化工学报 ›› 2021, Vol. 72 ›› Issue (9): 4665-4674.DOI: 10.11949/0438-1157.20210244
贺兴处( ),陈德珍(
),陈德珍( ),梅振飞,阿迪力·巴吐尔null,安青
),梅振飞,阿迪力·巴吐尔null,安青
收稿日期:2021-02-08
修回日期:2021-03-29
出版日期:2021-09-05
发布日期:2021-09-05
通讯作者:
陈德珍
作者简介:贺兴处(1994—),男,硕士研究生,基金资助:
Xingchu HE( ),Dezhen CHEN(
),Dezhen CHEN( ),Zhenfei MEI,Batuer ADILI,Qing AN
),Zhenfei MEI,Batuer ADILI,Qing AN
Received:2021-02-08
Revised:2021-03-29
Online:2021-09-05
Published:2021-09-05
Contact:
Dezhen CHEN
摘要:
结合反应分子动力学(ReaxFF MD)模拟方法和自行开发的全自动ReaxFF反应机理分析软件(automatic reaction mechanism analyzer, AutoRMA)深入探究了CaO催化聚乙烯(PE)热解及H2O对催化过程的影响,通过热解产物分析、热解过程反应路径追踪和C―C键及C―H键时序变化分析来揭示反应机理。结果显示,CaO作为催化剂提高了PE的热解反应速率,有效降低了PE热解过程中C―C键和C―H键断裂的活化能,使其分别从316.88 kJ/mol和430.13 kJ/mol降低到22.24 kJ/mol和30.87 kJ/mol,并促进了PE向轻质油和气体分子的转化;CaO对自由基和碳链上的非饱和碳原子的吸附及解离可以有效促进PE的热解;但H2O存在时由于其自身与CaO结合会抑制CaO的催化作用,同时H2O主要通过取代反应以羟基的形式存在于烃类产品中,水分含量(质量分数)为PE的50%时导致10%的油品中含有氧元素,降低产品质量,也会导致液相产品中存在大量的水分, 因此应避免热解的废PE携带水分。研究表明,ReaxFF MD结合AutoRMA有助于对PE等聚合物催化热解机理的深入理解,进而优化反应体系。
中图分类号:
贺兴处,陈德珍,梅振飞,阿迪力·巴吐尔null,安青. CaO催化PE热解及H2O对催化过程影响的ReaxFF MD研究与机理分析[J]. 化工学报, 2021, 72(9): 4665-4674.
Xingchu HE,Dezhen CHEN,Zhenfei MEI,Batuer ADILI,Qing AN. ReaxFF MD study on the pyrolysis of PE catalyzed by CaO and the effect of H2O on the catalytic process and mechanism analysis[J]. CIESC Journal, 2021, 72(9): 4665-4674.
| 体系 | PE(C300H602)单链/条 | CaO超晶胞(Ca545O544)/个 | H2O分子/个 |
|---|---|---|---|
| PE | 8 | — | — |
| PE-H2O | 8 | — | 900 |
| PE-CaO | 8 | 1 | — |
| PE-CaO-H2O | 8 | 1 | 900 |
表1 模型体系分子构成
Table 1 Molecular composition of model system
| 体系 | PE(C300H602)单链/条 | CaO超晶胞(Ca545O544)/个 | H2O分子/个 |
|---|---|---|---|
| PE | 8 | — | — |
| PE-H2O | 8 | — | 900 |
| PE-CaO | 8 | 1 | — |
| PE-CaO-H2O | 8 | 1 | 900 |
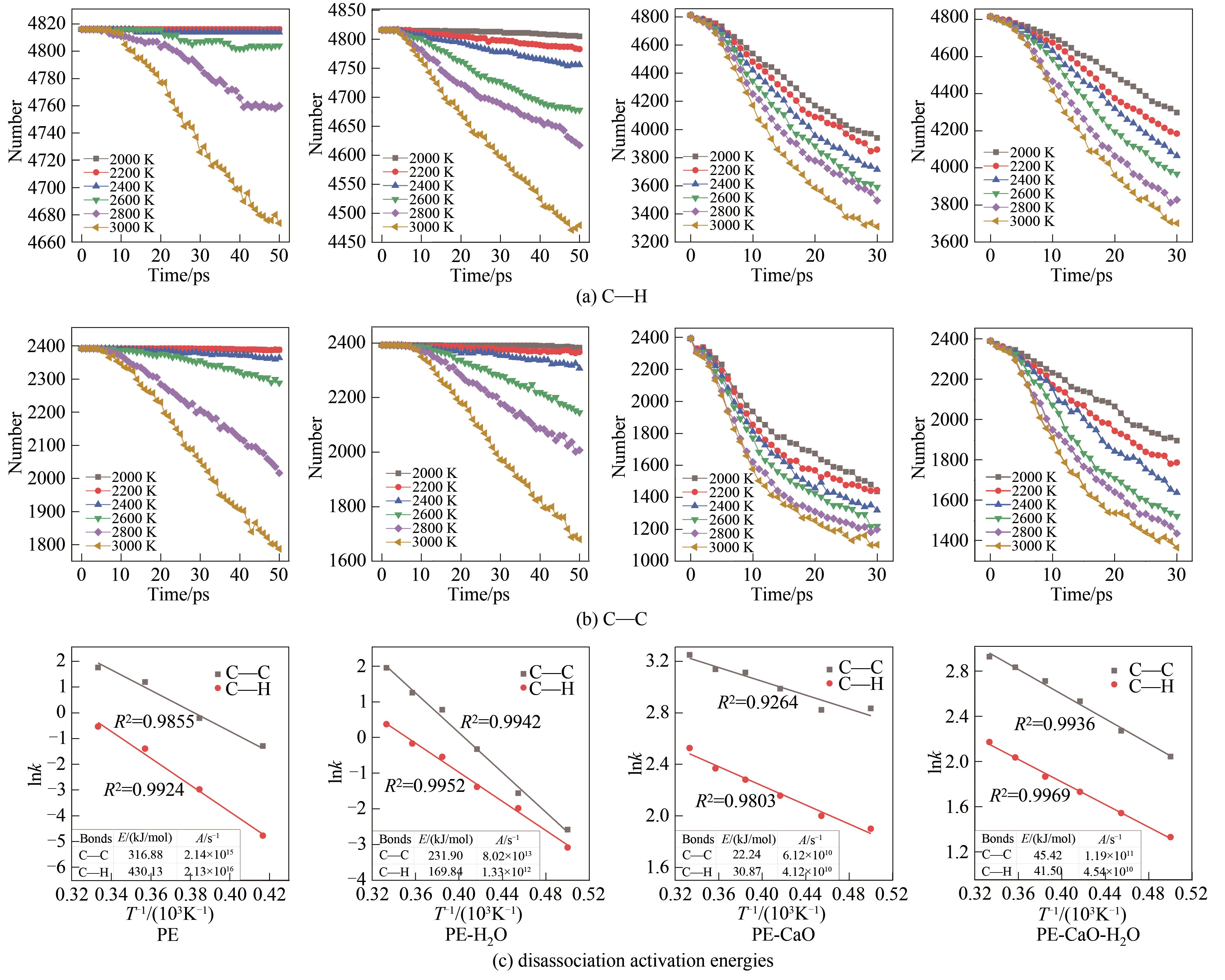
图7 不同温度下热解过程整个体系C―C键和C―H键的数量变化及动力学计算
Fig.7 The number change of C―C bond and C―H bond in the whole pyrolysis system at different temperatures and kinetics calculation
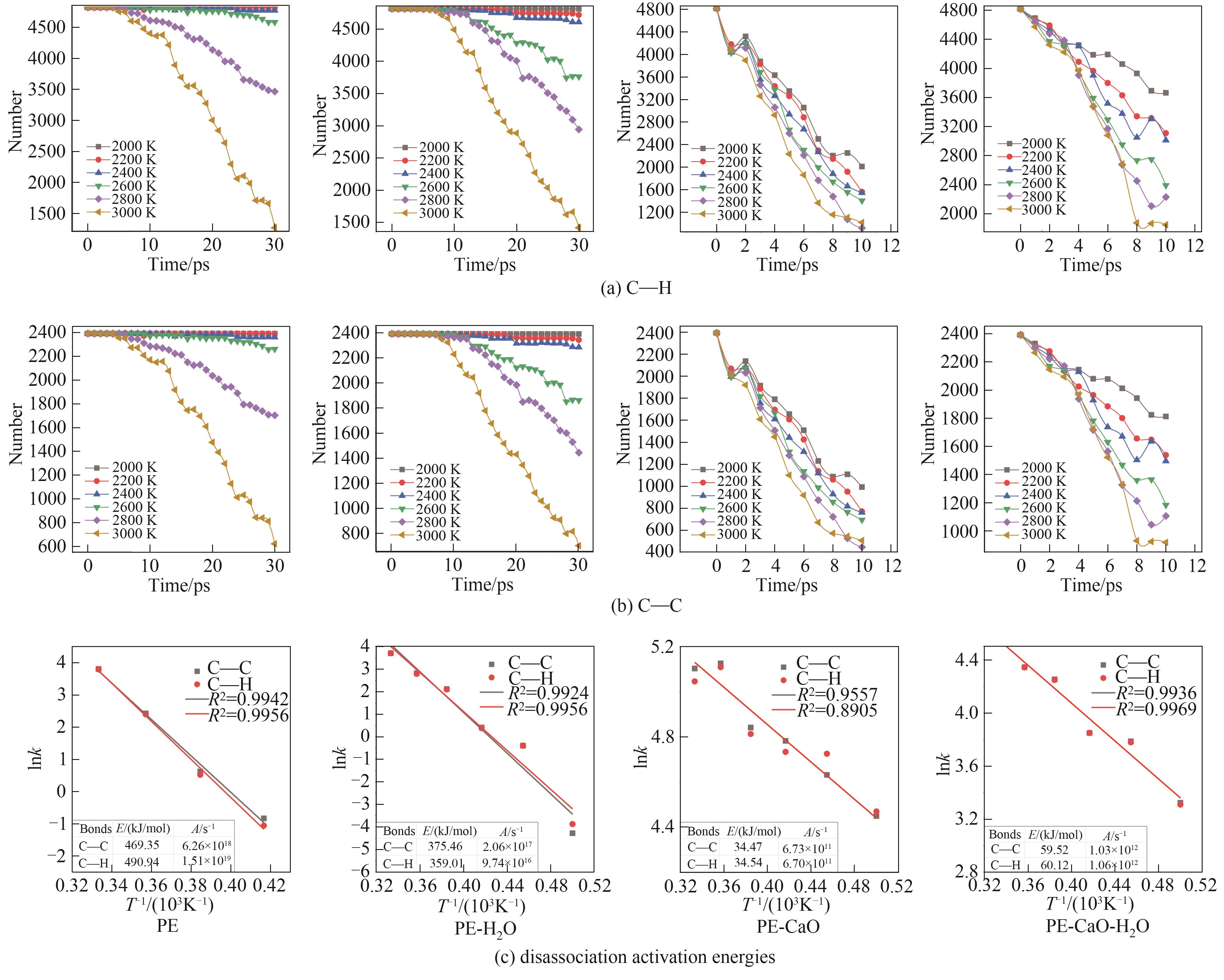
图8 不同温度下热解过程炭产物中C―C键和C―H键的数量变化及动力学计算
Fig.8 The number change of C―C bond and C―H bond in char product during pyrolysis at different temperatures and kinetics calculation
| 1 | 全球塑料垃圾达49亿吨热解处理技术成突破口[J]. 塑料科技, 2018, 46(8): 23. |
| Global plastic waste reaches4.9 billion tons, pyrolysis treatment technology becomes a breakthrough[J]. Plastics Science and Technology, 2018, 46(8): 23. | |
| 2 | Anuar Sharuddin S D, Abnisa F, Wan Daud W M A, et al. A review on pyrolysis of plastic wastes[J]. Energy Conversion and Management, 2016, 115: 308-326. |
| 3 | Kunwar B, Cheng H N, Chandrashekaran S R, et al. Plastics to fuel: a review[J]. Renewable and Sustainable Energy Reviews, 2016, 54: 421-428. |
| 4 | Aguado J, Serrano D P, Escola J M. Fuels from waste plastics by thermal and catalytic processes: a review[J]. Industrial & Engineering Chemistry Research, 2008, 47(21): 7982-7992. |
| 5 | Artetxe M, Lopez G, Amutio M, et al. Light olefins from HDPE cracking in a two-step thermal and catalytic process[J]. Chemical Engineering Journal, 2012, 207/208: 27-34. |
| 6 | Chen D Z, Yin L J, Wang H, et al. Pyrolysis technologies for municipal solid waste: a review[J]. Waste Management, 2014, 34(12): 2466-2486. |
| 7 | Williams P T, Williams E A. Interaction of plastics in mixed-plastics pyrolysis[J]. Energy & Fuels, 1999, 13(1): 188-196. |
| 8 | Zhou C B, Fang W J, Xu W Y, et al. Characteristics and the recovery potential of plastic wastes obtained from landfill mining[J]. Journal of Cleaner Production, 2014, 80: 80-86. |
| 9 | Bajus M, Hájeková E. Thermal cracking of the model seven components mixed plastics into oils/waxes[J]. Petroleum & Coal, 2010, 52(3): 164-172. |
| 10 | Scott D S, Czernik S R, Piskorz J, et al. Fast pyrolysis of plastic wastes[J]. Energy & Fuels, 1990, 4(4): 407-411. |
| 11 | Liu X L, Li X X, Liu J, et al. Study of high density polyethylene (HDPE) pyrolysis with reactive molecular dynamics[J]. Polymer Degradation and Stability, 2014, 104: 62-70. |
| 12 | Al-Salem S M, Lettieri P. Kinetic study of high density polyethylene (HDPE) pyrolysis[J]. Chemical Engineering Research and Design, 2010, 88(12): 1599-1606. |
| 13 | Gaca P, Drzewiecka M, Kaleta W, et al. Catalytic degradation of polyethylene over mesoporous molecular sieve MCM-41 modified with heteropoly compounds[J]. Polish Journal of Environmental Studies, 2008, 17(1): 25-31. |
| 14 | Ratnasari D K, Nahil M A, Williams P T. Catalytic pyrolysis of waste plastics using staged catalysis for production of gasoline range hydrocarbon oils[J]. Journal of Analytical and Applied Pyrolysis, 2017, 124: 631-637. |
| 15 | Zhang Y T, Ji G Z, Chen C S, et al. Liquid oils produced from pyrolysis of plastic wastes with heat carrier in rotary kiln[J]. Fuel Processing Technology, 2020, 206: 106455. |
| 16 | Arabiourrutia M, Elordi G, Lopez G, et al. Characterization of the waxes obtained by the pyrolysis of polyolefin plastics in a conical spouted bed reactor[J]. Journal of Analytical and Applied Pyrolysis, 2012, 94: 230-237. |
| 17 | Jha K K, Kannan T T M, Senthilvelan N. Optimization of catalytic pyrolysis process for change of plastic waste into fuel[J]. Materials Today: Proceedings, 2021, 39: 708-711. |
| 18 | Al-Salem S M, Antelava A, Constantinou A, et al. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW)[J]. Journal of Environmental Management, 2017, 197: 177-198. |
| 19 | Predel M, Kaminsky W. Pyrolysis of mixed polyolefins in a fluidised-bed reactor and on a pyro-GC/MS to yield aliphatic waxes[J]. Polymer Degradation and Stability, 2000, 70(3): 373-385. |
| 20 | Donaj P J, Kaminsky W, Buzeto F, et al. Pyrolysis of polyolefins for increasing the yield of monomers' recovery[J]. Waste Management, 2012, 32(5): 840-846. |
| 21 | Kaminsky W, Predel M, Sadiki A. Feedstock recycling of polymers by pyrolysis in a fluidised bed[J]. Polymer Degradation and Stability, 2004, 85(3): 1045-1050. |
| 22 | Qureshi M S, Oasmaa A, Pihkola H, et al. Pyrolysis of plastic waste: opportunities and challenges[J]. Journal of Analytical and Applied Pyrolysis, 2020, 152: 104804. |
| 23 | Joo H S, Guin J A. Continuous upgrading of a plastics pyrolysis liquid to an environmentally favorable gasoline range product[J]. Fuel Processing Technology, 1998, 57(1): 25-40. |
| 24 | Seth D, Sarkar A. Thermal pyrolysis of polypropylene: effect of reflux-condenser on the molecular weight distribution of products[J]. Chemical Engineering Science, 2004, 59(12): 2433-2445. |
| 25 | Bagri R, Williams P T. Catalytic pyrolysis of polyethylene[J]. Journal of Analytical and Applied Pyrolysis, 2002, 63(1): 29-41. |
| 26 | Chen Z Z, Zhang X R, Che L, et al. Effect of volatile reactions on oil production and composition in thermal and catalytic pyrolysis of polyethylene[J]. Fuel, 2020, 271: 117308. |
| 27 | Zheng Y W, Wang J D, Liu C, et al. Enhancing the aromatic hydrocarbon yield from the catalytic copyrolysis of xylan and LDPE with a dual-catalytic-stage combined CaO/HZSM-5 catalyst[J]. Journal of the Energy Institute, 2020, 93(5): 1833-1847. |
| 28 | Chen C, Jin Y Q, Chi Y. Effects of moisture content and CaO on municipal solid waste pyrolysis in a fixed bed reactor[J]. Journal of Analytical and Applied Pyrolysis, 2014, 110: 108-112. |
| 29 | Fan L L, Chen P, Zhang Y N, et al. Fast microwave-assisted catalytic co-pyrolysis of lignin and low-density polyethylene with HZSM-5 and MgO for improved bio-oil yield and quality[J]. Bioresource Technology, 2017, 225: 199-205. |
| 30 | Miandad R, Barakat M A, Rehan M, et al. Plastic waste to liquid oil through catalytic pyrolysis using natural and synthetic zeolite catalysts[J]. Waste Management, 2017, 69: 66-78. |
| 31 | Bai C, Liu L C, Sun H. Molecular dynamics simulations of methanol to olefin reactions in HZSM-5 zeolite using a ReaxFF force field[J]. The Journal of Physical Chemistry C, 2012, 116(12): 7029-7039. |
| 32 | Chen C, Zhao L L, Wu X, et al. Theoretical understanding of coal char oxidation and gasification using reactive molecular dynamics simulation[J]. Fuel, 2020, 260: 116300. |
| 33 | Su J, Fang C Q, Yang M N, et al. Catalytic pyrolysis of waste packaging polyethylene using AlCl3-NaCl eutectic salt as catalyst[J]. Journal of Analytical and Applied Pyrolysis, 2019, 139: 274-281. |
| 34 | van Duin A C T, Dasgupta S, Lorant F, et al. ReaxFF: a reactive force field for hydrocarbons[J]. The Journal of Physical Chemistry A, 2001, 105(41): 9396-9409. |
| 35 | Knyazev V D. Effects of chain length on the rates of C–C bond dissociation in linear alkanes and polyethylene[J]. The Journal of Physical Chemistry A, 2007, 111(19): 3875-3883. |
| 36 | 同济大学. 全自动ReaxFF反应机理分析软件[简称:AutoRMA] V1.0: 2021SR0108488[P].2021-01-20. |
| 37 | Si T, Huang K, Lin Y Y, et al. ReaxFF study on the effect of CaO on cellulose pyrolysis[J]. Energy & Fuels, 2019, 33(11): 11067-11077. |
| 38 | Sandia National Laboratories. LAMMPS[EB/OL]. . |
| 39 | Pitman M C, van Duin A C T. Dynamics of confined reactive water in smectite clay-zeolite composites[J]. Journal of the American Chemical Society, 2012, 134(6): 3042-3053. |
| 40 | Paajanen A, Vaari J. High-temperature decomposition of the cellulose molecule: a stochastic molecular dynamics study[J]. Cellulose, 2017, 24(7): 2713-2725. |
| 41 | Bhoi S, Banerjee T, Mohanty K. Molecular dynamic simulation of spontaneous combustion and pyrolysis of brown coal using ReaxFF[J]. Fuel, 2014, 136: 326-333. |
| 42 | Zhong Q F, Mao Q Y, Xiao J, et al. ReaxFF simulations of petroleum coke sulfur removal mechanisms during pyrolysis and combustion[J]. Combustion and Flame, 2018, 198: 146-157. |
| 43 | Zhang Z J, Guo L T, Zhang H Y, et al. Comparing product distribution and desulfurization during direct pyrolysis and hydropyrolysis of Longkou oil shale kerogen using reactive MD simulations[J]. International Journal of Hydrogen Energy, 2019, 44(47): 25335-25346. |
| 44 | Popov K V, Knyazev V D. Initial stages of the pyrolysis of polyethylene[J]. The Journal of Physical Chemistry A, 2015, 119(49): 11737-11760. |
| 45 | Liu Q Y, Hu C S, Peng B, et al. High H2/CO ratio syngas production from chemical looping co-gasification of biomass and polyethylene with CaO/Fe2O3 oxygen carrier[J]. Energy Conversion and Management, 2019, 199: 111951. |
| 46 | Zhang J L, Gu J T, Han Y, et al. Supercritical water oxidation vs supercritical water gasification: which process is better for explosive wastewater treatment?[J]. Industrial & Engineering Chemistry Research, 2015, 54(4): 1251-1260. |
| 47 | Zaker A, Chen Z, Zaheer-Uddin M, et al. Co-pyrolysis of sewage sludge and low-density polyethylene—a thermogravimetric study of thermo-kinetics and thermodynamic parameters[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104554. |
| [1] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [2] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [3] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [4] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [5] | 肖明堃, 杨光, 黄永华, 吴静怡. 浸没孔液氧气泡动力学数值研究[J]. 化工学报, 2023, 74(S1): 87-95. |
| [6] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [7] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [8] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [9] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [10] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [11] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [12] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [13] | 曾如宾, 沈中杰, 梁钦锋, 许建良, 代正华, 刘海峰. 基于分子动力学模拟的Fe2O3纳米颗粒烧结机制研究[J]. 化工学报, 2023, 74(8): 3353-3365. |
| [14] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [15] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号