(质量分数) |
| H | 12.81%(质量分数) |
| N | 0.19%(质量分数) |
| S | 0.45%(质量分数) |
| 项目 | 质量指标 | |
|---|---|---|
| LTD-主剂 | LTD-助剂 | |
| 灼烧减量(湿基)/%(质量分数) | <13.0 | <13.0 |
| Na2O | <0.30 | <0.30 |
| 筛分组成 | ||
| 0~20 μm | <3.0 | <3.0 |
| 0~40 μm | <18.0 | <15.0 |
| 0~149 μm | >88.0 | >81.0 |
| APS/μm | 65.0~80.0 | 65.0~80.0 |
| 孔体积/(ml/g) | >0.33 | >0.20 |
| 磨损指数(干基)/%(质量分数) | <2.0 | <3.5 |
| 表观密度/(g/ml) | 0.65~0.75 | 0.60~0.80 |
| 比表面积/(m2/g) | >220 | >80 |
| 微反活性(800℃, 4 h)/% | >75 | >30 |
表2 下行专用高活性催化剂
Table 2 The special catalyst with high activity and selectivity for DCP
| 项目 | 质量指标 | |
|---|---|---|
| LTD-主剂 | LTD-助剂 | |
| 灼烧减量(湿基)/%(质量分数) | <13.0 | <13.0 |
| Na2O | <0.30 | <0.30 |
| 筛分组成 | ||
| 0~20 μm | <3.0 | <3.0 |
| 0~40 μm | <18.0 | <15.0 |
| 0~149 μm | >88.0 | >81.0 |
| APS/μm | 65.0~80.0 | 65.0~80.0 |
| 孔体积/(ml/g) | >0.33 | >0.20 |
| 磨损指数(干基)/%(质量分数) | <2.0 | <3.5 |
| 表观密度/(g/ml) | 0.65~0.75 | 0.60~0.80 |
| 比表面积/(m2/g) | >220 | >80 |
| 微反活性(800℃, 4 h)/% | >75 | >30 |
| 项目 | DCP-Ⅱ | DCP-Ⅰ | 某炼厂提升管 |
|---|---|---|---|
| 反应温度/℃ | 600 | 550 | ~507 |
| 剂油比/(kg/kg) | 30 | 30 | ~8 |
| 停留时间/s | 0.40 | 0.40 | ~2 |
| 产品收率/%(质量分数) | |||
| 干气 | 5.30 | 3.02 | 4.02 |
| 氢气 | 0.05 | 0.02 | — |
| 甲烷 | 1.19 | 0.41 | 2.16 |
| 乙烷 | 0.45 | 0.21 | — |
| 乙烯 | 3.61 | 2.37 | 1.86 |
| 液化气 | 50.88 | 48.99 | 16.86 |
| 丙烷 | 2.16 | 1.78 | 1.37 |
| 丙烯 | 18.18 | 16.29 | 5.53 |
| 汽油 | 27.00 | 30.60 | 47.1 |
| 柴油 | 6.49 | 6.92 | 14.13 |
| 重油 | 5.30 | 6.03 | 10.32 |
| 焦炭 | 5.00 | 5.41 | 7.43 |
| 损失/%(质量分数) | 0.03 | -0.97 | 0.14 |
| 转化率/%(质量分数) | 88.21 | 87.05 | 75.55 |
表3 DCP与传统提升管产品分布的对比
Table 3 The comparison between product distribution of riser with DCP
| 项目 | DCP-Ⅱ | DCP-Ⅰ | 某炼厂提升管 |
|---|---|---|---|
| 反应温度/℃ | 600 | 550 | ~507 |
| 剂油比/(kg/kg) | 30 | 30 | ~8 |
| 停留时间/s | 0.40 | 0.40 | ~2 |
| 产品收率/%(质量分数) | |||
| 干气 | 5.30 | 3.02 | 4.02 |
| 氢气 | 0.05 | 0.02 | — |
| 甲烷 | 1.19 | 0.41 | 2.16 |
| 乙烷 | 0.45 | 0.21 | — |
| 乙烯 | 3.61 | 2.37 | 1.86 |
| 液化气 | 50.88 | 48.99 | 16.86 |
| 丙烷 | 2.16 | 1.78 | 1.37 |
| 丙烯 | 18.18 | 16.29 | 5.53 |
| 汽油 | 27.00 | 30.60 | 47.1 |
| 柴油 | 6.49 | 6.92 | 14.13 |
| 重油 | 5.30 | 6.03 | 10.32 |
| 焦炭 | 5.00 | 5.41 | 7.43 |
| 损失/%(质量分数) | 0.03 | -0.97 | 0.14 |
| 转化率/%(质量分数) | 88.21 | 87.05 | 75.55 |
| 项目 | 提升管 | 下行床 | 多级下行床 |
|---|---|---|---|
| 油气+催化剂流向 | 并流向上 | 并流向下 | 气固逆流 |
| 催化剂返混 | 有 | 基本无 | 基本无 |
| 反应推动力 | 小 | 小 | 大 |
| 芳烃竞争吸附 | 有限制 | 有限制 | 基本无 |
| 反应时间/s | 2~5 | < 1 | < 1 |
| 剂油比/(kg/kg) | < 15 | ~ 30 | ~30 |
| 甲烷氢与焦炭 | 高 | 低 | 低 |
| 二次反应 | 强 | 弱 | 弱 |
表4 提升管、下行式以及多级下行床反应器的对比
Table 4 The comparison between riser, single downer and multi-stage downer
| 项目 | 提升管 | 下行床 | 多级下行床 |
|---|---|---|---|
| 油气+催化剂流向 | 并流向上 | 并流向下 | 气固逆流 |
| 催化剂返混 | 有 | 基本无 | 基本无 |
| 反应推动力 | 小 | 小 | 大 |
| 芳烃竞争吸附 | 有限制 | 有限制 | 基本无 |
| 反应时间/s | 2~5 | < 1 | < 1 |
| 剂油比/(kg/kg) | < 15 | ~ 30 | ~30 |
| 甲烷氢与焦炭 | 高 | 低 | 低 |
| 二次反应 | 强 | 弱 | 弱 |
| 项目 | 数值 |
|---|---|
| 密度 (20℃) | 0.91 g/cm3 |
| 平均分子量 | 488 |
| 残炭 | 4.13%(质量分数) |
| 元素分析 | |
| C | 86.48%(质量分数) |
| H | 12.98%(质量分数) |
| S | 0.33%(质量分数) |
| N | 0.19%(质量分数) |
| 黏度 | |
| 80℃ | 46.76 mm2/s |
| 100℃ | 24.56 mm2/s |
| 金属含量 | |
| Fe | 3.6μg/g |
| Na | 1.7μg/g |
| Ni | 6.9μg/g |
| V | 2.3μg/g |
| Ca | 1.1μg/g |
| 烃族组成 | |
| 饱和烃 | 62%(质量分数) |
| 芳烃 | 23%(质量分数) |
| 胶质 | 13%(质量分数) |
| 沥青质 | 2%(质量分数) |
| 馏程分析 | |
| 初馏点 | 260℃ |
| 5% | 330℃ |
| 10% | 368℃ |
| 50% | 486℃ |
表5 实验原料油性质
Table 5 The feed-oil property for MDCPTM
| 项目 | 数值 |
|---|---|
| 密度 (20℃) | 0.91 g/cm3 |
| 平均分子量 | 488 |
| 残炭 | 4.13%(质量分数) |
| 元素分析 | |
| C | 86.48%(质量分数) |
| H | 12.98%(质量分数) |
| S | 0.33%(质量分数) |
| N | 0.19%(质量分数) |
| 黏度 | |
| 80℃ | 46.76 mm2/s |
| 100℃ | 24.56 mm2/s |
| 金属含量 | |
| Fe | 3.6μg/g |
| Na | 1.7μg/g |
| Ni | 6.9μg/g |
| V | 2.3μg/g |
| Ca | 1.1μg/g |
| 烃族组成 | |
| 饱和烃 | 62%(质量分数) |
| 芳烃 | 23%(质量分数) |
| 胶质 | 13%(质量分数) |
| 沥青质 | 2%(质量分数) |
| 馏程分析 | |
| 初馏点 | 260℃ |
| 5% | 330℃ |
| 10% | 368℃ |
| 50% | 486℃ |
| 项目 | 反应器形式 | |||
|---|---|---|---|---|
| DCP | DCP | DCP | MDCPTM | |
| 反应温度/℃ | 550 | 600 | 650 | 620/670 |
| 剂油比/(kg/kg) | 30 | 30 | 30 | 30 |
| 停留时间/s | 0.8 | 0.8 | 0.8 | 0.3/0.5 |
| 产品分布/%(质量分数) | ||||
| 甲烷氢 | 1.06 | 1.16 | 1.62 | 7.91 |
| 乙烯 | 2.74 | 2.88 | 3.43 | 16.06 |
| 丙烯 | 19.29 | 20.32 | 20.55 | 24.57 |
| 丁烯 | 16.66 | 17.47 | 19.98 | 10.91 |
| 轻质烯烃 | 38.69 | 40.68 | 43.96 | 51.54 |
| 汽油 | 27.84 | 26.93 | 26.19 | 14.27 |
| 汽油芳烃 | 61.47 | 67.11 | 69.58 | 80.78 |
| BTEX | 6.19 | 10.81 | 12.89 | 9.32 |
| 柴油+煤油 | 15.81 | 13.36 | 10.21 | 9.23 |
| 焦炭 | 6.99 | 7.65 | 8.60 | 9.54 |
表6 下行催化裂解(DCP)与多级逆流下行催化裂解(MDCPTM)产品分布的对比
Table 6 The comparison between product distribution of DCP with MDCPTM
| 项目 | 反应器形式 | |||
|---|---|---|---|---|
| DCP | DCP | DCP | MDCPTM | |
| 反应温度/℃ | 550 | 600 | 650 | 620/670 |
| 剂油比/(kg/kg) | 30 | 30 | 30 | 30 |
| 停留时间/s | 0.8 | 0.8 | 0.8 | 0.3/0.5 |
| 产品分布/%(质量分数) | ||||
| 甲烷氢 | 1.06 | 1.16 | 1.62 | 7.91 |
| 乙烯 | 2.74 | 2.88 | 3.43 | 16.06 |
| 丙烯 | 19.29 | 20.32 | 20.55 | 24.57 |
| 丁烯 | 16.66 | 17.47 | 19.98 | 10.91 |
| 轻质烯烃 | 38.69 | 40.68 | 43.96 | 51.54 |
| 汽油 | 27.84 | 26.93 | 26.19 | 14.27 |
| 汽油芳烃 | 61.47 | 67.11 | 69.58 | 80.78 |
| BTEX | 6.19 | 10.81 | 12.89 | 9.32 |
| 柴油+煤油 | 15.81 | 13.36 | 10.21 | 9.23 |
| 焦炭 | 6.99 | 7.65 | 8.60 | 9.54 |
| 1 | 林世雄. 石油炼制工程[M]. 3版. 北京: 石油工业出版社, 2000. |
| Lin S X. Petroleum Refining Engineering[M]. 3rd ed. Beijing: Petroleum Industry Press, 2000. | |
| 2 | Energy Informaion Adminstration U.S.. Petroleum supply annual[R]. Washington: U.S. Energy Information Administration, 2022. |
| 3 | Fahim M A, Alsahhaf T A, Elkilani A. Fundamentals of Petroleum Refining[M]. Amsterdam: Elsevier, 2010. |
| 4 | Wojciechowski B W, Corma A. Catalytic Cracking: Catalysts, Chemistry, and Kinetics[M]. New York : Marcel Dekker, 1986. |
| 5 | Vogt E C, Weckhuysen B M. Fluid catalytic cracking: recent developments on the grand old lady of zeolite catalysis[J]. Chemical Society Reviews, 2015, 44(20): 7342-7370. |
| 6 | 杨朝合, 陈小博, 李春义, 等. 催化裂化技术面临的挑战与机遇[J]. 中国石油大学学报(自然科学版), 2017, 41(6): 171-177. |
| Yang C H, Chen X B, Li C Y, et al. Challenges and opportunities of fluid catalytic cracking technology[J]. Journal of China University of Petroleum (Edition of Natural Science), 2017, 41(6): 171-177. | |
| 7 | 安超. 全球对二甲苯供需分析与预测[J]. 世界石油工业, 2021, 28(3): 37-43. |
| An C. Analysis and forecast of global p-xylene supply and demand[J]. World Petroleum Industry, 2021, 28(3): 37-43. | |
| 8 | 陈俊武. 催化裂化工艺与工程[M]. 2版. 北京: 中国石化出版社, 2005. |
| Chen J W. Catalytic Cracking Process and Engineering[M]. 2nd ed. Beijing: China Petrochemical Press, 2005. | |
| 9 | Wauquier J P, Smith D H. Crude Oil, Petroleum Products, Process Flowsheets[M]. Paris: Editions Technip, 1996. |
| 10 | Cheng W C, Kim G, Peters A W, et al. Environmental fluid catalytic cracking technology[J]. Catalysis Reviews, 1998, 40(1/2): 39-79. |
| 11 | Taufiqurrahmi N, Mohamed A R, Bhatia S. Deactivation and coke combustion studies of nanocrystalline zeolite beta in catalytic cracking of used palm oil[J]. Chemical Engineering Journal, 2010, 163(3): 413-421. |
| 12 | Ibáñez M, Valle B, Bilbao J, et al. Effect of operating conditions on the coke nature and HZSM-5 catalysts deactivation in the transformation of crude bio-oil into hydrocarbons[J]. Catalysis Today, 2012, 195(1): 106-113. |
| 13 | Doronin V P, Lipin P V, Sorokina T P. Effect of process conditions on the composition of products in the conventional and deep catalytic cracking of oil fractions[J]. Catalysis in Industry, 2012, 4(2): 100-104. |
| 14 | Deng R S, Wei F, Jin Y, et al. Experimental study of the deep catalytic cracking process in a downer reactor[J]. Industrial & Engineering Chemistry Research, 2002, 41(24): 6015-6019. |
| 15 | Fujiyama Y, Al-Tayyar M H, Dean C F, et al. Development of high-severity FCC process: an overview[J]. Studies in Surface Science and Catalysis, 2007, 166: 1-12. |
| 16 | Kärger J, Heink W, Pfeifer H, et al. N.M.R. evidence of the existence of surface barriers on zeolite crystallites[J]. Zeolites, 1982, 2(4): 275-278. |
| 17 | Karwacki L, Kox M H F, de Winter D A M, et al. Morphology-dependent zeolite intergrowth structures leading to distinct internal and outer-surface molecular diffusion barriers[J]. Nature Materials, 2009, 8(12): 959-965. |
| 18 | Krishna R. Diffusion in porous crystalline materials[J]. Chemical Society Reviews, 2012, 41(8): 3099-3118. |
| 19 | Kockmann N. History of Distillation[M]. Amsterdam: Elsevier, 2014: 1-43. |
| 20 | Greensfelder B S, Voge H H, Good G M. Catalytic and thermal cracking of pure hydrocarbons: mechanisms of reaction[J]. Industrial & Engineering Chemistry, 1949, 41(11): 2573-2584. |
| 21 | Miale J, Chen N Y, Weisz P. Catalysis by crystalline aluminosilicates (Ⅳ): Attainable catalytic cracking rate constants, and superactivity[J]. Journal of Catalysis, 1966, 6(2): 278-287. |
| 22 | Houdry E, Burt W F, Pew A, et al. The houdry process[J]. Oil and Gas Journal, Engineering and Operating Section, 1938, 37: 40-45. |
| 23 | Avidan A A, Shinnar R. Development of catalytic cracking technology. A lesson in chemical reactor design[J]. Industrial & Engineering Chemistry Research, 1990, 29(6): 931-942. |
| 24 | Eigenberger G, Ruppel W. Catalytic fixed-bed reactors[M]// Ullmann’s Encyclopedia of Industrial Chemistry. Berlin: Wiley-VCH, 2000. |
| 25 | 张执刚, 谢朝钢, 施至诚, 等. 催化热裂解制取乙烯和丙烯的工艺研究[J]. 石油炼制与化工, 2001(5): 21-24. |
| Zhang Z G, Xie C G, Shi Z C, et al. Study on catalytic pyrolysis process for ethylene and propylene production[J]. Petroleum Processing and Petrochemicals, 2001(5): 21-24. | |
| 26 | Magee J, Dolbear G. Catalytic cracking[M]// Petroleum Catalysis in Nontechnical Language. Tulsa: PennWell Publishing Company, 1998: 53-93. |
| 27 | Ward J W. Hydrocracking processes and catalysts[J]. Fuel Processing Technology, 1993, 35(1/2): 55-85. |
| 28 | Kunii D, Levenspiel O. Fluidization Engineering[M]. 2nd ed. Boston: Butterworths, 1991. |
| 29 | Wei J, Norman E. Lie algebraic solution of linear differential equations[J]. Journal of Mathematical Physics, 1963, 4: 575-581. |
| 30 | Weekman Jr V W, Nace D M. Kinetics of catalytic cracking selectivity in fixed, moving, and fluid bed reactors [J]. AIChE Journal, 1970, 16(3): 397-404. |
| 31 | Olah G A, Tolgyesi W S, Kuhn S J, et al. Stable carbonium ions. IV. 1a Secondary and tertiary alkyl and aralkyl oxocarbonium hexafluoroantimonates. Formation and identification of the trimethylcarbonium ion by decarbonylation of the tert-butyl oxocarbonium ion[J]. Journal of the American Chemical Society, 1963, 85(9): 1328-1334. |
| 32 | Theologos K N, Markatos N C. Advanced modeling of fluid catalytic cracking riser-type reactors[J]. AIChE Journal, 1993, 39(6): 1007-1017. |
| 33 | Degnan T F, Chitnis G K, Schipper P H. History of ZSM-5 fluid catalytic cracking additive development at Mobil[J]. Microporous and Mesoporous Materials, 2000, 35/36: 245-252. |
| 34 | Gholami Z, Gholami F, Tišler Z, et al. A review on production of light olefins via fluid catalytic cracking[J]. Energies, 2021, 14(4): 1089. |
| 35 | Biswas J, Maxwell I E. Recent process- and catalyst-related developments in fluid catalytic cracking[J]. Applied Catalysis, 1990, 63(1): 197-258. |
| 36 | Meng X H, Gao J S, Li L, et al. Advances in catalytic pyrolysis of hydrocarbons[J]. Petroleum Science and Technology, 2004, 22(9/10): 1327-1341. |
| 37 | Ino T, Fujiyama Y, Redhwi H, et al. A new FCC process upgrades gasoline and maximizes propylene[J]. Catalagram, 2004, 94: 45-49. |
| 38 | Eng C, Heidenreich S, Swart S, et al. Clean fuels and petrochemicals at SASOL via SUPERFLEXTM [C]//18th World Petroleum Congress. Johannesburg: OnePetro, 2005. |
| 39 | Yarulina I, Chowdhury A D, Meirer F, et al. Recent trends and fundamental insights in the methanol-to-hydrocarbons process[J]. Nature Catalysis, 2018, 1(6): 398-411. |
| 40 | Tian P, Wei Y X, Ye M, et al. Methanol to olefins (MTO): from fundamentals to commercialization[J]. ACS Catalysis, 2015, 5(3): 1922-1938. |
| 41 | Chen Y J, Zhou H Q, Zhu J, et al. Direct synthesis of a fluidizable SAPO-34 catalyst for a fluidized dimethyl ether-to-olefins process[J]. Catalysis Letters, 2008, 124(3): 297-303. |
| 42 | Zeeshan N, 汤效平, 朱杰, 等. 不同结构SAPO-34催化剂上1-己烯催化裂解制丙烯[J]. 催化学报, 2009, 30(10): 1049-1057. |
| Zeeshan N, Tang X P, Zhu J, et al. Catalytic cracking of 1- hexene to propylene over SAPO-34 catalysts with different structures[J]. Chinese Journal of Catalysis, 2009, 30(10): 1049-1057. | |
| 43 | 汤效平, 周华群, 魏飞, 等. 催化裂解多产丙烯过程热力学分析[J]. 石油学报(石油加工), 2008, 24(1): 22-27. |
| Tang X P, Zhou H Q, Wei F, et al. Thermodynamic analysis of propylene-enhancing FCC process[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2008, 24(1): 22-27. | |
| 44 | Zhou J, Fan W, Wang Y D, et al. The essential mass transfer step in hierarchical/nano zeolite: surface diffusion[J]. National Science Review, 2020, 7(11): 1630-1632. |
| 45 | Fasano M, Humplik T, Bevilacqua A, et al. Interplay between hydrophilicity and surface barriers on water transport in zeolite membranes[J]. Nature Communications, 2016, 7: 12762. |
| 46 | Shen B Y, Wang H Q, Xiong H, et al. Atomic imaging of zeolite-confined single molecules by electron microscopy[J]. Nature, 2022, 607(7920): 703-707. |
| 47 | Kärger J, Pfeifer H. N.M.R. self-diffusion studies in zeolite science and technology[J]. Zeolites, 1987, 7(2): 90-107. |
| 48 | Keil F J, Krishna R, Coppens M O. Modeling of diffusion in zeolites[J]. Reviews in Chemical Engineering, 2000, 16(2): 71-197. |
| 49 | Cai D L, Ma Y H, Hou Y L, et al. Establishing a discrete Ising model for zeolite deactivation: inspiration from the game of Go[J]. Catalysis Science & Technology, 2017, 7(12): 2440-2444. |
| 50 | Saravanan C, Jousse F, Auerbach S M. Ising model of diffusion in molecular sieves[J]. Physical Review Letters, 1998, 80(26): 5754-5757. |
| 51 | Chai Y C, Dai W L, Wu G J, et al. Confinement in a zeolite and zeolite catalysis[J]. Accounts of Chemical Research, 2021, 54(13): 2894-2904. |
| 52 | Sastre G, Corma A. The confinement effect in zeolites[J]. Journal of Molecular Catalysis A: Chemical, 2009, 305(1/2): 3-7. |
| 53 | Cai D L, Xiong H, Zhang C X, et al. Transport phenomena in zeolites in view of graph theory and pseudo-phase transition[J]. Small, 2020, 16(15): e1901979. |
| 54 | Cai D L, Hou Y L, Zhang C X, et al. Analyzing transfer properties of zeolites using small-world networks[J]. Nanoscale, 2018, 10(35): 16431-16433. |
| 55 | Li Y, Yu J H. New stories of zeolite structures: their descriptions, determinations, predictions, and evaluations[J]. Chemical Reviews, 2014, 114(14): 7268-7316. |
| 56 | Watts D J, Strogatz S H. Collective dynamics of ‘small-world’ networks[J]. Nature, 1998, 393(6684): 440-442. |
| 57 | Wang N, Hou Y L, Sun W J, et al. Modulation of b-axis thickness within MFI zeolite: correlation with variation of product diffusion and coke distribution in the methanol-to-hydrocarbons conversion[J]. Applied Catalysis B: Environmental, 2019, 243: 721-733. |
| 58 | Barbera K, Bonino F, Bordiga S, et al. Structure-deactivation relationship for ZSM-5 catalysts governed by framework defects[J]. Journal of Catalysis, 2011, 280(2): 196-205. |
| 59 | Aerts A, Kirschhock C E A, Martens J A. Methods for in situ spectroscopic probing of the synthesis of a zeolite[J]. Chemical Society Reviews, 2010, 39(12): 4626-4642. |
| 60 | Wang W, Hunger M. Reactivity of surface alkoxy species on acidic zeolite catalysts[J]. Accounts of Chemical Research, 2008, 41(8): 895-904. |
| 61 | Knops-Gerrits P P, De Vos D E, Feijen E J P, et al. Raman spectroscopy on zeolites[J]. Microporous Materials, 1997, 8(1/2): 3-17. |
| 62 | Coudurier G, Naccache C, Vedrine J C. Uses of I.R. spectroscopy in identifying ZSM zeolite structure[J]. Chemical Communications, 1982(24): 1413. |
| 63 | Palomino G T, Bordiga S, Zecchina A, et al. XRD, XAS, and IR characterization of copper-exchanged Y zeolite[J]. The Journal of Physical Chemistry B, 2000, 104(36): 8641-8651. |
| 64 | Cha W, Jeong N C, Song S, et al. Core-shell strain structure of zeolite microcrystals[J]. Nature Materials, 2013, 12(8): 729-734. |
| 65 | Stavitski E, Kox M, Swart I, et al. In situ synchrotron-based IR microspectroscopy to study catalytic reactions in zeolite crystals[J]. Angewandte Chemie, 2008, 120(19): 3599-3603. |
| 66 | Vjunov A, Fulton J L, Huthwelker T, et al. Quantitatively probing the Al distribution in zeolites[J]. Journal of the American Chemical Society, 2014, 136(23): 8296-8306. |
| 67 | Karwacki L, Stavitski E, Kox M H F, et al. Intergrowth structure of zeolite crystals as determined by optical and fluorescence microscopy of the template-removal process[J]. Angewandte Chemie, 2007, 46(38): 7228-7231. |
| 68 | Ristanović Z, Kerssens M M, Kubarev A V, et al. High-resolution single-molecule fluorescence imaging of zeolite aggregates within real-life fluid catalytic cracking particles[J]. Angewandte Chemie, 2015, 127(6): 1856-1860. |
| 69 | Ristanović Z, Hofmann J P, De Cremer G, et al. Quantitative 3D fluorescence imaging of single catalytic turnovers reveals spatiotemporal gradients in reactivity of zeolite H-ZSM-5 crystals upon steaming[J]. Journal of the American Chemical Society, 2015, 137(20): 6559-6568. |
| 70 | Kärger J, Binder T, Chmelik C, et al. Microimaging of transient guest profiles to monitor mass transfer in nanoporous materials[J]. Nature Materials, 2014, 13(4): 333-343. |
| 71 | Saint Remi J C, Lauerer A, Chmelik C, et al. The role of crystal diversity in understanding mass transfer in nanoporous materials[J]. Nature Materials, 2016, 15(4): 401-406. |
| 72 | Chmelka B F, Pearson J G, Liu S B, et al. NMR study of the distribution of aromatic molecules in NaY zeolite[J]. The Journal of Physical Chemistry, 1991, 95(1): 303-310. |
| 73 | Hong U, Kärger J, Kramer R, et al. PFG N.M.R. study of diffusion anisotropy in oriented ZSM-5 type zeolite crystallites[J]. Zeolites, 1991, 11(8): 816-821. |
| 74 | Li S H, Zheng A M, Su Y C, et al. Brønsted/Lewis acid synergy in dealuminated HY zeolite: a combined solid-state NMR and theoretical calculation study[J]. Journal of the American Chemical Society, 2007, 129(36): 11161-11171. |
| 75 | Ma D, Deng F, Fu R Q, et al. MAS NMR studies on the dealumination of zeolite MCM-22[J]. The Journal of Physical Chemistry B, 2001, 105(9): 1770-1779. |
| 76 | Fraissard J, Ito T. 129Xe N.M.R. study of adsorbed xenon: a new method for studying zeolites and metal-zeolites[J]. Zeolites, 1988, 8(5): 350-361. |
| 77 | Haw J F, Richardson B R, Oshiro I S, et al. Reactions of propene on zeolite HY catalyst studied by in situ variable temperature solid-state nuclear magnetic resonance spectroscopy[J]. Journal of the American Chemical Society, 1989, 111(6): 2052-2058. |
| 78 | de Vience S J, Pham L M, Lovchinsky I, et al. Nanoscale NMR spectroscopy and imaging of multiple nuclear species[J]. Nature Nanotechnology, 2015, 10(2): 129-134. |
| 79 | Ruska E. The development of the electron microscope and of electron microscopy[J]. Bioscience Reports, 1987, 7(8): 607-629. |
| 80 | Egerton R F. Analytical electron microscopy[M]//Physical Principles of Electron Microscopy. Boston, MA: Springer US, 2005: 155-175. |
| 81 | Chen Q L, Dwyer C, Sheng G, et al. Imaging beam-sensitive materials by electron microscopy[J]. Advanced Materials, 2020, 32(16): e1907619. |
| 82 | Lazić I, Bosch E G T, Lazar S. Phase contrast STEM for thin samples: integrated differential phase contrast[J]. Ultramicroscopy, 2016, 160: 265-280. |
| 83 | Lazic I, Bosch E G T, Lazar S, et al. Integrated differential phase contrast (iDPC)-direct phase imaging in STEM for thin samples[J]. Microscopy and Microanalysis, 2016, 22(S3): 36-37. |
| 84 | Yücelen E, Lazić I, Bosch E G T. Phase contrast scanning transmission electron microscopy imaging of light and heavy atoms at the limit of contrast and resolution[J]. Scientific Reports, 2018, 8: 2676. |
| 85 | Liu L M, Wang N, Zhu C Z, et al. Direct imaging of atomically dispersed molybdenum that enables location of aluminum in the framework of zeolite ZSM-5[J]. Angewandte Chemie, 2020, 59(2): 819-825. |
| 86 | Shen B Y, Chen X, Cai D L, et al. Atomic spatial and temporal imaging of local structures and light elements inside zeolite frameworks[J]. Advanced Materials, 2020, 32(4): e1906103. |
| 87 | Shen B Y, Chen X, Shen K, et al. Imaging the node-linker coordination in the bulk and local structures of metal-organic frameworks[J]. Nature Communications, 2020, 11: 2692. |
| 88 | Shen B Y, Chen X, Fan X Y, et al. Resolving atomic SAPO-34/18 intergrowth architectures for methanol conversion by identifying light atoms and bonds[J]. Nature Communications, 2021, 12: 2212. |
| 89 | Shen B Y, Chen X, Wang H Q, et al. A single-molecule van der Waals compass[J]. Nature, 2021, 592(7855): 541-544. |
| 90 | Arslan M T, Tian G, Ali B, et al. Highly selective conversion of CO2 or CO into precursors for kerosene-based aviation fuel via an aldol-aromatic mechanism[J]. ACS Catalysis, 2022, 12(3): 2023-2033. |
| 91 | Wang N, Li J, Sun W J, et al. Rational design of zinc/zeolite catalyst: selective formation of p-xylene from methanol to aromatics reaction[J]. Angewandte Chemie, 2022, 61(10): e202114786. |
| 92 | Xiong H, Liu Z Q, Chen X, et al. In situ imaging of the sorption-induced subcell topological flexibility of a rigid zeolite framework[J]. Science, 2022, 376(6592): 491-496. |
| 93 | Lan X, Shi X, Zhang Y, et al. Solids back-mixing behavior and effect of the mesoscale structure in CFB risers[J]. Industrial & Engineering Chemistry Research, 2013, 52(34): 11888-11896. |
| 94 | Mahmoudi S, Seville J P K, Baeyens J. The residence time distribution and mixing of the gas phase in the riser of a circulating fluidized bed[J]. Powder Technology, 2010, 203(2): 322-330. |
| 95 | Das A K, Baudrez E, Marin G B, et al. Three-dimensional simulation of a fluid catalytic cracking riser reactor[J]. Industrial & Engineering Chemistry Research, 2003, 42(12): 2602-2617. |
| 96 | Cheng Y, Wu C N, Zhu J X, et al. Downer reactor: from fundamental study to industrial application[J]. Powder Technology, 2008, 183(3): 364-384. |
| 97 | 郭慕孙, 李洪钟. 流态化手册[M]. 北京: 化学工业出版社, 2008. |
| Guo M S, Li H Z. Handbook of Fluidization[M]. Beijing: Chemical Industry Press, 2008. | |
| 98 | Deng R, Wei F, Jin Y, et al. Downer catalytic pyrolysis (DCP): a novel process for light olefins production[J]. Chemical Engineering & Technology, 2002, 25(7): 711. |
| 99 | Parthasarathi R S, Alabduljabbar S S. HS-FCC high-severity fluidized catalytic cracking: a newcomer to the FCC family[J]. Applied Petrochemical Research, 2014, 4(4): 441-444. |
| 100 | 金涌, 魏飞, 程易, 等. 气固并流折叠式快速流化床反应装置: 1265937A[P]. 2000-09-13. |
| Jin Y, Wei F, Cheng Y, et al. Gas and solid parallel- flow folding type quick fluidized-bed reactor: 1265937A[P]. 2000-09-13. | |
| 101 | Johnson A R, Gartside R J, Ross J L, et al. Low residence time catalytic cracking process: US5976355A[P]. 1999-11-02. |
| 102 | 祁春鸣, 俞青, 金涌, 等. 气-固并流下行惯性分离装置的研究[J]. 石油炼制与化工, 1989(12): 51-56. |
| Qi C M, Yu Q, Jin Y, et al. A novel inertial separator for gas-solid suspension in concurrent downflow[J]. Petrol Process, 1989(12): 51-56. | |
| 103 | 魏飞, 金涌, 钱震, 等. 附壁切割式气固快速分离装置: 1267564A[P]. 2000-09-27. |
| Wei F, Jin Y, Qian Z, et al. Wall-attached cutting type fast gas-solid separator: 1267564A[P]. 2000-09-27. |
| [1] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [2] | 杨越, 张丹, 郑巨淦, 涂茂萍, 杨庆忠. NaCl水溶液喷射闪蒸-掺混蒸发的实验研究[J]. 化工学报, 2023, 74(8): 3279-3291. |
| [3] | 董明, 徐进良, 刘广林. 超临界水非均质特性分子动力学研究[J]. 化工学报, 2023, 74(7): 2836-2847. |
| [4] | 郑志航, 马郡男, 闫子涵, 卢春喜. 提升管射流影响区内压力脉动特性研究[J]. 化工学报, 2023, 74(6): 2335-2350. |
| [5] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [6] | 李新亚, 邢雷, 蒋明虎, 赵立新. 倒锥注气强化井下油水分离水力旋流器性能研究[J]. 化工学报, 2023, 74(3): 1134-1144. |
| [7] | 颜少航, 赖天伟, 王彦武, 侯予, 陈双涛. 微间隙内R134a空化可视化实验研究[J]. 化工学报, 2023, 74(3): 1054-1061. |
| [8] | 章承浩, 罗京, 张吉松. 微反应器内基于氮氧自由基催化剂连续氧气/空气氧化反应的研究进展[J]. 化工学报, 2023, 74(2): 511-524. |
| [9] | 孔令菲, 陈延佩, 王维. 气固流态化中颗粒介尺度结构的动力学研究[J]. 化工学报, 2022, 73(6): 2486-2495. |
| [10] | 范小强, 黄正梁, 孙婧元, 王靖岱, 王晓飞, 胡晓波, 韩国栋, 阳永荣, 吴文清. 气液法流化床乙烯云聚合工艺开发及产品高性能化[J]. 化工学报, 2022, 73(6): 2742-2747. |
| [11] | 李铁男, 赵碧丹, 赵鹏, 张永民, 王军武. 气固流化床启动阶段挡板内构件受力特性的CFD-DEM模拟[J]. 化工学报, 2022, 73(6): 2649-2661. |
| [12] | 管小平, 杨宁. 基于介尺度稳定性条件的多相流曳力与群体平衡模型[J]. 化工学报, 2022, 73(6): 2427-2437. |
| [13] | 刘梦溪, 范怡平, 闫子涵, 姚秀颖, 卢春喜. 提升管进料区内气体射流流动行为的调控及工业应用[J]. 化工学报, 2022, 73(6): 2496-2513. |
| [14] | 石孝刚, 王成秀, 高金森, 蓝兴英. 提升管反应器介尺度结构影响规律的数值模拟研究[J]. 化工学报, 2022, 73(6): 2708-2721. |
| [15] | 兰文杰, 胡晓洁, 蔡迪宗. 界面探针法测量液滴与固体壁面间相互作用力[J]. 化工学报, 2022, 73(3): 1119-1126. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号