化工学报 ›› 2019, Vol. 70 ›› Issue (3): 1135-1143.DOI: 10.11949/j.issn.0438-1157.20181079
收稿日期:2018-09-26
修回日期:2018-12-10
出版日期:2019-03-05
发布日期:2019-03-05
通讯作者:
方桂英,阳庆元
作者简介:<named-content content-type="corresp-name">王磊</named-content>(1993—),男,硕士研究生,<email>evacolin@163.com</email>|方桂英(1964—),女,学士,副教授,<email>guiyingfang1012@163.com</email>|阳庆元(1976—),男,博士,教授,<email>qyyang@mail.buct.edu.cn</email>
基金资助:
Lei WANG1( ),Guiying FANG2(
),Guiying FANG2( ),Qingyuan YANG1(
),Qingyuan YANG1( )
)
Received:2018-09-26
Revised:2018-12-10
Online:2019-03-05
Published:2019-03-05
Contact:
Guiying FANG,Qingyuan YANG
摘要:
全球性温室效应形势的日趋严重,迫切需要研究和开发可用于CO2捕集的高性能材料。对于含有双铜船桨型片段(Cu2(COO)4)的MOF材料,因其结构中含有配位不饱和的Cu金属位点,在低压区域的CO2捕获方面展现出优异的性能。目前,大规模计算筛选工作主要是基于传统的分子力场,无法对此类Cu-MOFs中主客体分子间的相互作用进行准确描述。基于量化计算获得的精确分子力场,利用Monte Carlo分子模拟方法考察了常温常压条件下763个基于Cu-OMS的MOF材料对CO2存储和CO2/N2的分离行为。不仅筛选出潜在的高性能材料,而且揭示出了材料的结构与其性能之间的关系和具有优良性能的材料结构特征,可为面向特定应用的新材料设计和合成提供理论参考。
中图分类号:
王磊, 方桂英, 阳庆元. 金属-有机骨架材料CO2捕获性能的大规模计算筛选[J]. 化工学报, 2019, 70(3): 1135-1143.
Lei WANG, Guiying FANG, Qingyuan YANG. Performance of metal-organic frameworks for CO2 capture from large-scale computational screening[J]. CIESC Journal, 2019, 70(3): 1135-1143.
| Atom pair | D0/(kJ/mol) | R0/nm | α |
|---|---|---|---|
| Cu-CO2-O[ | 1.0113 | 0.3313 | 10.5781 |
表1 描述MOFs中Cu开放金属位点和CO2分子之间特殊作用的Morse势能参数
Table 1 Morse potential parameter for describing specific interactions between CO2 molecules and open Cu sites of MOFs
| Atom pair | D0/(kJ/mol) | R0/nm | α |
|---|---|---|---|
| Cu-CO2-O[ | 1.0113 | 0.3313 | 10.5781 |

图1 N2在Cu-BTC和Cu-TDPAT(a), ZJU-8a和ZJNU-40a(b)中的模拟吸附等温线与实验值对比
Fig.1 Comparison of simulated adsorption isotherms of N2 in Cu-BTC and Cu-TDPAT(a), ZJU-8a and ZJNU-40a(b) with experimental data
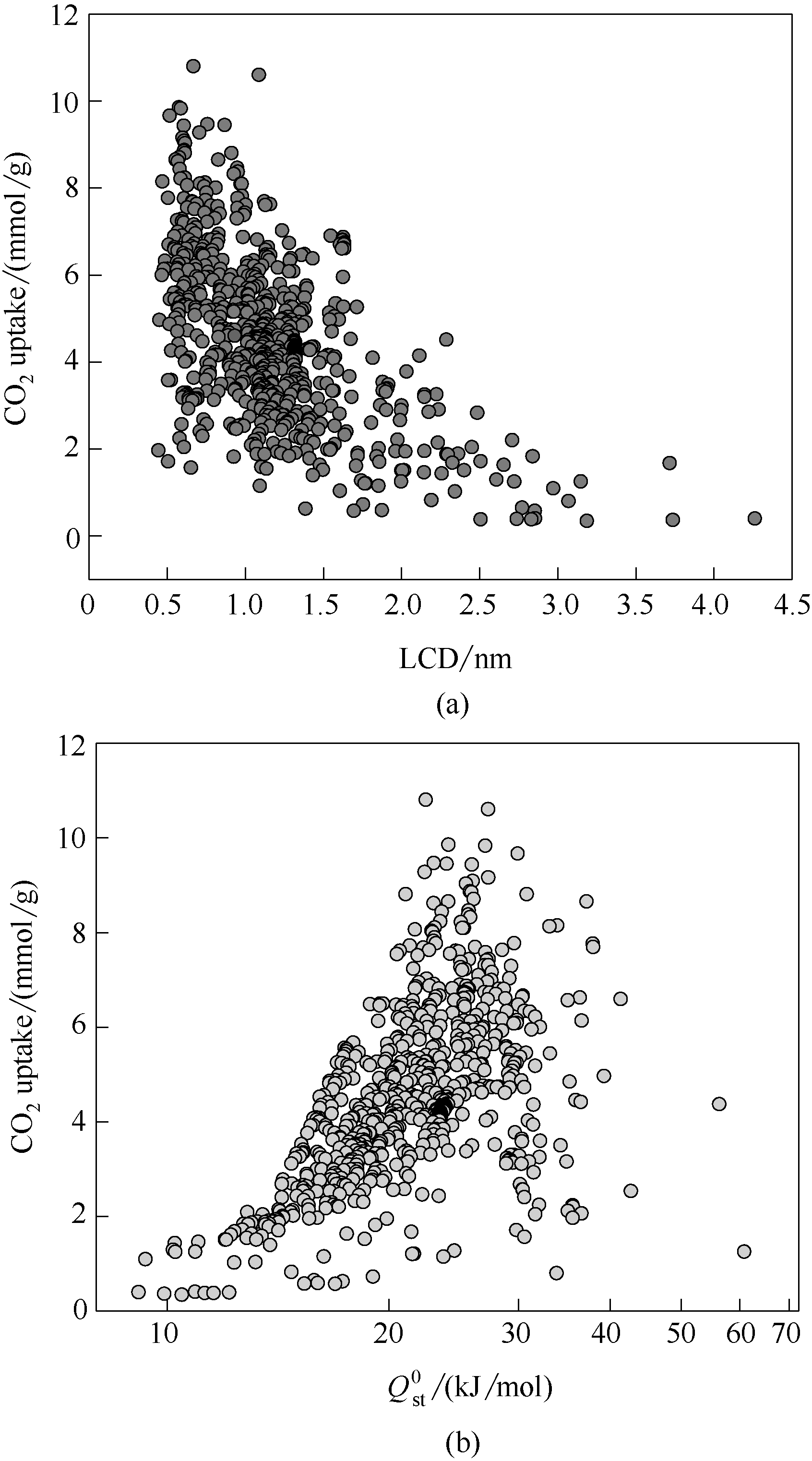
图2 常温常压下763个Cu-OMS MOFs中CO2吸附量的模拟构效关系:材料最大孔径(a),无限稀释吸附热(b)
Fig.2 Gravimetric CO2 uptakes of 763 MOFs with Cu-OMS versus largest cavity diameter (LCD) (a), zero-coverage isosteric heat of adsorption (Qst0) (b) under ambient conditions

图3 常温常压下763种Cu-OMS MOF材料的CO2吸附性能与材料孔体积(a)、材料孔隙率(b)、比表面积(c)、吸附度(d)之间的
Fig.3 Relationships of CO2 uptake of 763 Cu-OMS-containing MOFs under ambient conditions with pore volume(a), fraction(b), accessible surface area(c) and adsorbility (AD)(d)(Uptakes are colored by value of AD in (a), (b) and (c))

图5 常温常压下763种Cu-OMS MOF材料的CO2/N2分离选择性与1/ΔAD的关系
Fig.5 Relationship between CO2/N2 selectivity of 763 Cu-OMS-containing MOFs and 1/ΔAD at ambient conditions

图6 298 K和0.1 MPa下MOF材料的CO2/N2(15:85)选择性与其CO2吸附量之间的关系(正方形代表本工作中筛选出的两种前景材料;三角形代表目前文献中已经报道的最佳材料)
Fig.6 CO2/N2 (15:85) separation performance of 763 MOFs with Cu-OMS at 298K and 0.1 MPa(Squares represent top 2 MOFs identified in this work; triangles denote some of best MOFs experimentally reported in literature)

图7 298 K和0.1 MPa条件下CO2和N2在NEYRIU材料的微观吸附结构(蓝色代表N2分子)
Fig.7 Microscopic adsorption structures of CO2 and N2 molecules in NEYRIU and 298 K and 0.1 MPa(N2 molecule is colored by blue)
| 1 | BaeY S, SnurrR Q. Development and evaluation of porous materials for carbon dioxide separation and capture[J]. Angew. Chem. Int. Ed., 2011, 50(49): 11586-11596. |
| 2 | QuadrelliR, PetersonS. The energy-climate challenge: recent trends in CO2 emissions from fuel combustion[J]. Energy Policy, 2007, 35(11): 5938-5952. |
| 3 | YangQ Y, ZhongC L, ChenJ F. Computational study of CO2 storage in metal-organic frameworks[J]. J. Phys. Chem. C, 2008, 112(5): 1562-1569. |
| 4 | XiangS C, HeY B, ZhangZ G, et al. Microporous metal-organic framework with potential for carbon dioxide capture at ambient conditions[J]. Nat. Commun., 2012, 3: 954. |
| 5 | LinZ D, LvZ Q, ZhouX, et al. Postsynthetic strategy to prepare ACN@Cu-BTCs with enhanced water vapor stability and CO2/CH4 separation selectivity[J]. Ind. Eng. Chem. Res., 2018, 57(10): 3765-3772. |
| 6 | SalehM, LeeH M, KempK C, et al. Highly stable CO2/N2 and CO2/CH4 selectivity in hyper-cross-linked heterocyclic porous polymers[J]. ACS Appl. Mater. Interfaces, 2014, 6(10): 7325-7333. |
| 7 | HaszeldineR S. Carbon capture and storage: how green can black be?[J]. Science, 2009, 325: 1647-1652. |
| 8 | FurukawaH, CordovaK E, O'keeffeM, et al. The chemistry and applications of metal-organic frameworks[J]. Science, 2013, 341: 1230444. |
| 9 | Gómez-GualdrónD A, ColónY J, ZhangX, et al. Evaluating topologically diverse metal-organic frameworks for cryo-adsorbed hydrogen storage[J]. Energy Environ. Sci., 2016, 9(10): 3279-3289. |
| 10 | NugentP, BelmabkhoutY, BurdS D, et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation[J]. Nature, 2013, 495: 80-84. |
| 11 | HeY B, ZhouW, QianG D, et al. Methane storage in metal-organic frameworks[J]. Chem. Soc. Rev., 2014, 43(16): 5657-5678. |
| 12 | MasonJ A, VeenstraM, LongJ R. Evaluating metal-organic frameworks for natural gas storage[J]. Chem. Sci., 2014, 5(1): 32-51. |
| 13 | ZornozaB, TellezC, CoronasJ, et al. Metal organic framework based mixed matrix membranes: an increasingly important field of research with a large application potential[J]. Microporous Mesoporous Mater., 2013, 166: 67-78. |
| 14 | HuangC, SunR, LuH, et al. A pilot trial for fast deep desulfurization on MOF-199 by simultaneous adsorption-separation via hydrocyclones[J]. Sep. Purif. Technol., 2017, 182: 110-117. |
| 15 | LiuY, LiuH, HuY, et al. Density functional theory for adsorption of gas mixtures in metal-organic frameworks[J]. J. Phys. Chem. B, 2010, 114(8): 2820-2827. |
| 16 | LeeJ Y, FarhaO K, RobertsJ, et al. Metal-organic framework materials as catalysts[J]. Chem. Soc. Rev., 2009, 38(5): 1450-1459. |
| 17 | CaskeyS R, Wong-FoyA G, MatzgerA J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores[J]. J. Am. Chem. Soc., 2008, 130(33): 10870-10871. |
| 18 | ZhaoX, WangY X, LiD S, et al. Metal-organic frameworks for separation[J]. Adv. Mater., 2018: 1705189. |
| 19 | AdilK, BelmabkhoutY, PillaiR S, et al. Gas/vapour separation using ultra-microporous metal-organic frameworks: insights into the structure/separation relationship[J]. Chem. Soc. Rev., 2017, 46(11): 3402-3430. |
| 20 | AltintasC, AvciG, DaglarH, et al. Database for CO2 separation performances of MOFs based on computational materials screening[J]. ACS Appl. Mater. Interfaces, 2018, 10(20): 17257-17268. |
| 21 | WatanabeT, ShollD S. Accelerating applications of metal-organic frameworks for gas adsorption and separation by computational screening of materials[J]. Langmuir, 2012, 28(40): 14114-14128. |
| 22 | LiS, ChungY G, Snurr, R Q. High-throughput screening of metal-organic frameworks for CO2 capture in the presence of water[J]. Langmuir, 2016, 32(40): 10368-10376. |
| 23 | QiaoZ, PengC, ZhouJ, et al. High-throughput computational screening of 137953 metal-organic frameworks for membrane separation of a CO2/N2/CH4 mixture[J]. J. Mater. Chem. A, 2016, 4(41): 15904-15912. |
| 24 | ChenY, WangB, WangX Q, et al. A copper(Ⅱ)-paddlewheel metal-organic framework with exceptional hydrolytic stability and selective adsorption and detection ability of aniline in water[J]. ACS Appl. Mater. Interfaces, 2017, 9(32): 27027-27035. |
| 25 | KimH K, YunW S, KimM B, et al. A chemical route to activation of open metal sites in the copper-based metal-organic framework materials HKUST-1 and Cu-MOF-2[J]. J. Am. Chem. Soc., 2015, 137(31): 10009-10015. |
| 26 | LinX, TelepeniI, BlakeA J, et al. High capacity hydrogen adsorption in Cu(Ⅱ) tetracarboxylate framework materials: the role of pore size, ligand functionalization, and exposed metal sites[J]. J.Am. Chem. Soc., 2009, 131(6): 2159-2171. |
| 27 | ZhangC, Lan, Y S, GuoX Y, et al. Materials genomics-guided ab initio screening of MOFs with open copper sites for acetylene storage[J]. AIChE J., 2018, 64(4): 1389-1398. |
| 28 | ChenB L, XiangS C, QianG D. Metal-organic frameworks with functional pores for recognition of small molecules[J]. Accounts Chem. Res., 2010, 43(8): 1115-1124. |
| 29 | LiuX P, XiaoZ Y, XuJ. A NbO-type copper metal–organic framework decorated with carboxylate groups exhibiting highly selective CO2 adsorption and separation of organic dyes[J]. J.Mater. Chem. A, 2016, 4(36): 13844-13851. |
| 30 | WillemsT F, RycroftC H, KaziM, et al. Algorithms and tools for high-throughput geometry-based analysis of crystalline porous materials[J]. Microporous Mesoporous Mater., 2012, 149(1): 134-141. |
| 31 | ZhangC, WangL, MaurinG, et al. In silico screening of MOFs with open copper sites for C2H2/CO2 separation[J]. AIChE J., 2018, 64(11): 4089-4096. |
| 32 | PotoffJ J, SiepmannJ I. Vapor-liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen[J]. AIChE J., 2001, 47(7): 1676-1682. |
| 33 | HarrisJ G, YungK H. Carbon dioxide's liquid-vapor coexistence curve and critical properties as predicted by a simple molecular model[J]. J. Phys. Chem., 1995, 99(31): 12021-12024. |
| 34 | RappeA K, CasewitC J, ColwellK S, et al. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations[J]. J. Am. Chem. Soc., 1992, 114(25): 10024-10035. |
| 35 | EwaldP P. Die Berechnung optischer und elektrostatischer Gitterpotentiale[J]. Ann. Phys., 1921, 396(3): 253-287. |
| 36 | VlugtT J H, Garcia-PerezE, DubbeldamD, et al. Computing the heat of adsorption using molecular simulations: the effect of strong coulombic interactions[J]. J. Chem. Theory Comput., 2008, 4(7): 1107-1118. |
| 37 | WellsB A, Bruin-DickasonC D, ChaffeeA L. Charge equilibration based on atomic ionization in metal-organic frameworks[J]. J. Phys. Chem. C, 2015, 119(1): 456-466. |
| 38 | BrenemanC M, WibergK B. Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis[J]. J. Comput. Chem., 1990, 11(3): 361-373. |
| 39 | QiaoZ, WangN, JiangJ, et al. Design of amine-functionalized metal-organic frameworks for CO2 separation: the more amine, the better? [J]. Chem. Commun., 2016, 52(5): 974-977. |
| 40 | ScottH S, ShivannaM, BajpaiA, et al. Highly selective separation of C2H2 from CO2 by a new dichromate-based hybrid ultramicroporous material[J]. ACS Appl. Mater. Interfaces, 2017, 9(39): 33395-33400. |
| 41 | ChowdhuryP, BikkinaC, MeisterD, et al. Comparison of adsorption isotherms on Cu-BTC metal organic frameworks synthesized from different routes[J]. Microporous Mesoporous Mater., 2009, 117: 406-413. |
| 42 | LiB Y, ZhangZ J, LiY, et al. Enhanced binding affinity, remarkable selectivity, and high capacity of CO2 by dual functionalization of a rht-type metal-organic framework[J]. Angew. Chem. Int. Ed., 2012, 51(6): 1412-1415. |
| 43 | CaiJ F, Wang, H Z, WangH L, et al. An amino-decorated Nbo-type metal-organic framework for high C2H2 storage and selective CO2 capture[J]. RSC Adv., 2015, 5(94): 77417-77422. |
| 44 | SongC L, HeY B, LiB, et al. Enhanced CO2 sorption and selectivity by functionalization of a Nbo-type metal-organic framework with polarized benzothiadiazole moieties[J]. Chem. Commun., 2014, 50(81): 12105-12108. |
| 45 | LinL C, BergerA H, MartinR L, et al. In silico screening of carbon-capture materials[J]. Nat. Mater., 2012, 11(7): 633-641. |
| 46 | WuD, WangC C, LiuB, et al. Large-scale computational screening of metal-organic frameworks for CH4/H2 separation[J]. AIChE J., 2012, 58(7): 2078-2084. |
| 47 | BoldogI, BereciartuaP J, BulánekR, et al. 10-Vertex closo-carborane: a unique ligand platform for porous coordination polymers[J]. CrystEngComm, 2016, 18(12): 2036-2040. |
| 48 | PangJ D, LiuC P, HuangY G, et al. Visualizing the dynamics of temperature- and solvent-responsive soft crystals[J]. Angew. Chem. Int. Ed., 2016, 55(26): 7478-7482. |
| 49 | WuH, SimmonsJ M, SrinivasG, et al. Adsorption sites and binding nature of CO2 in prototypical metal-organic frameworks: a combined neutron diffraction and first-principles study[J]. J. Phys. Chem. Lett., 2010, 1(13): 1946-1951. |
| 50 | ChenK J, MaddenD G, PhamT, et al. Tuning pore size in square-lattice coordination networks for size-selective sieving of CO2[J]. Angew. Chem. Int. Ed., 2016, 55(35): 10268-10272. |
| 51 | HuangS L, WengL H, JinG X. Bottom-up synthesis of coordination polymers based on carborane backbones and Cu2(CO2)4 paddle-wheel: ligand metathesis with metallotecons[J]. Dalton Trans., 2012, 41(38): 11657-11662. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [3] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [4] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [5] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [6] | 赵佳佳, 田世祥, 李鹏, 谢洪高. SiO2-H2O纳米流体强化煤尘润湿性的微观机理研究[J]. 化工学报, 2023, 74(9): 3931-3945. |
| [7] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [8] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [9] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [10] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [11] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [12] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [13] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [14] | 董明, 徐进良, 刘广林. 超临界水非均质特性分子动力学研究[J]. 化工学报, 2023, 74(7): 2836-2847. |
| [15] | 文兆伦, 李沛睿, 张忠林, 杜晓, 侯起旺, 刘叶刚, 郝晓刚, 官国清. 基于自热再生的隔壁塔深冷空分工艺设计及优化[J]. 化工学报, 2023, 74(7): 2988-2998. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号