化工学报 ›› 2022, Vol. 73 ›› Issue (10): 4507-4517.DOI: 10.11949/0438-1157.20220531
收稿日期:2022-04-13
修回日期:2022-08-08
出版日期:2022-10-05
发布日期:2022-11-02
通讯作者:
赵之平
作者简介:王玉杰(1980—),女,博士,高级工程师,wangyuj.bjhy@sinopec.com
基金资助:
Yujie WANG1,2( ), Shenhui LI2, Zhiping ZHAO2(
), Shenhui LI2, Zhiping ZHAO2( )
)
Received:2022-04-13
Revised:2022-08-08
Online:2022-10-05
Published:2022-11-02
Contact:
Zhiping ZHAO
摘要:
为了探究有机金属框架MOF-74能否作为一种优良的固体吸附剂,分离H2/He混合物中H2,并达到提纯He的目的,采用分子模拟的手段研究了H2、He及H2/He混合物在M-MOF-74(M=Mg、Co、Ni、Cu、Zn)上的吸附性能及吸附机理。结果表明,在1 bar(1 bar=105 Pa)压力和25℃条件下,纯H2对纯He在Ni-MOF-74上的选择性达6.58,而Mg-MOF-74对H2的吸附量最大,其值为0.19 mmol·cm-3,为He吸附量的6.46倍。当H2/He混合物的浓度发生变化时,对其在M-MOF-74上的吸附分离因子没有较大影响,说明浓度变化不会影响M-MOF-74上吸附位点容纳H2和He的能力。吸附位点和吸附热分析表明,MOF-74上的金属离子未饱和位点能够显著增强其对H2的吸附能力。其结果对判断M-MOF-74是否具有分离H2/He混合物的潜力,以及定量分析MOFs金属未饱和位点对H2/He混合物分离的贡献提供了一定的理论基础。
中图分类号:
王玉杰, 李申辉, 赵之平. M-MOF-74吸附分离H2/He混合物的分子模拟研究[J]. 化工学报, 2022, 73(10): 4507-4517.
Yujie WANG, Shenhui LI, Zhiping ZHAO. Molecular simulation study on adsorption and separation of H2/He mixtures by M-MOF-74[J]. CIESC Journal, 2022, 73(10): 4507-4517.
| 名称 | CSD号 | 孔径/nm | 孔隙率/% | 比表面积/(m2·m-3) | 晶胞参数/nm;(°) |
|---|---|---|---|---|---|
| Mg-MOF-74 | 1863524 | 1.1 | 0.724 | 1943.2 | 2.6×2.6×0.69;90×90×120 |
| Co-MOF-74 | 1494752 | 1.1 | 0.721 | 1818.5 | 2.6×2.6×0.67;90×90×120 |
| Ni-MOF-74 | 1494751 | 1.1 | 0.725 | 1834.4 | 2.6×2.6×0.67;90×90×120 |
| Cu-MOF-74 | — | 1.1 | 0.722 | 1878.8 | 2.6×2.6×0.69;90×90×120 |
| Zn-MOF-74 | 1863522 | 1.1 | 0.723 | 1922.4 | 2.6×2.6×0.69;90×90×120 |
表1 M-MOF-74的结构特点
Table 1 Structural features of M-MOF-74
| 名称 | CSD号 | 孔径/nm | 孔隙率/% | 比表面积/(m2·m-3) | 晶胞参数/nm;(°) |
|---|---|---|---|---|---|
| Mg-MOF-74 | 1863524 | 1.1 | 0.724 | 1943.2 | 2.6×2.6×0.69;90×90×120 |
| Co-MOF-74 | 1494752 | 1.1 | 0.721 | 1818.5 | 2.6×2.6×0.67;90×90×120 |
| Ni-MOF-74 | 1494751 | 1.1 | 0.725 | 1834.4 | 2.6×2.6×0.67;90×90×120 |
| Cu-MOF-74 | — | 1.1 | 0.722 | 1878.8 | 2.6×2.6×0.69;90×90×120 |
| Zn-MOF-74 | 1863522 | 1.1 | 0.723 | 1922.4 | 2.6×2.6×0.69;90×90×120 |
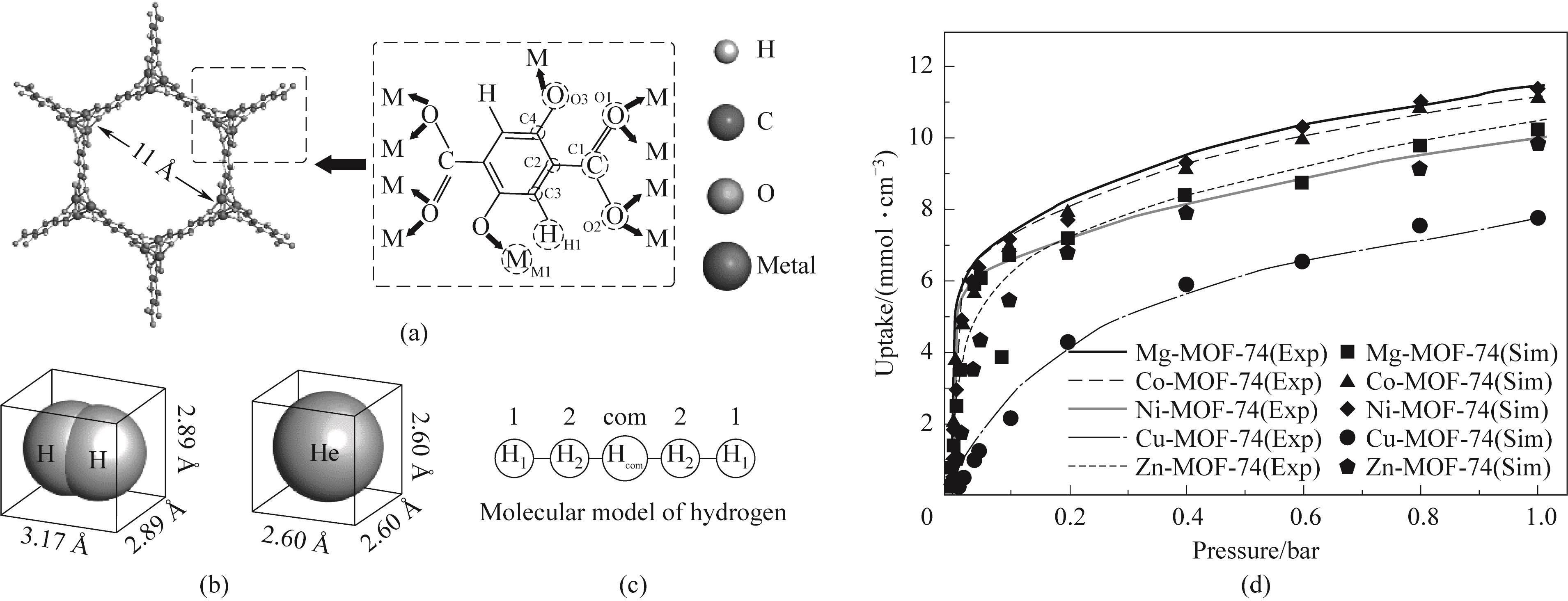
图1 (a)M-MOF-74的结构;(b)H2和He的分子尺寸;(c)H2的五点模型; (d) M-MOF-74对H2的吸附等温线(其中Exp代表实验测得的吸附等温线,数据来自文献[36-38]; Sim代表本文模拟得到的数据; 1? =0.1 nm)
Fig.1 (a) Structure of M-MOF-74; (b) Molecular dimensions of hydrogen and helium; (c) Five-point model of hydrogen; (d) Adsorption isotherms of H2 in M-MOF-74 (where Exp represents the experimentally measured adsorption isotherm, and the data are from Refs. [36-38]; Sim represents the data get from the simulation of this work)
| 名称 | 临界温度/K | 临界压力/bar | 分子动力学直径/nm | 极化率/(10-25 cm3) | 电四极矩/(10-40 C·m-2) | 摩尔质量/(g·mol-1) |
|---|---|---|---|---|---|---|
| He | 5.2 | 2.28 | 0.260 | 2.08 | 0 | 4.003 |
| H2 | 33.2 | 13.15 | 0.289 | 7.87 | 2.21 | 2.016 |
表2 H2与He的物化性质
Table 2 Physicochemical properties of H2 and He
| 名称 | 临界温度/K | 临界压力/bar | 分子动力学直径/nm | 极化率/(10-25 cm3) | 电四极矩/(10-40 C·m-2) | 摩尔质量/(g·mol-1) |
|---|---|---|---|---|---|---|
| He | 5.2 | 2.28 | 0.260 | 2.08 | 0 | 4.003 |
| H2 | 33.2 | 13.15 | 0.289 | 7.87 | 2.21 | 2.016 |
| 名称 | 势陷深度ε/K | 平衡距离σ/nm | 原子电荷q/e | ||||
|---|---|---|---|---|---|---|---|
| Mg | Co | Ni | Zn | Cu | |||
| Mg | 55.857 | 0.260 | 1.678 | ||||
| Co | 7.045 | 0.255 | 1.420 | ||||
| Ni | 7.545 | 0.252 | 1.501 | ||||
| Zn | 62.399 | 0.246 | 1.492 | ||||
| Cu | 2.516 | 0.311 | 1.128 | ||||
| C1 | 47.856 | 0.347 | 0.93030 | 0.93940 | 0.88260 | 0.87980 | 1.14250 |
| C2 | 47.856 | 0.347 | -0.48150 | -0.52700 | -0.44910 | -0.26750 | -0.53740 |
| C3 | 47.856 | 0.347 | 0.46750 | 0.49000 | 0.44070 | 0.40420 | 0.55200 |
| C4 | 47.856 | 0.347 | -0.38240 | -0.40320 | -0.43910 | -0.37410 | -0.33180 |
| O1 | 48.158 | 0.303 | -0.87140 | -0.79450 | -0.78000 | -0.82960 | -0.76640 |
| O2 | 48.158 | 0.303 | -0.76800 | -0.58940 | -0.66470 | -0.75710 | -0.72140 |
| O3 | 48.158 | 0.303 | -0.80540 | -0.72910 | -0.71650 | -0.77420 | -0.68620 |
表3 M-MOF-74的力场参数及原子电荷
Table 3 Force field parameters and atomic charges of M-MOF-74
| 名称 | 势陷深度ε/K | 平衡距离σ/nm | 原子电荷q/e | ||||
|---|---|---|---|---|---|---|---|
| Mg | Co | Ni | Zn | Cu | |||
| Mg | 55.857 | 0.260 | 1.678 | ||||
| Co | 7.045 | 0.255 | 1.420 | ||||
| Ni | 7.545 | 0.252 | 1.501 | ||||
| Zn | 62.399 | 0.246 | 1.492 | ||||
| Cu | 2.516 | 0.311 | 1.128 | ||||
| C1 | 47.856 | 0.347 | 0.93030 | 0.93940 | 0.88260 | 0.87980 | 1.14250 |
| C2 | 47.856 | 0.347 | -0.48150 | -0.52700 | -0.44910 | -0.26750 | -0.53740 |
| C3 | 47.856 | 0.347 | 0.46750 | 0.49000 | 0.44070 | 0.40420 | 0.55200 |
| C4 | 47.856 | 0.347 | -0.38240 | -0.40320 | -0.43910 | -0.37410 | -0.33180 |
| O1 | 48.158 | 0.303 | -0.87140 | -0.79450 | -0.78000 | -0.82960 | -0.76640 |
| O2 | 48.158 | 0.303 | -0.76800 | -0.58940 | -0.66470 | -0.75710 | -0.72140 |
| O3 | 48.158 | 0.303 | -0.80540 | -0.72910 | -0.71650 | -0.77420 | -0.68620 |
| 文献 | H2吸附热/(kJ·mol-1) | ||||
|---|---|---|---|---|---|
Mg- MOF-74 | Co- MOF-74 | Ni- MOF-74 | Cu-MOF-74 | Zn-MOF-74 | |
| [ | -10.1 | -10.7 | -12.9 | — | -8.8 |
| [ | -10.3 | — | — | — | — |
| [ | — | — | — | — | -8.8 |
| [ | — | — | — | — | -8.3 |
| 本文 | -10.41 | -10.44 | -11.71 | -8.41 | -9.17 |
表4 本文计算得到的吸附热与实验值的对比
Table 4 Comparison of heat of adsorption calculated in this work with experimental values
| 文献 | H2吸附热/(kJ·mol-1) | ||||
|---|---|---|---|---|---|
Mg- MOF-74 | Co- MOF-74 | Ni- MOF-74 | Cu-MOF-74 | Zn-MOF-74 | |
| [ | -10.1 | -10.7 | -12.9 | — | -8.8 |
| [ | -10.3 | — | — | — | — |
| [ | — | — | — | — | -8.8 |
| [ | — | — | — | — | -8.3 |
| 本文 | -10.41 | -10.44 | -11.71 | -8.41 | -9.17 |

图4 M-MOF-74上OMS位点对H2和He在M-MOF-74上吸附量及H2/He选择性的影响
Fig.4 Effects of OMS sites on M-MOF-74 on the adsorption capacity of H2 and He and ideal selectivity of H2/He on M-MOF-74

图7 (a)H2在M-MOF-74上的金属离子吸附位点及RDG分析(其中蓝色代表一定的配位作用,绿色代表弱的范德华相互作用),等值面为0.2;(b)H2在M-MOF-74上的苯环吸附位点;(c)H2和He到M-MOF-74的平衡距离;(d)H2和He与M-MOF-74的结合能
Fig.7 (a) Metal ion adsorption sites and RDG analysis of H2 on M-MOF-74 (Blue represents certain coordination and green represents weak van der Waals interactions), isosurface at 0.2; (b) H2 adsorption sites on the benzene ring on M-MOF-74; (c) Equilibrium distances of H2 and He to M-MOF-74; (d) Binding energy of H2 and He to M-MOF-74
| 1 | 秦胜飞, 李济远. 世界氦气供需现状及发展趋势[J]. 石油知识, 2021(5): 44-45. |
| Qin S F, Li J Y. Current situation and development trend of helium supply and demand in the world [J]. Petroleum Knowledge, 2021(5): 44-45. | |
| 2 | Xiong L, Peng N, Liu L, et al. Helium extraction and nitrogen removal from LNG boil-off gas[J]. IOP Conference Series: Materials Science and Engineering, 2017, 171: 012003. |
| 3 | 邢国海. 天然气提取氦气技术现状与发展[J]. 天然气工业, 2008, 28(8): 114-116, 149. |
| Xing G H. Status quo and development of the technology on helium gas abstracted from natural gas[J]. Natural Gas Industry, 2008, 28(8): 114-116, 149. | |
| 4 | 李均方, 何琳琳, 柴露华. 天然气提氦技术现状及建议[J]. 石油与天然气化工, 2018, 47(4): 41-44. |
| Li J F, He L L, Chai L H. Present situation and suggestion of helium extraction from natural gas[J]. Chemical Engineering of Oil & Gas, 2018, 47(4): 41-44. | |
| 5 | Rufford T E, Chan K I, Huang S H, et al. A review of conventional and emerging process technologies for the recovery of helium from natural gas[J]. Adsorption Science & Technology, 2014, 32(1): 49-72. |
| 6 | Antunes R, B?hml?nder A, Bükki-Deme A, et al. Experimental investigation of the ideal selectivity of MFI-ZSM-5 zeolite-type membranes for a first evaluation of the separation of hydrogen isotopologues from helium[J]. Separation and Purification Technology, 2019, 212: 767-773. |
| 7 | Simplicio M, Afonso M D, Borisevich O, et al. Permeation of single gases and binary mixtures of hydrogen and helium through a MFI zeolite hollow fibres membrane for application in nuclear fusion[J]. Separation and Purification Technology, 2014, 122: 199-205. |
| 8 | Favvas E P, Heliopoulos N S, Papageorgiou S K, et al. Helium and hydrogen selective carbon hollow fiber membranes: the effect of pyrolysis isothermal time[J]. Separation and Purification Technology, 2015, 142: 176-181. |
| 9 | He T, Pachfule P, Wu H, et al. Hydrogen carriers[J]. Nature Reviews Materials, 2016, 1: 16059. |
| 10 | Wang X S, Ma S Q, Forster P, et al. Enhancing H2 uptake by "close-packing" alignment of open copper sites in metal-organic frameworks[J]. Angewandte Chemie, 2008, 120(38): 7373-7376. |
| 11 | Bae Y S, Snurr R Q. Optimal isosteric heat of adsorption for hydrogen storage and delivery using metal-organic frameworks[J]. Microporous and Mesoporous Materials, 2010, 132(1/2): 300-303. |
| 12 | Pham T, Forrest K A, Eckert J, et al. Dramatic effect of the electrostatic parameters on H2 sorption in an M-MOF-74 analogue[J]. Crystal Growth & Design, 2016, 16(2): 867-874. |
| 13 | Valenzano L, Civalleri B, Chavan S, et al. Computational and experimental studies on the adsorption of CO, N2, and CO2 on Mg-MOF-74[J]. The Journal of Physical Chemistry C, 2010, 114(25): 11185-11191. |
| 14 | Liu A Q, Peng X, Jin Q B, et al. Adsorption and diffusion of benzene in Mg-MOF-74 with open metal sites[J]. ACS Applied Materials & Interfaces, 2019, 11(4): 4686-4700. |
| 15 | Su X, Bromberg L, Martis V, et al. Postsynthetic functionalization of Mg-MOF-74 with tetraethylenepentamine: structural characterization and enhanced CO2 adsorption[J]. ACS Applied Materials & Interfaces, 2017, 9(12): 11299-11306. |
| 16 | Perry J J, Teich-McGoldrick S L, Meek S T, et al. Noble gas adsorption in metal-organic frameworks containing open metal sites[J]. The Journal of Physical Chemistry C, 2014, 118(22): 11685-11698. |
| 17 | Qiao Z W, Xu Q S, Jiang J W. High-throughput computational screening of metal-organic framework membranes for upgrading of natural gas[J]. Journal of Membrane Science, 2018, 551: 47-54. |
| 18 | Tang H J, Jiang J W. In silico screening and design strategies of ethane-selective metal-organic frameworks for ethane/ethylene separation[J]. AIChE Journal, 2021, 67(3): e17025. |
| 19 | Yeo B C, Kim D, Kim H, et al. High-throughput screening to investigate the relationship between the selectivity and working capacity of porous materials for propylene/propane adsorptive separation[J]. The Journal of Physical Chemistry C, 2016, 120(42): 24224-24230. |
| 20 | Moghadam P Z, Li A, Wiggin S B, et al. Development of a Cambridge structural database subset: a collection of metal-organic frameworks for past, present, and future[J]. Chemistry of Materials, 2017, 29(7): 2618-2625. |
| 21 | Chung Y G, Haldoupis E, Bucior B J, et al. Advances, updates, and analytics for the computation-ready, experimental metal-organic framework database: core MOF 2019[J]. Journal of Chemical & Engineering Data, 2019, 64(12): 5985-5998. |
| 22 | Willems T F, Rycroft C H, Kazi M, et al. Algorithms and tools for high-throughput geometry-based analysis of crystalline porous materials[J]. Microporous and Mesoporous Materials, 2012, 149(1): 134-141. |
| 23 | Dubbeldam D, Calero S, Ellis D E, et al. RASPA: molecular simulation software for adsorption and diffusion in flexible nanoporous materials[J]. Molecular Simulation, 2016, 42(2): 81-101. |
| 24 | Eggimann B L, Sunnarborg A J, Stern H D, et al. An online parameter and property database for the TraPPE force field[J]. Molecular Simulation, 2014, 40(1/2/3): 101-105. |
| 25 | Belof J L, Stern A C, Space B. An accurate and transferable intermolecular diatomic hydrogen potential for condensed phase simulation[J]. Journal of Chemical Theory and Computation, 2008, 4(8): 1332-1337. |
| 26 | Rappe A K, Casewit C J, Colwell K S, et al. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations[J]. Journal of the American Chemical Society, 1992, 114(25): 10024-10035. |
| 27 | Pham T, Forrest K A, Banerjee R, et al. Understanding the H2 sorption trends in the M-MOF-74 series (M = Mg, Ni, Co, Zn)[J]. The Journal of Physical Chemistry C, 2015, 119(2): 1078-1090. |
| 28 | Souers P C. Hydrogen Properties for Fusion Energy[M]. Berkeley: University of California Press, 1986. |
| 29 | Stogryn D E, Stogryn A P. Molecular multipole moments[J]. Molecular Physics, 1966, 11(4): 371-393. |
| 30 | National Institute of Standards and Technology. NIST Chemistry WebBook, SRD 69 [EB/OL]. 2018-07-15. . |
| 31 | Auerbach S M, Carrado K A, Dutta P K. Handbook of Zeolite Science and Technology[M]. Boca Raton: CRC Press, 2003. |
| 32 | Zhou W, Wu H, Yildirim T. Enhanced H2 adsorption in isostructural metal–organic frameworks with open metal sites: strong dependence of the binding strength on metal ions[J]. Journal of the American Chemical Society, 2008, 130(46): 15268-15269. |
| 33 | Sumida K, Brown C M, Herm Z R, et al. Hydrogen storage properties and neutron scattering studies of Mg2(dobdc): a metal-organic framework with open Mg2+ adsorption sites[J]. Chemical Communications (Cambridge, England), 2011, 47(4): 1157-1159. |
| 34 | Liu Y, Kabbour H, Brown C M, et al. Increasing the density of adsorbed hydrogen with coordinatively unsaturated metal centers in metal-organic frameworks[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2008, 24(9): 4772-4777. |
| 35 | Rowsell J L C, Yaghi O M. Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal-organic frameworks[J]. Journal of the American Chemical Society, 2006, 128(4): 1304-1315. |
| 36 | Dietzel P D, Georgiev P A, Eckert J, et al. Interaction of hydrogen with accessible metal sites in the metal–organic frameworks M2(dhtp) (CPO-27-M; M = Ni, Co, Mg)[J]. Chemical Communications, 2010, 46(27): 4962-4964. |
| 37 | Jesse L C R, Yaghi O M. Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal-organic frameworks[J]. Journal of the American Chemical Society, 2006, 128(4): 1304-1315. |
| 38 | Rosnes M H, Opitz M, Frontzek M, et al. Intriguing differences in hydrogen adsorption in CPO-27 materials induced by metal substitution[J]. Journal of Materials Chemistry A, 2015, 3(9): 4827. |
| 39 | Johnson E R, Keinan S, Mori-Sánchez P, et al. Revealing noncovalent interactions[J]. Journal of the American Chemical Society, 2010, 132(18): 6498-6506. |
| 40 | Lu T, Chen F W. Multiwfn: a multifunctional wavefunction analyzer[J]. Journal of Computational Chemistry, 2012, 33(5): 580-592. |
| 41 | Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics[J]. Journal of Molecular Graphics, 1996, 14(1): 33-38. |
| 42 | Liao P Q, Huang N Y, Zhang W X, et al. Controlling guest conformation for efficient purification of butadiene[J]. Science, 2017, 356(6343): 1193-1196. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [5] | 赵佳佳, 田世祥, 李鹏, 谢洪高. SiO2-H2O纳米流体强化煤尘润湿性的微观机理研究[J]. 化工学报, 2023, 74(9): 3931-3945. |
| [6] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [7] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [8] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [9] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [10] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [11] | 董明, 徐进良, 刘广林. 超临界水非均质特性分子动力学研究[J]. 化工学报, 2023, 74(7): 2836-2847. |
| [12] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [13] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| [14] | 刘远超, 蒋旭浩, 邵钶, 徐一帆, 钟建斌, 李耑. 几何尺寸及缺陷对石墨炔纳米带热输运特性的影响[J]. 化工学报, 2023, 74(6): 2708-2716. |
| [15] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号