化工学报 ›› 2025, Vol. 76 ›› Issue (S1): 258-267.DOI: 10.11949/0438-1157.20250004
• 分离工程 • 上一篇
收稿日期:2025-01-01
修回日期:2025-02-20
出版日期:2025-06-25
发布日期:2025-06-26
通讯作者:
叶翠平
作者简介:裴星亮(1999—),男,硕士研究生,peixingliang1367@link.tyut.edu.cn
基金资助:
Xingliang PEI1( ), Cuiping YE1,2(
), Cuiping YE1,2( ), Yingli PEI3, Wenying LI3
), Yingli PEI3, Wenying LI3
Received:2025-01-01
Revised:2025-02-20
Online:2025-06-25
Published:2025-06-26
Contact:
Cuiping YE
摘要:
MIL-53是一类吸附分离二甲苯异构体的重要材料,其中MIL-53(Cr)对邻二甲苯(OX)和对二甲苯(PX)具有较高的分离选择性,而对OX和间二甲苯(MX)选择性低。为了改善MIL-53(Cr)对OX和MX的选择性,利用碱前体KNO3和金属中心Cr3+之间的氧化还原作用在MIL-53(Cr)上直接制备强碱性位点,以碱改性的MIL-53(Cr)为吸附剂,对OX、PX和MX进行液相吸附分离。结果表明,在MIL-53(Cr)中引入碱性位不改变材料的独特结构。由于引入的碱性位占用了部分金属位点,导致材料对二甲苯的吸附量有所下降。OX/PX的吸附选择性不变,而碱性位点的增加导致材料对MX的吸附阻力增大,吸附量减少,OX/MX的吸附选择性从MIL-53(Cr)的2.3提高到K-MIL-53(Cr)的4.1。K-MIL-53(Cr)具有良好的稳定性和再生能力,吸附循环5次,吸附量仅下降5.7%,且材料结构保持不变。
中图分类号:
裴星亮, 叶翠平, 裴赢丽, 李文英. 碱改性MIL-53(Cr)选择性吸附分离二甲苯异构体[J]. 化工学报, 2025, 76(S1): 258-267.
Xingliang PEI, Cuiping YE, Yingli PEI, Wenying LI. Selective adsorption and separation of xylene isomers by alkali-modified MIL-53(Cr)[J]. CIESC Journal, 2025, 76(S1): 258-267.
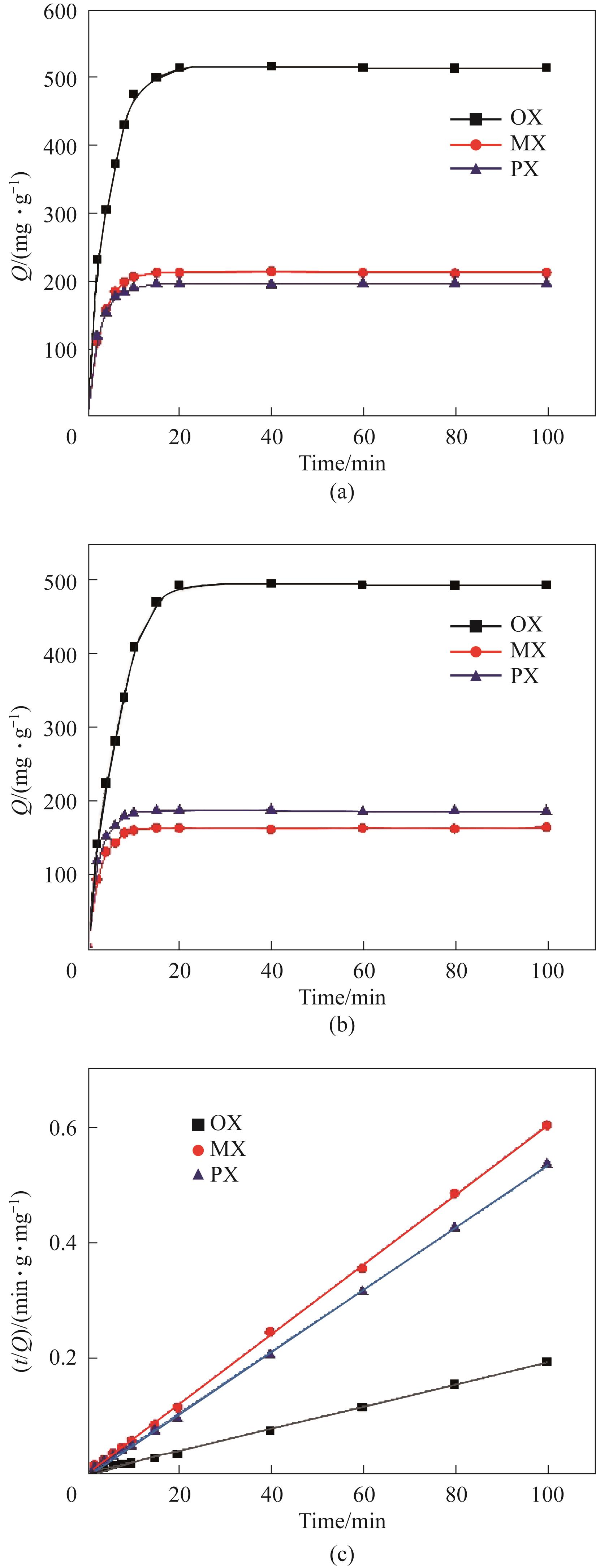
图3 单组分二甲苯在(a)MIL-53(Cr)和(b)K-MIL-53(Cr)上吸附量随时间的变化;(c) K-MIL-53(Cr)二甲苯吸附过程的准二级动力学模型拟合
Fig.3 Time-dependent adsorption of single-component xylene on (a)MIL-53(Cr) and (b)K-MIL-53(Cr);(c) Quasi-second-order kinetic model fitting for the xylene adsorption process of K-MIL-53(Cr)
| 二甲苯 | C0/(mol·L-1) | 准一级动力学模型 | 准二级动力学模型 | ||||
|---|---|---|---|---|---|---|---|
| k1/(g·mg-1·h-1) | Qe/(mg·g-1) | R2 | k2/(g·mg-1·h-1) | Qe/(mg·g-1) | R2 | ||
| OX | 0.5 | 0.0773 | 138.02 | 0.7887 | 0.0005 | 497.51 | 0.9979 |
| MX | 0.5 | 0.0600 | 24.05 | 0.6740 | 0.0101 | 164.21 | 0.9996 |
| PX | 0.5 | 0.0601 | 27.39 | 0.6666 | 0.0291 | 187.82 | 0.9995 |
表1 K-MIL-53(Cr)吸附二甲苯异构体的动力学拟合参数
Table 1 Kinetic fitting results of xylene isomers adsorption on K-MIL-53(Cr)
| 二甲苯 | C0/(mol·L-1) | 准一级动力学模型 | 准二级动力学模型 | ||||
|---|---|---|---|---|---|---|---|
| k1/(g·mg-1·h-1) | Qe/(mg·g-1) | R2 | k2/(g·mg-1·h-1) | Qe/(mg·g-1) | R2 | ||
| OX | 0.5 | 0.0773 | 138.02 | 0.7887 | 0.0005 | 497.51 | 0.9979 |
| MX | 0.5 | 0.0600 | 24.05 | 0.6740 | 0.0101 | 164.21 | 0.9996 |
| PX | 0.5 | 0.0601 | 27.39 | 0.6666 | 0.0291 | 187.82 | 0.9995 |
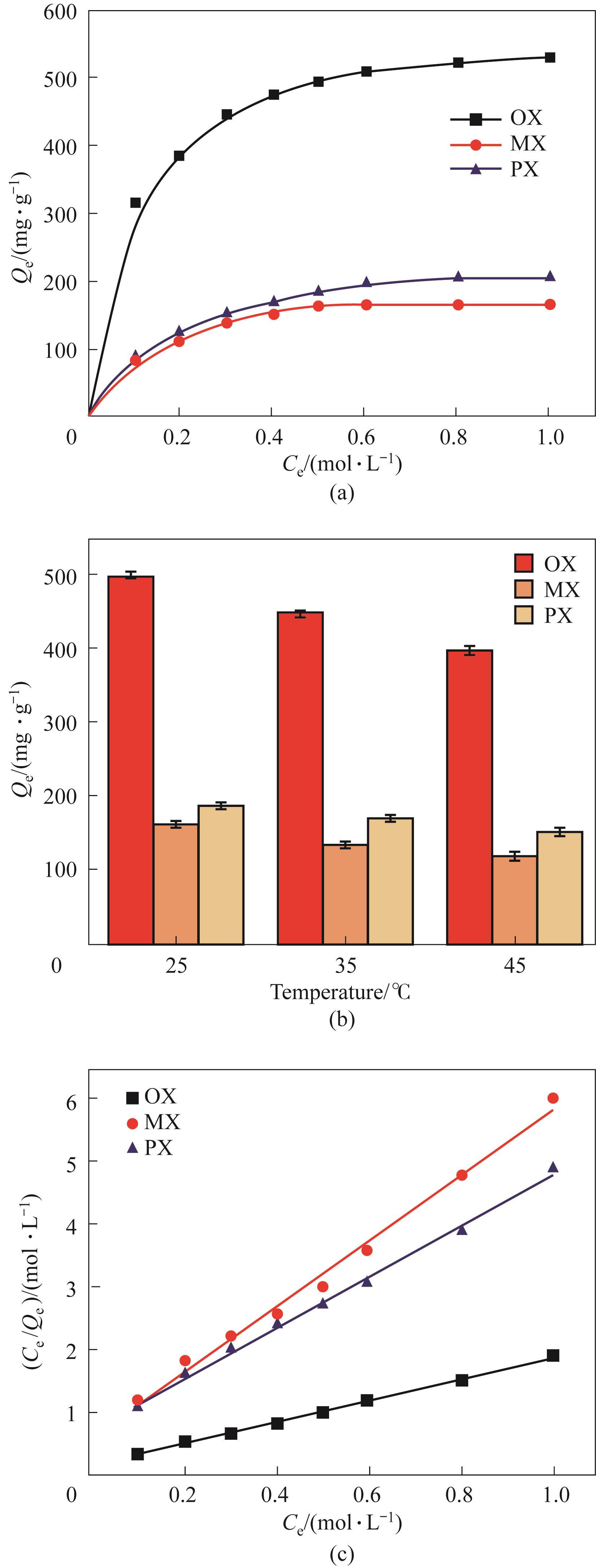
图4 (a) 25℃下K-MIL-53(Cr)对单组分二甲苯的吸附性能;(b)温度的影响;(c) Langmuir拟合曲线
Fig.4 (a) Single component adsorption of K-MIL-53(Cr) for xylene at 25℃; (b) Impact of temperature; (c) Langmuir adsorption isotherm fitting curves
| 吸附质 | 温度/℃ | Langmuir吸附等温线 | Freundlich吸附等温线 | ||||
|---|---|---|---|---|---|---|---|
| Qm/(mg·g-1) | KL/(L·mol-1) | R2 | KF/(mg·g-1·(L·mol-1)1/n ) | R2 | |||
| OX | 25 | 581.39 | 11.23 | 0.9985 | 562.34 | 0.23 | 0.9506 |
| MX | 25 | 164.20 | 6.91 | 0.9913 | 175.27 | 0.31 | 0.9007 |
| PX | 25 | 187.70 | 7.86 | 0.9962 | 213.87 | 0.37 | 0.9612 |
表2 K-MIL-53(Cr)上单组分二甲苯的Langmuir和Freundlich吸附等温线拟合参数
Table 2 Parameters of Langmuir and Freundlich adsorption model for single-xylene isomer on K-MIL-53(Cr)
| 吸附质 | 温度/℃ | Langmuir吸附等温线 | Freundlich吸附等温线 | ||||
|---|---|---|---|---|---|---|---|
| Qm/(mg·g-1) | KL/(L·mol-1) | R2 | KF/(mg·g-1·(L·mol-1)1/n ) | R2 | |||
| OX | 25 | 581.39 | 11.23 | 0.9985 | 562.34 | 0.23 | 0.9506 |
| MX | 25 | 164.20 | 6.91 | 0.9913 | 175.27 | 0.31 | 0.9007 |
| PX | 25 | 187.70 | 7.86 | 0.9962 | 213.87 | 0.37 | 0.9612 |
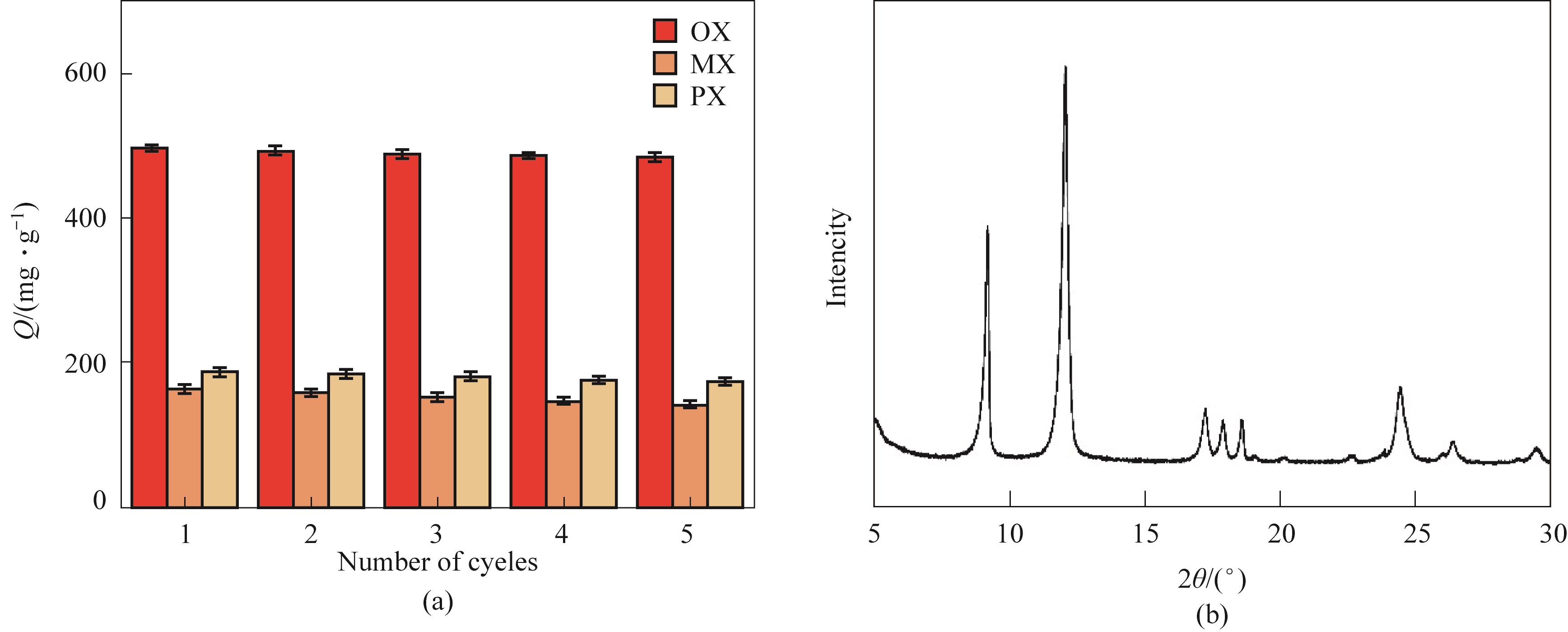
图6 (a) K- MIL-53(Cr)在25℃下对二甲苯的吸附循环性能; (b) 5次吸附循环后K-MIL-53(Cr)的XRD谱图
Fig.6 (a) Cycle performance of K-MIL-53(Cr) at 25℃; (b) XRD pattern of K-MIL-53(Cr) after 5 cycles
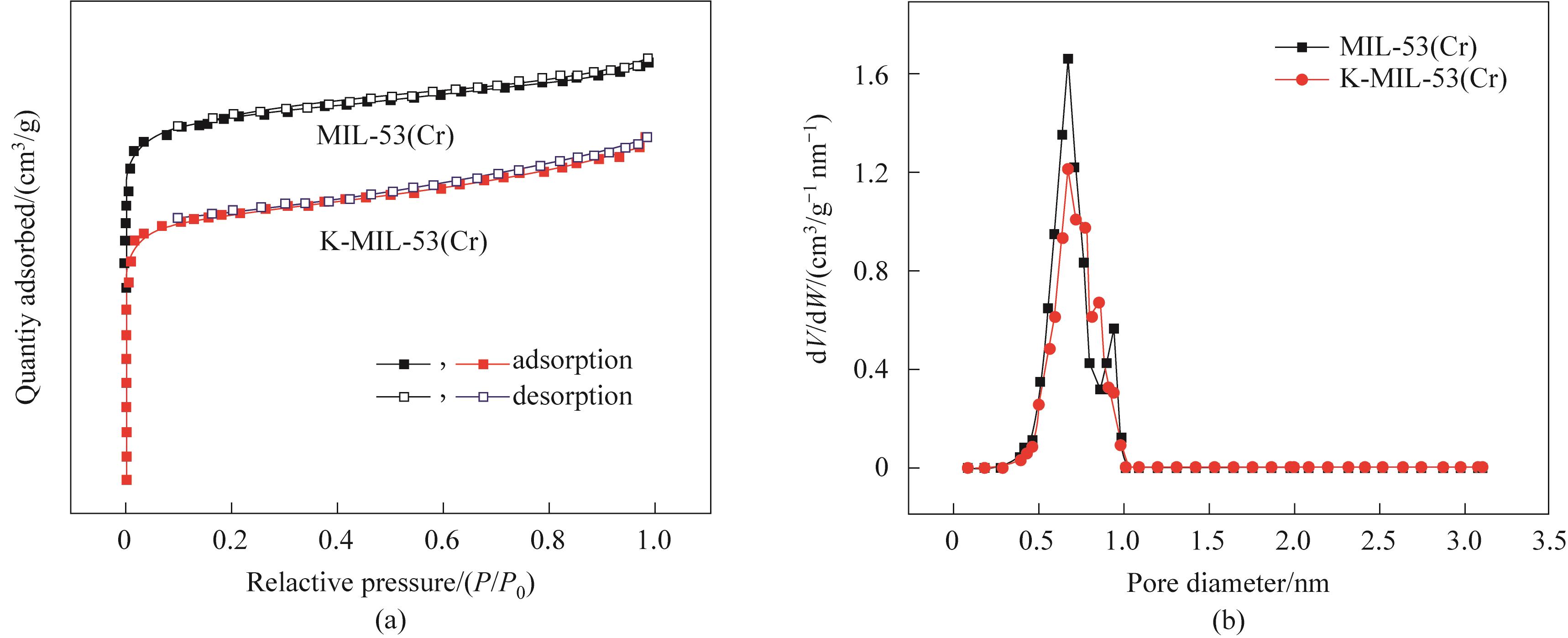
图7 (a) MIL-53(Cr)和K-MIL-53(Cr)在77 K下的N2吸脱附等温线;(b) MIL-53(Cr)和K-MIL-53(Cr)的微孔孔径分布
Fig.7 (a) N2 adsorption and desorption isotherms of MIL-53(Cr) and K-MIL-53(Cr) at 77 K;(b) Micropore size distribution of MIL-53(Cr) and K-MIL-53(Cr)
| Item | MIL-53(Cr) | K-MIL-53(Cr) |
|---|---|---|
| specific surface area(N2)/(m2·g-1) | 1004 | 832 |
| pore volume/(cm3·g-1) | 0.53 | 0.49 |
| most probable aperture/nm | 0.69 | 0.69 |
表3 MIL-53(Cr)和K-MIL-53 (Cr)的BET比表面积、孔体积和最可几孔径
Table 3 BET specific surface area, pore volume and most probable aperture of MIL-53(Cr) and K-MIL-53 (Cr)
| Item | MIL-53(Cr) | K-MIL-53(Cr) |
|---|---|---|
| specific surface area(N2)/(m2·g-1) | 1004 | 832 |
| pore volume/(cm3·g-1) | 0.53 | 0.49 |
| most probable aperture/nm | 0.69 | 0.69 |
| 1 | Yousefzadeh Borzehandani M, Abdulmalek E, Abdul Rahman M B, et al. First-principles investigation of dimethyl-functionalized MIL-53(Al) metal-organic framework for adsorption and separation of xylene isomers[J]. Journal of Porous Materials, 2021, 28(2): 579-591. |
| 2 | Yang Y, Bai P, Guo X. Separation of xylene isomers: a review of recent advances in materials[J]. Industrial & Engineering Chemistry Research, 2017, 56(50): 14725-14753. |
| 3 | 刘莹, 郑芳, 杨启炜, 等. 二甲苯异构体吸附分离研究进展[J]. 化工学报, 2024, 75(4): 1081-1095. |
| Liu Y, Zheng F, Yang Q W, et al. Recent progress in adsorption and separation of xylene isomers [J].CIESC Journal, 2024, 75(4): 1081-1095. | |
| 4 | 郝西维, 刘秋芳, 刘弓, 等. 对二甲苯生产技术开发进展及展望[J]. 洁净煤技术, 2016, 22(5): 25-30. |
| Hao X W, Liu Q F, Liu G, et al. Progress and prospect of p‐xylene production technologies[J]. Clean Coal Technology, 2016, 22(5): 25-30. | |
| 5 | Gonzalez M I, Kapelewski M T, Bloch E D, et al. Separation of xylene isomers through multiple metal site interactions in metal-organic frameworks[J]. Journal of the American Chemical Society, 2018, 140(9): 3412-3422. |
| 6 | 钱庆玲. 大环衍生多孔有机聚合物吸附分离二甲苯异构体的研究[D]. 合肥: 中国科学技术大学, 2023. |
| Qian Q L. Adsorption separation of xylene lsomers by macrocycle-based porous organic polymers[D]. Hefei: University of Science and Technology of China, 2023. | |
| 7 | Torres-Knoop A, Krishna R, Dubbeldam D. Separating xylene isomers by commensurate stacking of p‐xylene within channels of MAF‐X8[J]. Angewandte Chemie International Edition, 2014, 53(30): 7774-7778. |
| 8 | Moreira M A, Santos J C, Ferreira A F P, et al. Selective liquid phase adsorption and separation of ortho-xylene with the microporous MIL-53(Al) [J]. Separation Science and Technology, 2011, 46(13): 1995-2003. |
| 9 | Khosravi-Nikou M, Shahmoradi A, Shariati A, et al. Experimental and techno-economic investigation of industrial para-xylene plant revamping to produce meta-xylene [J]. Scientific Reports, 2023, 13(1): 12534. |
| 10 | Lippi M, Cametti M. Highly dynamic 1D coordination polymers for adsorption and separation applications [J]. Coordination Chemistry Reviews, 2021, 430: 213661. |
| 11 | Li L, Guo L, Olson D H, et al. Discrimination of xylene isomers in a stacked coordination polymer [J]. Science, 2022, 377(6603): 335-339. |
| 12 | Lannoeye J, van de Voorde B, Bozbiyik B, et al. An aliphatic copper metal-organic framework as versatile shape selective adsorbent in liquid phase separations[J]. Microporous and Mesoporous Materials, 2016, 226: 292-298. |
| 13 | Sui H, Jiang P, Li X, et al. Binary adsorption equilibrium and breakthrough of n-butyl acetate and p-xylene on granular activated carbon[J]. Industrial & Engineering Chemistry Research, 2019, 58(19): 8279-8289. |
| 14 | Yu L, Zhang J, Ullah S, et al. Separating xylene isomers with a calcium metal-organic framework[J]. Angewandte Chemie International Edition, 2023, 62(41): 130-135. |
| 15 | Yao J, Chen Q, Sheng Y, et al. pH-controlled crystal growth of copper/gemini surfactant complexes with bipyridine groups [J]. Crystengcomm, 2017, 19(39): 5835-5843. |
| 16 | Zhang C, Qin Y, Duan L, et al. pH-dependent formation of three porous In(Ⅲ)-MOFs: framework diversity and selective gas adsorption [J]. Dalton Transactions, 2022, 51(2): 473-477. |
| 17 | Zhao M, Yuan K, Wang Y, et al. Metal-organic frameworks as selectivity regulators for hydrogenation reactions [J]. Nature, 2016, 539(7627): 76-80. |
| 18 | Reinsch H, Stassen I, Bueken B, et al. First examples of aliphatic zirconium MOFs and the influence of inorganic anions on their crystal structures [J]. Crystengcomm, 2015, 17(2): 331-337. |
| 19 | Khudozhitkov A E, Jobic H, Freude D, et al. Ultraslow dynamics of a framework linker in MIL-53 (Al) as a sensor for different isomers of xylene [J]. The Journal of Physical Chemistry C, 2016, 120(38): 21704-21709. |
| 20 | Lu P, Yu D, Lu J.A method for modifying dynamically classes in the object-oriented dynamic programming environment[J]. ACM SIGPLAN Notices, 1997, 32(9): 57-60. |
| 21 | Yang H, Hu Y. Separation of para-xylene and meta-xylene by extraction process using aqueous cyclodextrins solution [J]. Chemical Engineering and Processing-Process Intensification, 2017, 116: 114-120. |
| 22 | Serre C, Millange F, Thouvenot C, et al. Very large breathing effect in the first nanoporous chromium(Ⅲ)-based solids: MIL-53 or CrⅢ(OH)·{O2C-C6H4-CO2}·{HO2C-C6H4-CO2H} x ·H2O y [J]. Journal of the American Chemical Society, 2002, 124(45): 13519-13526. |
| 23 | Moïse J C, Bellat J P. Effect of preadsorbed water on the adsorption of p-xylene and m-xylene mixtures on BaX and BaY zeolites [J]. Journal of Physical Chemistry B, 2005, 109(36): 17239-17244. |
| 24 | Li Y X, Jiang W J, Tan P, et al. What matters to the adsorptive desulfurization performance of metal-organic frameworks? [J]. Journal of Physical Chemistry C, 2015, 119(38): 21969-21977. |
| 25 | Liu X, Pang H, Liu X, et al. Orderly porous covalent organic frameworks-based materials: superior adsorbents for pollutants removal from aqueous solutions [J]. The Innovation, 2021, 2(1): 100076-100104. |
| 26 | He Z, Yang Y, Bai P, et al. Metal-organic framework MIL-53(Cr) as a superior adsorbent: highly efficient separation of xylene isomers in liquid phase [J]. Journal of Industrial and Engineering Chemistry, 2019, 77: 262-272. |
| 27 | Ren J, Musyoka N M, Langmi H W, et al. Modulated synthesis of chromium-based metal-organic framework (MIL-101) with enhanced hydrogen uptake [J]. International Journal of Hydrogen Energy, 2014, 39(23): 12018-12023. |
| 28 | Mounfield W P, Walton K S. Effect of synthesis solvent on the breathing behavior of MIL-53(Al) [J]. Journal of Colloid and Interface Science, 2015, 447: 33-39. |
| 29 | Ortiz G, Chaplais G, Paillaud J-L, et al. New insights into the hydrogen bond network in Al-MIL-53 and Ga-MIL-53 [J]. The Journal of Physical Chemistry C, 2014, 118(38): 22021-22029. |
| 30 | Liu W, Zhu L, Jiang Y, et al. Direct fabrication of strong basic sites on ordered nanoporous materials: exploring the possibility of metal-organic frameworks [J]. Chemistry of Materials, 2018, 30(5): 1686-1694. |
| 31 | Rasouli M, Yaghobi N, Chitsazan S, et al. Effect of nanocrystalline zeolite Na-Y on meta-xylene separation [J]. Microporous and Mesoporous Materials, 2012, 152: 141-147. |
| 32 | Jiang J, Sandler S I. Shape versus inverse-shape selective adsorption of alkane isomers in carbon nanotubes [J]. The Journal of Chemical Physics, 2006, 124(2): 024717. |
| [1] | 胡嘉朗, 姜明源, 金律铭, 张永刚, 胡鹏, 纪红兵. 机器学习辅助MOFs高通量计算筛选及气体分离研究进展[J]. 化工学报, 2025, 76(5): 1973-1996. |
| [2] | 茅雨洁, 路晓飞, 锁显, 杨立峰, 崔希利, 邢华斌. 工业气体中微量氧深度脱除催化剂研究进展[J]. 化工学报, 2025, 76(5): 1997-2010. |
| [3] | 徐泽海, 刘超, 张国亮. 聚合物基疏水渗透汽化膜及其溶剂回收应用[J]. 化工学报, 2025, 76(5): 2055-2069. |
| [4] | 张耀辉, 班宇杰, 杨维慎. 以蒸气加工法制备和修饰金属-有机框架膜[J]. 化工学报, 2025, 76(5): 2070-2086. |
| [5] | 郭彭涛, 王婷, 薛波, 应允攀, 刘大欢. 用于CH4/N2分离的多吸附位点超微孔MOF[J]. 化工学报, 2025, 76(5): 2304-2312. |
| [6] | 花敬贤, 罗宇荣, 顾亚伟, 吴婷婷, 潘宜昌, 邢卫红. 超薄取向ZIF-8膜的制备及乙烯/乙烷高效分离[J]. 化工学报, 2025, 76(5): 2209-2218. |
| [7] | 向昕辰, 鲁丹, 赵影, 姚之侃, 寇瑞强, 郑丹军, 周志军, 张林. 聚酰胺纳滤膜表面季铵化提高荷正电性及其锂镁分离性能[J]. 化工学报, 2025, 76(5): 2377-2386. |
| [8] | 尤潇楠, 范小强, 杨遥, 王靖岱, 阳永荣. 超临界乙烯和高压聚乙烯混合物的减压分离过程建模方法[J]. 化工学报, 2025, 76(2): 695-706. |
| [9] | 杨晨, 毛伟, 董兴宗, 田松, 赵锋伟, 吕剑. 选择性加氢脱氯合成烯烃研究进展[J]. 化工学报, 2025, 76(1): 53-70. |
| [10] | 唐宇昊, 张迎迎, 赵智伟, 鲁梦悦, 张飞飞, 王小青, 杨江峰. 弱极性超微孔Sc/In-CPM-66A用于CH4/N2吸附分离性能[J]. 化工学报, 2024, 75(9): 3210-3220. |
| [11] | 周文轩, 刘珍, 张福建, 张忠强. 高通量-高截留率时间维度膜法水处理机理研究[J]. 化工学报, 2024, 75(7): 2583-2593. |
| [12] | 王涛虹, 王超, 李政, 刘莹, 田歌, 常刚刚, 阳晓宇, 鲍宗必. 固载Cu(Ⅰ)的π络合MOF吸附剂用于乙烷/乙烯的选择性分离[J]. 化工学报, 2024, 75(7): 2565-2573. |
| [13] | 吴哲明, 张碧云, 郑仁朝. 腈水解酶立体选择性改造及其合成布瓦西坦[J]. 化工学报, 2024, 75(7): 2633-2643. |
| [14] | 张林, 张子怡, 李勇, 童少平. Fe-MOF-74前体制备铁-碳/氮复合材料及其活化过硫酸盐性能[J]. 化工学报, 2024, 75(5): 1882-1889. |
| [15] | 孟园, 倪善, 刘亚锋, 王文杰, 赵越, 朱育丹, 杨良嵘. 功能化多孔氮化碳材料对铀的吸附性能研究[J]. 化工学报, 2024, 75(4): 1616-1629. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号