化工学报 ›› 2023, Vol. 74 ›› Issue (5): 2046-2056.DOI: 10.11949/0438-1157.20230061
王蕾1,2( ), 王磊1,2(
), 王磊1,2( ), 白云龙1, 何柳柳1,2
), 白云龙1, 何柳柳1,2
收稿日期:2023-01-30
修回日期:2023-03-09
出版日期:2023-05-05
发布日期:2023-06-29
通讯作者:
王磊
作者简介:王蕾(1992—),女,博士研究生,136997223@qq.com
基金资助:
Lei WANG1,2( ), Lei WANG1,2(
), Lei WANG1,2( ), Yunlong BAI1, Liuliu HE1,2
), Yunlong BAI1, Liuliu HE1,2
Received:2023-01-30
Revised:2023-03-09
Online:2023-05-05
Published:2023-06-29
Contact:
Lei WANG
摘要:
采用亲水的SA为成膜材料,PEG为致孔剂,制备了掺杂自制锆氧化物包覆的锂离子筛前体LMZO的膜状锂离子筛,研究其对锂的吸附性能以及循环稳定性能等,并对其进行了吸附动力学及吸附等温模型分析。结果表明:SA浓度为30 mg·ml-1,PEG含量为1%,LMZO含量为55%时,制得的膜状锂离子筛对Li+的吸附性能最好,为1202.17 mg·m-2。用0.1 mol·L-1 HCl溶液解吸,平衡时锂解吸率可达66.55%,锰溶损率仅为0.345%。在卤水中进行了10次循环吸附解吸后,吸附量降至1116.64 mg·m-2,吸附量仅损失了7.0%。在含有多种复杂离子如Na+、K+、Mg2+和Ca2+的卤水中,SA膜状锂离子筛对Li+有很高的选择性,其吸附过程更符合伪二级动力学方程及Langmuir吸附等温模型。膜状锂离子筛对于从盐湖卤水等液态锂资源中提取锂具有很大的开发潜力。
中图分类号:
王蕾, 王磊, 白云龙, 何柳柳. SA膜状锂离子筛的制备及其锂吸附性能[J]. 化工学报, 2023, 74(5): 2046-2056.
Lei WANG, Lei WANG, Yunlong BAI, Liuliu HE. Preparation of SA lithium ion sieve membrane and its adsorptive properties[J]. CIESC Journal, 2023, 74(5): 2046-2056.
| 金属离子 | 初始浓度/(g·L-1) |
|---|---|
| Li+ | 0.11 |
| Mg2+ | 10.98 |
| Ca2+ | 0.063 |
| K+ | 4.04 |
| Na+ | 4.98 |
| Mn2+ | — |
表1 青海昆特依盐湖卤水水质成分
Table 1 The components of the Qinghai Kunty salt lake brine
| 金属离子 | 初始浓度/(g·L-1) |
|---|---|
| Li+ | 0.11 |
| Mg2+ | 10.98 |
| Ca2+ | 0.063 |
| K+ | 4.04 |
| Na+ | 4.98 |
| Mn2+ | — |
| Gl添加量/% | 卤水通量/(L·m2·h-1) |
|---|---|
| 0 | 334.51 |
| 1 | 361.98 |
| 2 | 383.85 |
| 3 | 402.37 |
| 4 | 414.73 |
| 5 | 429.63 |
表2 不同Gl添加量的SA膜状锂离子筛的膜通量参数
Table 2 Membrane flux performance of SA lithium ion sieve membrane with different glycerol contents
| Gl添加量/% | 卤水通量/(L·m2·h-1) |
|---|---|
| 0 | 334.51 |
| 1 | 361.98 |
| 2 | 383.85 |
| 3 | 402.37 |
| 4 | 414.73 |
| 5 | 429.63 |
| PEG含量/% | 卤水通量/ (L·m2·h-1) | 比表面积/ (m2·g-1) | 孔隙体积/ (cm3·g-1) |
|---|---|---|---|
| 0 | 378.15 | 11.608 | 0.0206 |
| 0.5 | 501.98 | 14.815 | 0.0293 |
| 1 | 668.37 | 17.095 | 0.0379 |
| 2 | 796.02 | 19.362 | 0.0404 |
| 4 | 959.33 | 21.298 | 0.0495 |
表3 不同PEG添加量的SA膜状锂离子筛的膜通量参数及比表面积
Table 3 Membrane flux performance and BET surface area of SA lithium ion sieve membrane with different PEG contents
| PEG含量/% | 卤水通量/ (L·m2·h-1) | 比表面积/ (m2·g-1) | 孔隙体积/ (cm3·g-1) |
|---|---|---|---|
| 0 | 378.15 | 11.608 | 0.0206 |
| 0.5 | 501.98 | 14.815 | 0.0293 |
| 1 | 668.37 | 17.095 | 0.0379 |
| 2 | 796.02 | 19.362 | 0.0404 |
| 4 | 959.33 | 21.298 | 0.0495 |
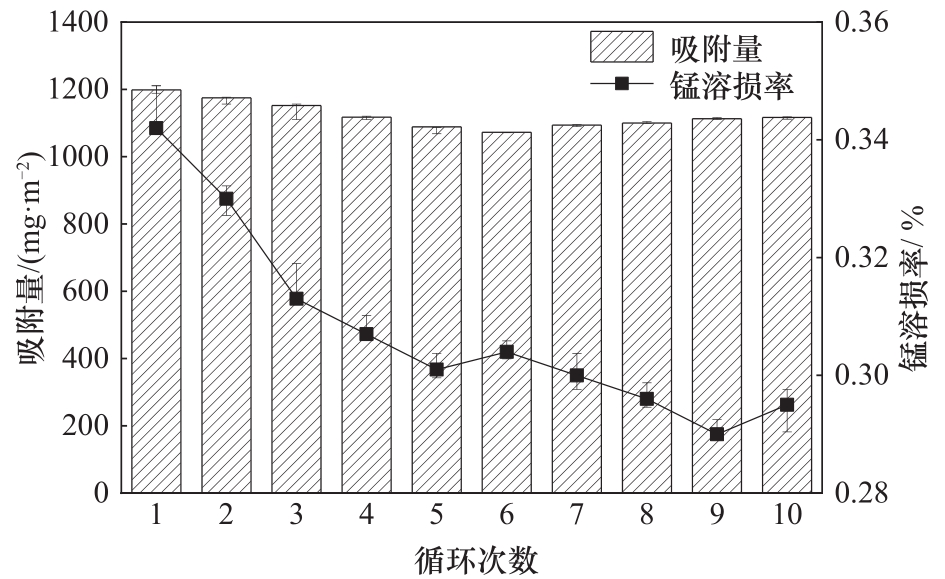
图10 SA膜状锂离子筛循环过程的锰溶损率及锂吸附量
Fig.10 Dissolution loss rate of Mn2+ and lithium adsorption capacity of SA lithium ion sieve membrane in the cycling process
| 阳离子 | C0/(mg·L-1) | Ce/(mg·L-1) | Q/(mg·m-2) | Q/(mmol·m-2) | Kd/(L·m-2) | CF/(L·m-2) | |
|---|---|---|---|---|---|---|---|
| Li+ | 112.701 | 76.922 | 1202.17 | 173.22 | 15.628 | 1.00 | 10.667 |
| Mg2+ | 10983.090 | 10867.487 | 3884.26 | 159.81 | 0.357 | 43.78 | 0.354 |
| K+ | 4043.177 | 4031.080 | 406.46 | 10.42 | 0.101 | 154.73 | 0.101 |
| Na+ | 4985.966 | 4979.104 | 902.56 | 39.24 | 0.182 | 85.87 | 0.181 |
表4 膜状锂离子筛从卤水中分离锂离子的性能
Table 4 Performance of SA lithium ion sieve membrane in the separation of Li+ from other cations in brine
| 阳离子 | C0/(mg·L-1) | Ce/(mg·L-1) | Q/(mg·m-2) | Q/(mmol·m-2) | Kd/(L·m-2) | CF/(L·m-2) | |
|---|---|---|---|---|---|---|---|
| Li+ | 112.701 | 76.922 | 1202.17 | 173.22 | 15.628 | 1.00 | 10.667 |
| Mg2+ | 10983.090 | 10867.487 | 3884.26 | 159.81 | 0.357 | 43.78 | 0.354 |
| K+ | 4043.177 | 4031.080 | 406.46 | 10.42 | 0.101 | 154.73 | 0.101 |
| Na+ | 4985.966 | 4979.104 | 902.56 | 39.24 | 0.182 | 85.87 | 0.181 |
| 1 | Rahman M A, Wang X J, Wen C E. A review of high energy density lithium-air battery technology[J]. Journal of Applied Electrochemistry, 2014, 44(1): 5-22. |
| 2 | Kesler S E, Gruber P W, Medina P A, et al. Global lithium resources: relative importance of pegmatite, brine and other deposits[J]. Ore Geology Reviews, 2012, 48: 55-69. |
| 3 | Speirs J, Contestabile M, Houari Y, et al. The future of lithium availability for electric vehicle batteries[J]. Renewable and Sustainable Energy Reviews, 2014, 35: 183-193. |
| 4 | Delgado M A, Valencia C, Sánchez M C, et al. Thermorheological behaviour of a lithium lubricating grease[J]. Tribology Letters, 2006, 23(1): 47-54. |
| 5 | Xu X, Chen Y M, Wan P Y, et al. Extraction of lithium with functionalized lithium ion-sieves[J]. Progress in Materials Science, 2016, 84: 276-313. |
| 6 | 赵旭, 张琦, 武海虹, 等. 盐湖卤水提锂[J]. 化学进展, 2017, 29(7): 796-808. |
| Zhao X, Zhang Q, Wu H H, et al. Extraction of lithium from salt lake brine[J]. Progress in Chemistry, 2017, 29(7): 796-808. | |
| 7 | Zhao B, Qian Z Q, Qiao Y J, et al. The Li(H2O) n dehydration behavior influences the Li+ ion adsorption on H4Ti5O12 with different facets exposed[J]. Chemical Engineering Journal, 2023, 451: 138870. |
| 8 | 卞维柏, 潘建明. 选择性吸附提锂材料的研究进展[J]. 化工进展, 2020, 39(6): 2206-2217. |
| Bian W B, Pan J M. Research progress in selective adsorption materialsfor lithium extraction[J]. Chemical Industry and Engineering Progress, 2020, 39(6): 2206-2217. | |
| 9 | Liu L F, Zhang H W, Zhang Y S, et al. Lithium extraction from seawater by manganese oxide ion sieve MnO2·0.5H2O[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2015, 468: 280-284. |
| 10 | Zandevakili S, Ranjbar M, Ehteshamzadeh M. Recovery of lithium from Urmia Lake by a nanostructure MnO2 ion sieve[J]. Hydrometallurgy, 2014, 149: 148-152. |
| 11 | Orooji Y, Nezafat Z, Nasrollahzadeh M, et al. Recent advances in nanomaterial development for lithium ion-sieving technologies[J]. Desalination, 2022, 529: 115624. |
| 12 | 杨曼丽, 石云龙, 于志浩, 等. 海藻酸钠/聚丙烯酸/二氧化硅纳米复合膜的制备及性能研究[J]. 食品科技, 2019, 44(4): 274-280. |
| Yang M L, Shi Y L, Yu Z H, et al. Preparation and properties investigation of sodium alginate/polyacrylic acid/SiO2 nanocomposite films[J]. Food Science and Technology, 2019, 44(4): 274-280. | |
| 13 | Tedeschi G, Benitez J J, Ceseracciu L, et al. Sustainable fabrication of plant cuticle-like packaging films from tomato pomace agro-waste, beeswax, and alginate[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 14955-14966. |
| 14 | Rye P D, Tøndervik A, Sletta H, et al. Alginate oligomers and their use as active pharmaceutical drugs[M]//Rehm B, Moradali M. Alginates and Their Biomedical Applications. Singapore: Springer, 2018: 237-256. |
| 15 | Tansaz S, Singh R, Cicha I, et al. Soy protein-based composite hydrogels: physico-chemical characterization and in vitro cytocompatibility[J]. Polymers, 2018, 10(10): 1159. |
| 16 | Vijayalakshmi K, Devi B M, Latha S, et al. Batch adsorption and desorption studies on the removal of lead (Ⅱ) from aqueous solution using nanochitosan/sodium alginate/microcrystalline cellulose beads[J]. International Journal of Biological Macromolecules, 2017, 104: 1483-1494. |
| 17 | 何荣军, 杨爽, 孙培龙, 等. 海藻酸钠/壳聚糖微胶囊的制备及其应用研究进展[J]. 食品与机械, 2010, 26(2): 166-169, 173. |
| He R J, Yang S, Sun P L, et al. Progress in preparation of sodium alginate & chitosan microcapsules and its applications[J]. Food & Machinery, 2010, 26(2): 166-169, 173. | |
| 18 | Dalheim M Ø, Ulset A S T, Jenssen I B, et al. Degradation kinetics of peptide-coupled alginates prepared via the periodate oxidation reductive amination route[J]. Carbohydrate Polymers, 2017, 157: 1844-1852. |
| 19 | Cerciello A, Del Gaudio P, Granata V, et al. Synergistic effect of divalent cations in improving technological properties of cross-linked alginate beads[J]. International Journal of Biological Macromolecules, 2017, 101: 100-106. |
| 20 | 于长江, 董心雨, 王苗, 等. 海藻酸钙/生物炭复合材料的制备及其对Pb(Ⅱ)的吸附性能和机制[J]. 环境科学, 2018, 39(8): 3719-3728. |
| Yu C J, Dong X Y, Wang M, et al. Preparation and characterization of a calcium alginate/biochar microsphere and its adsorption characteristics and mechanisms for Pb(Ⅱ)[J]. Environmental Science, 2018, 39(8): 3719-3728. | |
| 21 | 姚温浩, 于飞, 马杰. 海藻酸盐复合凝胶吸附材料的合成及其在水处理中的应用[J]. 化学进展, 2018, 30(11): 1722-1733. |
| Yao W H, Yu F, Ma J. Preparation of alginate composite gel and its application in water treatment[J]. Progress in Chemistry, 2018, 30(11): 1722-1733. | |
| 22 | Shen W, An Q D, Xiao Z Y, et al. Alginate modified graphitic carbon nitride composite hydrogels for efficient removal of Pb(Ⅱ), Ni(Ⅱ) and Cu(Ⅱ) from water[J]. International Journal of Biological Macromolecules, 2020, 148: 1298-1306. |
| 23 | 陈翀宇, 付广义, 赵媛媛, 等. 给水厂残泥-海藻酸钠胶珠对磷的吸附特性[J]. 环境化学, 2019, 38(3): 599-606. |
| Chen C Y, Fu G Y, Zhao Y Y, et al. Adsorption characteristics of phosphorus on drinking water treatment residuals-sodium alginate beads[J]. Environmental Chemistry, 2019, 38(3): 599-606. | |
| 24 | Bai C L, Wang L, Zhu Z Y. Adsorption of Cr(Ⅲ) and Pb(Ⅱ) by graphene oxide/alginate hydrogel membrane: characterization, adsorption kinetics, isotherm and thermodynamics studies[J]. International Journal of Biological Macromolecules, 2020, 147: 898-910. |
| 25 | Wang L, Wang L, Wang J, et al. Synthesis of zirconium-coated lithium ion sieve with enhanced cycle stability[J]. Separation and Purification Technology, 2022, 303: 121933. |
| 26 | Park M J, Nisola G M, Beltran A B, et al. Recyclable composite nanofiber adsorbent for Li+ recovery from seawater desalination retentate[J]. Chemical Engineering Journal, 2014, 254: 73-81. |
| 27 | Nisola G M, Limjuco L A, Vivas E L, et al. Macroporous flexible polyvinyl alcohol lithium adsorbent foam composite prepared via surfactant blending and cryo-desiccation[J]. Chemical Engineering Journal, 2015, 280: 536-548. |
| 28 | 屠洁, 刘冠卉, 李前龙, 等. 壳聚糖-甘油-丝素共混膜的制备及性能研究[J]. 食品与机械, 2010, 26(6): 34-36, 53. |
| Tu J, Liu G H, Li Q L, et al. Preparation of chitosan-glycerin-silk fibroin blend film and its property mensuration[J]. Food & Machinery, 2010, 26(6): 34-36, 53. | |
| 29 | 蒋世全, 邓靖. 肉桂精油-海藻酸钠可食性抗菌膜的研制[J]. 包装学报, 2010, 2(4): 75-78. |
| Jiang S Q, Deng J. Study on edible antimicrobial cinnamon oil-sodium alginate film[J]. Packaging Journal, 2010, 2(4): 75-78. | |
| 30 | Qin Y T, Zhu Z H, Kang G D, et al. Plasticizer-assisted interfacial polymerization for fabricating advanced reverse osmosis membranes[J]. Journal of Membrane Science, 2021, 619: 118788. |
| 31 | Sothornvit R, Krochta J M. Plasticizer effect on mechanical properties of β-lactoglobulin films[J]. Journal of Food Engineering, 2001, 50(3): 149-155. |
| 32 | Lim L T, Mine Y, Tung M A. Barrier and tensile properties of transglutaminase cross-linked gelatin films as affected by relative humidity, temperature, and glycerol content[J]. Journal of Food Science, 1999, 64(4): 616-622. |
| 33 | 徐玲, 秀芝, 王帆, 等. 以聚乙二醇为软模板制备微孔-介孔硅铝分子筛及其催化性能[J]. 吉林大学学报(理学版), 2022, 60(3): 743-747. |
| Xu L, Xiu Z, Wang F, et al. Preparation of micro-mesoporous aluminosilicate molecular sieves using polyethylene glycol as soft template and their catalytic performance[J]. Journal of Jilin University (Science Edition), 2022, 60(3): 743-747. | |
| 34 | Zhou J, Hua Z L, Liu Z C, et al. Direct synthetic strategy of mesoporous ZSM-5 zeolites by using conventional block copolymer templates and the improved catalytic properties[J]. ACS Catalysis, 2011, 1(4): 287-291. |
| 35 | Seo S J, Kim I Y, Choi Y J, et al. Enhanced liver functions of hepatocytes cocultured with NIH 3T3 in the alginate/galactosylated chitosan scaffold[J]. Biomaterials, 2006, 27(8): 1487-1495. |
| 36 | 李沁华, 韦丽萍, 莫小慧. 聚乙二醇相对分子质量及用量对海藻酸钙支架多孔结构及性能的调控[J]. 中国组织工程研究与临床康复, 2008, 12(1): 65-68. |
| Li Q H, Wei L P, Mo X H. Regulation effect of polyethylene glycol relative molecular weight and dosage on porous structure and property of calcium alginate scaffold[J]. Journal of Clinical Rehabilitative Tissue Engineering Research, 2008, 12(1): 65-68. | |
| 37 | 向川, 卫小春, 杜靖远. 聚乳酸和海藻酸钙体外复合培养人成骨细胞的对比研究[J]. 华中科技大学学报(医学版), 2005, 34(3): 334-338. |
| Xiang C, Wei X C, Du J Y. Comparative study of polylactic acid vs calcium alginate cultured with osteoblasts from human periosteum in vitro [J]. Acta Medicinae Universitatis Scientiae et Technolgiae Huazhong, 2005, 34(3): 334-338. | |
| 38 | Zhu G R, Wang P, Qi P F, et al. Adsorption and desorption properties of Li+ on PVC-H1.6Mn1.6O4 lithium ion-sieve membrane[J]. Chemical Engineering Journal, 2014, 235: 340-348. |
| 39 | Qi G C, Hai C X, Zhou Y. Synthesis of PVDF-H1.6Mn1.6O4 lithium ion-sieve membrane for lithium extraction[J]. Journal of Synthetic Crystals, 2019, 48(3): 418-427, 442. |
| 40 | 石西昌, 余亮良, 陈白珍, 等. 锂锰氧化物离子筛结构和掺杂研究进展[J]. 中国锰业, 2009, 27(3): 17-20. |
| Shi X C, Yu L L, Chen B Z, et al. Research progress on structure and doping of lithium manganese oxide ion-sieve[J]. China’s Manganese Industry, 2009, 27(3): 17-20. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 邵苛苛, 宋孟杰, 江正勇, 张旋, 张龙, 高润淼, 甄泽康. 水平方向上冰中受陷气泡形成和分布实验研究[J]. 化工学报, 2023, 74(S1): 161-164. |
| [3] | 吴延鹏, 李晓宇, 钟乔洋. 静电纺丝纳米纤维双疏膜油性细颗粒物过滤性能实验分析[J]. 化工学报, 2023, 74(S1): 259-264. |
| [4] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [5] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [6] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [7] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [8] | 齐聪, 丁子, 余杰, 汤茂清, 梁林. 基于选择吸收纳米薄膜的太阳能温差发电特性研究[J]. 化工学报, 2023, 74(9): 3921-3930. |
| [9] | 何松, 刘乔迈, 谢广烁, 王斯民, 肖娟. 高浓度水煤浆管道气膜减阻两相流模拟及代理辅助优化[J]. 化工学报, 2023, 74(9): 3766-3774. |
| [10] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [11] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [12] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [13] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [14] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [15] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号