化工学报 ›› 2021, Vol. 72 ›› Issue (1): 468-482.DOI: 10.11949/0438-1157.20201100
赵贞尧1,2( ),张保财1,2(
),张保财1,2( ),李锋1,2(
),李锋1,2( ),宋浩1,2(
),宋浩1,2( )
)
收稿日期:2020-08-03
修回日期:2020-10-10
出版日期:2021-01-05
发布日期:2021-01-05
通讯作者:
李锋,宋浩
作者简介:赵贞尧(1995—),男,硕士研究生,基金资助:
ZHAO Zhenyao1,2( ),ZHANG Baocai1,2(
),ZHANG Baocai1,2( ),LI Feng1,2(
),LI Feng1,2( ),SONG Hao1,2(
),SONG Hao1,2( )
)
Received:2020-08-03
Revised:2020-10-10
Online:2021-01-05
Published:2021-01-05
Contact:
LI Feng,SONG Hao
摘要:
以产电微生物为核心的微生物电催化系统在能源、环境等诸多领域有着广泛的应用,然而自然环境中野生型产电微生物可利用底物谱窄、底物摄取代谢强度弱,胞内电子池容量小、还原力再生效率差,胞外电子传递速率慢、电子通量小,这已成为限制其工业化应用的主要瓶颈。本文基于产电微生物介导的化学能到电能的能量转化路径,总结阐明了产电微生物的胞内电子生成过程与胞外电子传递机制,系统综述了近五年国内外利用合成生物学增强产电微生物底物摄取利用、强化胞内电子生成、加速胞外电子传递方面的研究进展,并对未来设计构建高效产电细胞研究进行了展望。
中图分类号:
赵贞尧, 张保财, 李锋, 宋浩. 产电细胞的合成生物学设计构建[J]. 化工学报, 2021, 72(1): 468-482.
ZHAO Zhenyao, ZHANG Baocai, LI Feng, SONG Hao. Design and construction of exoelectrogens by synthetic biology[J]. CIESC Journal, 2021, 72(1): 468-482.

图2 产电菌的底物摄取范围拓宽(a) 构建代谢木糖产电的工程希瓦氏菌[47]; (b)构建希瓦氏菌-肺炎克雷伯氏菌合成菌群产电[55]; (c)构建酿酒酵母菌-希瓦氏菌合成菌群产电[56]; (d)构建蓝藻-希瓦氏菌合成菌群产电[57]
Fig.2 Extension of substrate scope of electrogenic bacteria(a) construction of S. oneidensis for metabolizing xylose[47]; (b) synthetic Klebsiella pneumoniae-Shewanella oneidensis consortium for power generation[55]; (c) synthetic S. cerevisiae -S. oneidensis consortium for power generation[56]; (d) synthetic S. cyanobacteria-S. oneidensis consortium for power generation[57]

图3 产电菌胞内电子生成和传递的强化(a)敲除ldhA以扩充大肠杆菌可释放电子池[58]; (b) 敲除arcA以扩充大肠杆菌可释放电子池[59]; (c)模块化强化NADH再生路径以强化希瓦氏菌胞内还原力再生[60]; (d)改造希瓦氏菌从头合成NAD+以强化希瓦氏菌胞内还原力再生[61]
Fig.3 Generation and transform of intracellular electronics in electrogenic bacteria(a) knockdown ldhA in E. coli for augmenting intracellular electron pool[58]; (b) knockdown arcA in E. coli for augmenting intracellular electron pool[59]; (c) modular engineering regeneration pathway of NADH in S. oneidensis for intracellular reducing power regeneration[60]; (d) engineering de novo biosynthesis of NAD(H/+) in S. oneidensis for intracellular reducing power regeneration[61]
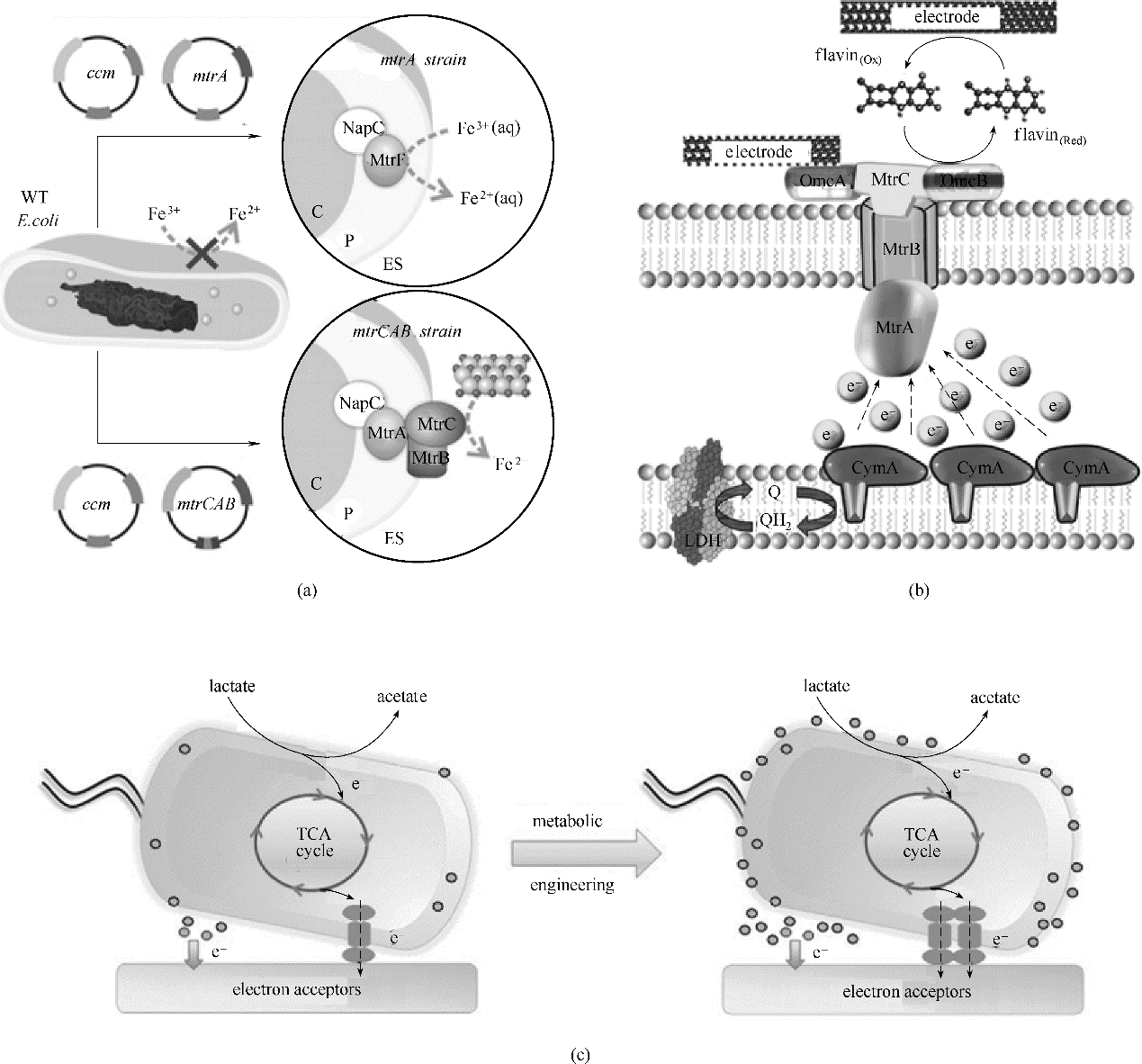
图4 强化导电元件提升电活性微生物产电能力(a)异源表达mtrCAB的大肠杆菌用于还原可溶性金属氧化物[68]; (b) 过表达cymA加强希瓦氏菌的胞外电子传递[70]; (c)共表达mtrC-mtrA-mtrB和ribD-ribC-ribBA-ribE增强希瓦氏菌的胞外电子传递[72]
Fig.4 Strengthen the conductive components for enhanceing the electricity generation capacity of electroactive microorganisms(a) overexpressing mtrCAB in E.coli for reducing soluble metal oxide[68]; (b) overexpressing cymA in S. oneidensis for enhancing extracellular electron transfer[70]; (c) coexpression of mtrC-mtrA-mtrB and ribD-ribC-ribBA-ribE in S. oneidensis for enhanceing extracellular electron transfer[72]

图5 强化导电生物膜提升微生物电活性(a) 构建近红外光响应性大肠杆菌生物膜系统催化吲哚转化为色氨酸[80]; (b)在 MFC中构建基于近红外光响应的c-di-GMP逻辑门[81]
Fig.5 Strengthen the conductive biofilm to improve the electrical activity of microorganisms(a) construction of NIR-light-responsive E. coli biofilm system for catalyzing indole into tryptophan[80]; (b) construction of NIR light responsive c-di-GMP logic gate in MFC[81]
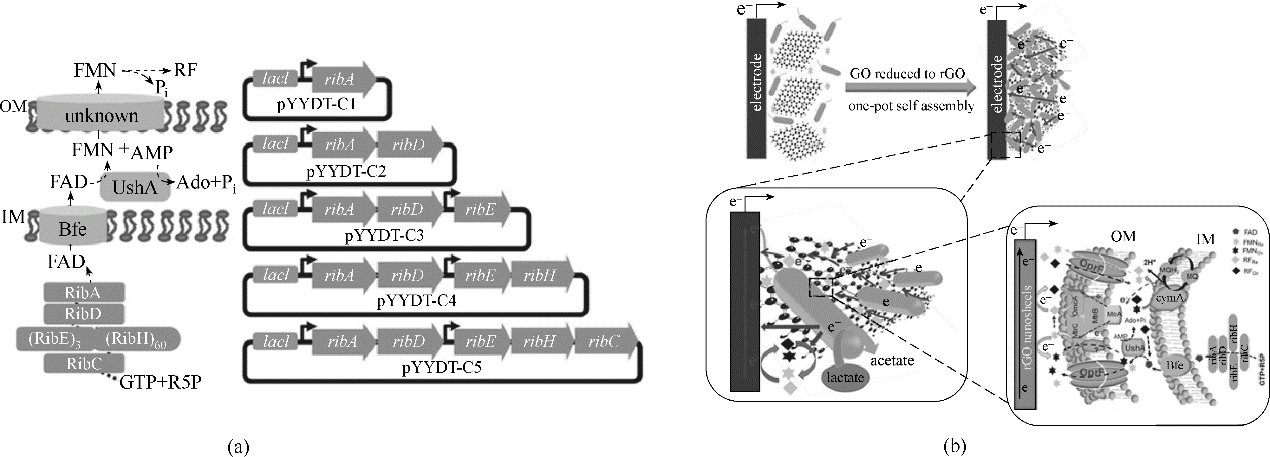
图6 强化电子传递载体提升电活性微生物产电能力(a) 构建黄素生物合成途径以强化希瓦氏菌胞外电子传递[89]; (b) 构建核黄素-自组装三维rGO杂化生物膜以强化MFC的电流输出能力[90]
Fig.6 Strengthen the electron shuttle for enhancing the electrical activity of microorganisms(a) construction of a synthetic flavin biosynthesis pathway in S. oneidensis for enhancing extracellular electron transfer[89]; (b) construction of flavins-self-assembled three-dimensional (3D) rGO biohybrid biofilm for enhancing power generation of MFC[90]
| 1 | Li M, Zhou M, Tian X, et al. Microbial fuel cell (MFC) power performance improvement through enhanced microbial electrogenicity [J]. Biotechnol Advances, 2018, 36(4): 1316-1327. |
| 2 | Sun M, Zhai L F, Li W W, et al. Harvest and utilization of chemical energy in wastes by microbial fuel cells [J]. Chemical Society Reviews, 2016, 45(10): 2847-2870. |
| 3 | Lu L, Guest J S, Peters C A, et al. Wastewater treatment for carbon capture and utilization [J]. Nature Sustainability, 2018, 1(12): 750-758. |
| 4 | Logan B E, Rabaey K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies [J]. Science, 2012, 337(6095): 686-690. |
| 5 | Logan B E, Rossi R, Ragab A A, et al. Electroactive microorganisms in bioelectrochemical systems [J]. Nature Reviews Microbiology, 2019, 17(5): 307-319. |
| 6 | Koch C, Harnisch F. Is there a specific ecological niche for electroactive microorganisms? [J]. ChemElectroChem, 2016, 3(9): 1282-1295. |
| 7 | Nealson K H, Rowe A R. Electromicrobiology: realities, grand challenges, goals and predictions [J]. Microbial Biotechnology, 2016, 9(5): 595-600. |
| 8 | Zou L, Qiao Y, Li C M. Boosting microbial electrocatalytic kinetics for high power density: insights into synthetic biology and advanced nanoscience [J]. Electrochemical Energy Reviews, 2018, 1(4): 567-598. |
| 9 | Madsen C S, Teravest M A. NADH dehydrogenases Nuo and Nqr1 contribute to extracellular electron transfer by Shewanella oneidensis MR-1 in bioelectrochemical systems [J]. Scientific Reports, 2019, 9(1): 14959. |
| 10 | Ishiki K, Shiigi H. Kinetics of intracellular electron generation in Shewanella oneidensis MR-1 [J]. Analytical Chemistry, 2019, 91(22): 14401-14406. |
| 11 | Hunt K A, Flynn J M, Naranjo B, et al. Substrate-level phosphorylation is the primary source of energy conservation during anaerobic respiration of Shewanella oneidensis strain MR-1 [J]. Journal of Bacteriology, 2010, 192(13): 3345-3351. |
| 12 | Tao L, Xie M, Chiew G G, et al. Improving electron trans-inner membrane movements in microbial electrocatalysts [J].Chemical Communications, 2016, 52(37): 6292-6295. |
| 13 | Nakayama Y, Hayashi M, Unemoto T. Identication of six subunits constituting Na+-translocating NADH-quinone reductase from the marine Vibrio alginolyticus [J]. FEBS Letters, 1998, 422: 240-242. |
| 14 | Shi L, Dong H, Reguera G, et al. Extracellular electron transfer mechanisms between microorganisms and minerals [J]. Nature Reviews Microbiology, 2016, 14(10): 651-662. |
| 15 | Richardson D J, Butt J N, Fredrickson J K, et al. The ‘porin-cytochrome' model for microbe-to-mineral electron transfer [J]. Molecular Microbiology, 2012, 85(2): 201-212. |
| 16 | Edwards M J, White G F, Butt J N, et al. The crystal structure of a biological insulated transmembrane molecular wire [J]. Cell, 2020, 181(3): 665-673. |
| 17 | Leang C, Coppi M V, Lovley D R. OmcB, a c-type polyheme cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens [J]. Journal of Bacteriology, 2003, 185(7): 2096-2103. |
| 18 | Shi L, Fredrickson J K, Zachara J M. Genomic analyses of bacterial porin-cytochrome gene clusters [J]. Frontiers in Microbiology, 2014, 5: 657 |
| 19 | Liu Y, Fredrickson J K, Zachara J M, et al. Direct involvement of ombB, omaB, and omcB genes in extracellular reduction of Fe(III) by Geobacter sulfurreducens PCA [J]. Frontiers in Microbiology, 2015, 6: 1075. |
| 20 | Qian X, Reguera G, Mester T, et al. Evidence that OmcB and OmpB of Geobacter sulfurreducens are outer membrane surface proteins [J]. FEMS Microbiology Letters, 2007, 277(1): 21-27. |
| 21 | Lloyd J R, Leang C, Hodges Myerson A L, et al. Biochemical and genetic characterization of PpcA, a periplasmic c-type cytochrome in Geobacter sulfurreducens [J]. Biochemical Journal, 2003, 369(1): 153-161. |
| 22 | Lovley D R, Malvankar N S. Seeing is believing: novel imaging techniques help clarify microbial nanowire structure and function [J]. Environmental Microbiology, 2015, 17(7): 2209-2215. |
| 23 | Walker D J, Adhikari R Y, Holmes D E, et al. Electrically conductive pili from pilin genes of phylogenetically diverse microorganisms [J]. ISME Journal, 2018, 12(1): 48-58. |
| 24 | Sure S, Ackland M L, Torriero A a J, et al. Microbial nanowires: an electrifying tale [J]. Microbiology, 2016, 162(12): 2017-2028. |
| 25 | Reguera G, Mccarthy K D, Mehta T, et al. Extracellular electron transfer via microbial nanowires [J]. Nature, 2005, 435(7045): 1098-1101. |
| 26 | Reardon P N, Mueller K T. Structure of the type IVa major pilin from the electrically conductive bacterial nanowires of Geobacter sulfurreducens [J]. Journal of Biological Chemistry, 2013, 288(41): 29260-29266. |
| 27 | Tan Y, Adhikari R Y, Malvankar N S, et al. Expressing the geobacter metallireducens PilA in Geobacter sulfurreducens yields Pili with exceptional conductivity [J]. mBio, 2017, 8(1): e02203-e02216. |
| 28 | Lampa-Pastirk S, Veazey J P, Walsh K A, et al. Thermally activated charge transport in microbial protein nanowires [J]. Scientific Reports, 2016, 6(1): 23517. |
| 29 | Tan Y, Adhikari R Y, Malvankar N S, et al. Synthetic biological protein nanowires with high conductivity [J]. Small, 2016, 12(33): 4481-4485. |
| 30 | Malvankar N S, Vargas M, Nevin K P, et al. Tunable metallic-like conductivity in microbial nanowire networks [J]. Nature Nanotechnology, 2011, 6(9): 573-579. |
| 31 | Strycharz-Glaven S M, Snider R M, Guiseppi-Elie A, et al. On the electrical conductivity of microbial nanowires and biofilms [J]. Energy & Environmental Science, 2011, 4(11): 4366-4379 |
| 32 | Marsili E, Baron D B, Shikhare I D, et al. Shewanella secretes flavins that mediate extracellular electron transfer [J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(10): 3968-3973. |
| 33 | Von Canstein H, Ogawa J, Shimizu S, et al. Secretion of flavins by Shewanella species and their role in extracellular electron transfer [J]. Applied and Environmental Microbiology, 2008, 74(3): 615-623. |
| 34 | Pham T H, Boon N, De Maeyer K, et al. Use of Pseudomonas species producing phenazine-based metabolites in the anodes of microbial fuel cells to improve electricity generation [J]. Applied and Environmental Microbiology, 2008, 80(6): 985-993. |
| 35 | Mcanulty M J, Poosarla V G, Kim K Y, et al. Electricity from methane by reversing methanogenesis [J]. Nature Communications, 2017, 8: 15419. |
| 36 | Lovley D, Woodard J C, Philips E J P, et al. Humic substances as electron acceptors for microbial respiration [J]. Nature, 1996, 382(6590): 445-448. |
| 37 | Okamoto A, Hashimoto K, Nealson K H, et al. Rate enhancement of bacterial extracellular electron transport involves bound flavin semiquinones [J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(19): 7856-7861. |
| 38 | Huang L, Tang J, Chen M, et al. Two modes of riboflavin-mediated extracellular electron transfer in Geobacter uraniireducens [J]. Frontiers in Microbiology, 2018, 9: 2886. |
| 39 | Okamoto A, Saito K, Inoue K, et al. Uptake of self-secreted flavins as bound cofactors for extracellular electron transfer in Geobacter species [J]. Energy & Environmental Science, 2014, 7(4): 1357-1361. |
| 40 | Okamoto A, Nakamura R, Nealson K H, et al. Bound flavin model suggests similar electron-transfer mechanisms in Shewanella and Geobacter [J]. ChemElectroChem, 2014, 1(11): 1808-1812. |
| 41 | Edwards M J, White G F, Norman M, et al. Redox linked flavin sites in extracellular decaheme proteins involved in microbe-mineral electron transfer [J]. Scientific Reports, 2015, 5(1): 11677. |
| 42 | Breuer M, Rosso K M, Blumberger J. Flavin binding to the deca-heme cytochrome MtrC: insights from computational molecular simulation [J]. Biophysical Journal, 2015, 109(12): 2614-2624. |
| 43 | Smith J A, Tremblay P L, Shrestha P M, et al. Going wireless: Fe(III) oxide reduction without pili by Geobacter sulfurreducens strain JS-1 [J]. Applied and Environmental Microbiology, 2014, 80(14): 4331-4340. |
| 44 | Flynn C M, Hunt K A, Gralnick J A, et al. Construction and elementary mode analysis of a metabolic model for Shewanella oneidensis MR-1 [J]. Biosystems, 2012, 107(2): 120-128. |
| 45 | Hau H H, Gralnick J A. Ecology and biotechnology of the genus Shewanella [J]. Annual Review of Microbiology, 2007, 61: 237-258. |
| 46 | Sekar R, Shin H D, Dichristina T J. Activation of an otherwise silent xylose metabolic pathway in Shewanella oneidensis [J]. Applied and Environmental Microbiology, 2016, 82(13): 3996-4005. |
| 47 | Li F, Li Y, Sun L, et al. Engineering Shewanella oneidensis enables xylose-fed microbial fuel cell [J]. Biotechnology for Biofuels, 2017, 10(1): 196. |
| 48 | Howard E C, Hamdan L J, Lizewski S E, et al. High frequency of glucose-utilizing mutants in Shewanella oneidensis MR-1 [J]. FEMS Microbiology Letters, 2012, 327(1): 9-14. |
| 49 | Choi D, Lee S B, Kim S, et al. Metabolically engineered glucose-utilizing Shewanella strains under anaerobic conditions [J]. Bioresource Technology, 2014, 154: 59-66. |
| 50 | Johnson E T, Baron D B, Naranjo B, et al. Enhancement of survival and electricity production in an engineered bacterium by light-driven proton pumping [J]. Applied and Environmental Microbiology, 2010, 76(13): 4123-4129. |
| 51 | Matsuda S, Liu H, Kouzuma A, et al. Electrochemical gating of tricarboxylic acid cycle in electricity-producing bacterial cells of Shewanella [J]. PLoS One, 2013, 8(8): e72901. |
| 52 | Wang Y, Lv M, Meng Q, et al. Facile one-step strategy for highly boosted microbial extracellular electron transfer of the genus Shewanella [J]. ACS Nano, 2016, 10(6): 6331-6337. |
| 53 | Wen Q, Kong F, Ren Y, et al. Improved performance of microbial fuel cell through addition of rhamnolipid [J]. Electrochemistry Communications, 2010, 12(12): 1710-1713. |
| 54 | Li F, Yin C, Sun L, et al. Synthetic Klebsiella pneumoniae-Shewanella oneidensis consortium enables glycerol-fed high-performance microbial fuel cells [J]. Biotechnology Journal, 2018, 13(5): e1700491. |
| 55 | Li F, An X, Wu D, et al. Engineering microbial consortia for high-performance cellulosic hydrolyzates-fed microbial fuel cells [J]. Frontiers in Microbiology, 2019, 10: 409. |
| 56 | Lin T, Bai X, Hu Y, et al. Synthetic Saccharomyces cerevisiae-Shewanella oneidensis consortium enables glucose-fed high-performance microbial fuel cell [J]. AIChE Journal, 2017, 63(6): 1830-1838. |
| 57 | Zhu H, Meng H, Zhang W, et al. Development of a longevous two-species biophotovoltaics with constrained electron flow [J]. Nature Communications, 2019, 10(1): 4282. |
| 58 | Yong Y C, Yu Y Y, Yang Y, et al. Increasing intracellular releasable electrons dramatically enhances bioelectricity output in microbial fuel cells [J]. Electrochemistry Communications, 2012, 19: 13-16. |
| 59 | Liu J, Yong Y C, Song H, et al. Activation enhancement of citric acid cycle to promote bioelectrocatalytic activity of arcA knockout Escherichia coli toward high-performance microbial fuel cell [J]. ACS Catalysis, 2012, 2(8): 1749-1752. |
| 60 | Li F, Li Y, Sun L, et al. Modular engineering intracellular NADH regeneration boosts extracellular electron transfer of Shewanella oneidensis MR-1 [J]. ACS Synthetic Biology, 2018, 7(3): 885-895. |
| 61 | Li F, Li Y X, Cao Y X, et al. Modular engineering to increase intracellular NAD(H/+) promotes rate of extracellular electron transfer of Shewanella oneidensis [J]. Nature Communications, 2018, 9(1): 3637. |
| 62 | Vamshi K K, Venkata M S. Purification and characterization of NDH-2 protein and elucidating its role in extracellular electron transport and bioelectrogenic activity [J]. Frontiers in Microbiology, 2019, 10: 880. |
| 63 | Tao L, Xie M, Chiew G G, et al. Improving electron trans-inner membrane movements in microbial electrocatalysts [J]. Chemical Communications, 2016, 52(37): 6292-6295. |
| 64 | Li F, Wang L, Liu C, et al. Engineering exoelectrogens by synthetic biology strategies [J]. Current Opinion in Electrochemistry, 2018, 10: 37-45. |
| 65 | Jensen H M, Teravest M A, Kokish M G, et al. CymA and exogenous flavins improve extracellular electron transfer and couple it to cell growth in Mtr-expressing Escherichia coli [J]. ACS Synthetic Biology, 2016, 5(7): 679-688. |
| 66 | Teravest M A, Zajdel T J, Ajo-Franklin C M. The Mtr pathway of Shewanella oneidensis MR-1 couples substrate utilization to current production in Escherichia coli [J]. ChemElectroChem, 2014, 1(11): 1874-1879. |
| 67 | Goldbeck C P, Jensen H M, Teravest M A, et al. Tuning promoter strengths for improved synthesis and function of electron conduits in Escherichia coli [J]. ACS Synthetic Biology, 2013, 2(3): 150-159. |
| 68 | Jensen H M, Albers A E, Malley K R, et al. Engineering of a synthetic electron conduit in living cells [J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(45): 19213-19218. |
| 69 | Sekar N, Jain R, Yan Y, et al. Enhanced photo-bioelectrochemical energy conversion by genetically engineered cyanobacteria [J]. Biotechnology and Bioengineering, 2016, 113(3): 675-679. |
| 70 | Vellingiri A, Song Y E, Munussami G, et al. Overexpression of c-type cytochrome, CymA in Shewanella oneidensis MR-1 for enhanced bioelectricity generation and cell growth in a microbial fuel cell [J]. Journal of Chemical Technology & Biotechnology, 2019, 94(7): 2115-2122. |
| 71 | Delgado V P, Paquete C M, Sturm G, et al. Improvement of the electron transfer rate in Shewanella oneidensis MR-1 using a tailored periplasmic protein composition [J]. Bioelectrochemistry, 2019, 129: 18-25. |
| 72 | Min D, Cheng L, Zhang F, et al. Enhancing extracellular electron transfer of Shewanella oneidensis MR-1 through coupling improved flavin synthesis and metal-reducing conduit for pollutant degradation [J]. Environmental Science & Technology, 2017, 51(9): 5082-5089. |
| 73 | Liu X, Tremblay P L, Malvankar N S, et al. A Geobacter sulfurreducens strain expressing Pseudomonas aeruginosa type IV pili localizes OmcS on pili but is deficient in Fe(III) oxide reduction and current production [J]. Applied and Environmental Microbiology, 2014, 80(3): 1219-1224. |
| 74 | Jana P S, Katuri K, Kavanagh P, et al. Charge transport in films of Geobacter sulfurreducens on graphite electrodes as a function of film thickness [J]. Physical Chemistry Chemical Physics, 2014, 16(19): 9039-9046. |
| 75 | Nevin K P, Richter H, Covalla S F, et al. Power output and columbic efficiencies from biofilms of Geobacter sulfurreducens comparable to mixed community microbial fuel cells [J]. Environmental Microbiology, 2008, 10(10): 2505-2514. |
| 76 | Reguera G, Nevin K P, Nicoll J S, et al. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells [J]. Applied and Environmental Microbiology, 2006, 72(11): 7345-7348. |
| 77 | Romling U, Galperin M Y, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger [J]. Microbiol and Molecular Biology Reviews, 2013, 77(1): 1-52. |
| 78 | Leang C, Malvankar N S, Franks A E, et al. Engineering Geobacter sulfurreducens to produce a highly cohesive conductive matrix with enhanced capacity for current production [J]. Energy & Environmental Science, 2013, 6(6): 1901. |
| 79 | Liu T, Yu Y Y, Deng X P, et al. Enhanced Shewanella biofilm promotes bioelectricity generation [J]. Biotechnology and Bioengineering, 2015, 112(10): 2051-2019. |
| 80 | Hu Y, Liu X, Ren A T M, et al. Optogenetic modulation of a catalytic biofilm for the biotransformation of indole into tryptophan [J]. ChemSusChem, 2019, 12(23): 5142-5148. |
| 81 | Hu Y, Wu Y, Mukherjee M, et al. A near-infrared light responsive c-di-GMP module-based AND logic gate in Shewanella oneidensis [J]. Chemical Communications, 2017, 53(10): 1646-1648. |
| 82 | Di Martino P. Extracellular polymeric substances, a key element in understanding biofilm phenotype [J]. AIMS Microbiology, 2018, 4(2): 274-288. |
| 83 | Ding Y, Peng N, Du Y, et al. Disruption of putrescine biosynthesis in Shewanella oneidensis enhances biofilm cohesiveness and performance in Cr(Ⅵ) immobilization [J]. Applied and Environmental Microbiology, 2014, 80(4): 1498-1506. |
| 84 | Kouzuma A, Meng X Y, Kimura N, et al. Disruption of the putative cell surface polysaccharide biosynthesis gene SO3177 in Shewanella oneidensis MR-1 enhances adhesion to electrodes and current generation in microbial fuel cells [J]. Applied and Environmental Microbiology, 2010, 76(13): 4151-4157. |
| 85 | Godeke J, Heun M, Bubendorfer S, et al. Roles of two Shewanella oneidensis MR-1 extracellular endonucleases [J]. Applied and Environmental Microbiology, 2011, 77(15): 5342-5351. |
| 86 | Wang V B, Chua S L, Cao B, et al. Engineering PQS biosynthesis pathway for enhancement of bioelectricity production in Pseudomonas aeruginosa microbial fuel cells [J]. PLoS One, 2013, 8(5): e63129. |
| 87 | Chen S, Jing X, Tang J, et al. Quorum sensing signals enhance the electrochemical activity and energy recovery of mixed-culture electroactive biofilms [J]. Biosensors and Bioelectronics, 2017, 97: 369-376. |
| 88 | Jing X, Liu X, Deng C, et al. Chemical signals stimulate Geobacter soli biofilm formation and electroactivity [J]. Biosensors and Bioelectronics, 2019, 127: 1-9. |
| 89 | Yang Y, Ding Y, Hu Y, et al. Enhancing bidirectional electron transfer of Shewanella oneidensis by a synthetic flavin pathway [J]. ACS Synthetic Biology, 2015, 4(7): 815-823. |
| 90 | Lin T, Ding W, Sun L, et al. Engineered Shewanella oneidensis-reduced graphene oxide biohybrid with enhanced biosynthesis and transport of flavins enabled a highest bioelectricity output in microbial fuel cells [J]. Nano Energy, 2018, 50: 639-648. |
| 91 | Liu T, Yu Y Y, Chen T, et al. A synthetic microbial consortium of Shewanella and Bacillus for enhanced generation of bioelectricity [J]. Biotechnology and Bioengineering, 2017, 114(3): 526-532. |
| 92 | Yang Y, Wu Y, Hu Y, et al. Engineering electrode-attached microbial consortia for high-performance xylose-fed microbial fuel cell [J]. ACS Catalysis, 2015, 5(11): 6937-6945. |
| 93 | Liu Y, Ding M, Ling W, et al. A three-species microbial consortium for power generation [J]. Energy & Environmental Science, 2017, 10(7): 1600-1609. |
| 94 | Bosire E M, Rosenbaum M A. Electrochemical potential influences phenazine production, electron transfer and consequently electric current generation by Pseudomonas aeruginosa [J]. Frontiers in Microbiology, 2017, 8: 892. |
| 95 | Qiao Y J, Qiao Y, Zou L, et al. Biofilm promoted current generation of Pseudomonas aeruginosa microbial fuel cell via improving the interfacial redox reaction of phenazines [J]. Bioelectrochemistry, 2017, 117: 34-39. |
| 96 | Yong X Y, Yan Z Y, Shen H B, et al. An integrated aerobic-anaerobic strategy for performance enhancement of Pseudomonas aeruginosa-inoculated microbial fuel cell [J]. Bioresource Technology, 2017, 241: 1191-1196. |
| 97 | Hernandez M E, Kappler A, Newman D K. Phenazines and other redox-active antibiotics promote microbial mineral reduction [J]. Applied and Environmental Microbiology, 2004, 70(2): 921-928. |
| 98 | Venkataraman A, Rosenbaum M A, Perkins S D, et al. Metabolite-based mutualism between Pseudomonas aeruginosa PA14 and Enterobacter aerogenes enhances current generation in bioelectrochemical systems [J]. Energy & Environmental Science, 2011, 4(11): 4550. |
| 99 | Schmitz S, Nies S, Wierckx N, et al. Engineering mediator-based electroactivity in the obligate aerobic bacterium Pseudomonas putida KT2440 [J]. Frontiers in Microbiology, 2015, 6: 284. |
| 100 | Yong X Y, Shi D Y, Chen Y L, et al. Enhancement of bioelectricity generation by manipulation of the electron shuttles synthesis pathway in microbial fuel cells [J]. Bioresource Technology, 2014, 152: 220-224. |
| 101 | Venkataraman A, Rosenbaum M, Arends J B A, et al. Quorum sensing regulates electric current generation of Pseudomonas aeruginosa PA14 in bioelectrochemical systems [J]. Electrochemistry Communications, 2010, 12(3): 459-462. |
| 102 | Yong Y C, Yu Y Y, Li C M, et al. Bioelectricity enhancement via overexpression of quorum sensing system in Pseudomonas aeruginosa-inoculated microbial fuel cells [J]. Biosensors and Bioelectronics, 2011, 30(1): 87-92. |
| [1] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [2] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [3] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [4] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [5] | 谭卓涛, 齐思雨, 许梦蛟, 戴杰, 朱晨杰, 应汉杰. 辅酶自循环的氧化还原级联体系在生物催化过程中的应用:机遇与挑战[J]. 化工学报, 2023, 74(1): 45-59. |
| [6] | 刘昕, 戈钧, 李春. 光驱动微生物杂合系统提高生物制造水平[J]. 化工学报, 2023, 74(1): 330-341. |
| [7] | 胡阳, 孙彦. 酶分子的自驱动及其介导的微纳马达[J]. 化工学报, 2023, 74(1): 116-132. |
| [8] | 刘雪, 张莉娟, 赵广荣. 大肠杆菌偏利共培养系统合成大豆苷元[J]. 化工学报, 2022, 73(9): 4015-4024. |
| [9] | 安绍杰, 许洪峰, 李思, 许远航, 李佳锡. 利用分子机器的组装与分解构建pH敏感性谷胱甘肽过氧化物人工酶[J]. 化工学报, 2022, 73(8): 3669-3678. |
| [10] | 张劢, 田瑶, 郭之旗, 王叶, 窦广进, 宋浩. 光催化-生物杂合系统设计优化用于燃料和化学品绿色合成[J]. 化工学报, 2022, 73(7): 2774-2789. |
| [11] | 孙甲琛, 孙文涛, 孙慧, 吕波, 李春. 甘草黄酮合酶Ⅱ催化甘草素特异性合成7,4′-二羟基黄酮[J]. 化工学报, 2022, 73(7): 3202-3211. |
| [12] | 王靖楠, 庞建, 秦磊, 郭超, 吕波, 李春, 王超. 丁烯基多杀菌素高产菌株的选育和改造策略[J]. 化工学报, 2022, 73(2): 566-576. |
| [13] | 王淋, 付乾, 肖帅, 李卓, 李俊, 张亮, 朱恂, 廖强. 高效可见光响应微生物/光电化学耦合人工光合作用系统[J]. 化工学报, 2022, 73(2): 887-893. |
| [14] | 黄明, 朱亮, 丁紫霞, 毛一婷, 马中青. 生物质三组分与低密度聚乙烯共催化热解制取轻质芳烃的协同作用机理[J]. 化工学报, 2022, 73(2): 699-711. |
| [15] | 孙怡, 张腾, 吕波, 李春. 胞内生物传感器提高微生物细胞工厂的精细调控[J]. 化工学报, 2022, 73(2): 521-534. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号