CIESC Journal ›› 2021, Vol. 72 ›› Issue (11): 5779-5789.DOI: 10.11949/0438-1157.20210835
• Energy and environmental engineering • Previous Articles Next Articles
Jin XU1,2( ),Jiedong ZHU1,2,Juanli LI1,2,Mengqiu LIU1,2,Heluo GONG1,2
),Jiedong ZHU1,2,Juanli LI1,2,Mengqiu LIU1,2,Heluo GONG1,2
Received:2021-06-21
Revised:2021-08-20
Online:2021-11-12
Published:2021-11-05
Contact:
Jin XU
许劲1,2( ),朱杰东1,2,李卷利1,2,刘孟秋1,2,龚河洛1,2
),朱杰东1,2,李卷利1,2,刘孟秋1,2,龚河洛1,2
通讯作者:
许劲
作者简介:许劲(1968—),女,博士,教授,基金资助:CLC Number:
Jin XU, Jiedong ZHU, Juanli LI, Mengqiu LIU, Heluo GONG. Potential of phosphorus recovery from sludge-based hydrochar by wet chemical method[J]. CIESC Journal, 2021, 72(11): 5779-5789.
许劲, 朱杰东, 李卷利, 刘孟秋, 龚河洛. 湿化学法回收污泥水热炭中磷的潜能研究[J]. 化工学报, 2021, 72(11): 5779-5789.
Add to citation manager EndNote|Ris|BibTeX
| 样品 | 工业分析①/%(质量) | 元素分析①/%(质量) | H/C | O/C | 炭产率/% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ash | VM | FC | C | H | N | S | O | ||||
| 污泥 | 56.48 | 40.57 | 2.95 | 20.16 | 3.5 | 2.9 | 0.9 | 16.07 | 2.08 | 0.6 | — |
| 水热炭 | 72.86 | 24.32 | 2.82 | 14.59 | 2.31 | 1.47 | 0.53 | 8.24 | 1.9 | 0.42 | 74.67 |
Table 1 Basic physicochemical properties of sludge and hydrochar
| 样品 | 工业分析①/%(质量) | 元素分析①/%(质量) | H/C | O/C | 炭产率/% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ash | VM | FC | C | H | N | S | O | ||||
| 污泥 | 56.48 | 40.57 | 2.95 | 20.16 | 3.5 | 2.9 | 0.9 | 16.07 | 2.08 | 0.6 | — |
| 水热炭 | 72.86 | 24.32 | 2.82 | 14.59 | 2.31 | 1.47 | 0.53 | 8.24 | 1.9 | 0.42 | 74.67 |
| 样品 | 金属含量/(mg/g)① | 重金属含量/(mg/kg)① | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | Al | Ca | Mg | Pb | Cu | Cr | Mn | Zn | |
| 污泥 | 92.15 | 41.84 | 18.99 | 10.58 | 29.04 | 73.12 | 50.11 | 552.53 | 474.62 |
| 水热炭 | 98.97 | 47.53 | 20.35 | 11.23 | 37.66 | 94.54 | 61.54 | 669.73 | 589.24 |
Table 2 Contents of metals and heavy metals in sludge and hydrochar
| 样品 | 金属含量/(mg/g)① | 重金属含量/(mg/kg)① | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | Al | Ca | Mg | Pb | Cu | Cr | Mn | Zn | |
| 污泥 | 92.15 | 41.84 | 18.99 | 10.58 | 29.04 | 73.12 | 50.11 | 552.53 | 474.62 |
| 水热炭 | 98.97 | 47.53 | 20.35 | 11.23 | 37.66 | 94.54 | 61.54 | 669.73 | 589.24 |
| 酸类型 | 相关参数 | 单因素 | 变量 |
|---|---|---|---|
盐酸 柠檬酸 | 水热炭质量:0.4 g 液固比:50 ml/g 酸浸时间:720 min | 酸浓度 | 0.01、0.05、0.1、0.15、0.3、0.5、0.8、1 mol/L |
酸体积:20 ml 盐酸浓度:0.3 mol/L 柠檬酸浓度:0.1 mol/L 酸浸时间:720 min | 液固比 | 10、15、25、50、100 ml/g | |
液固比:50 ml/g 盐酸浓度:0.3 mol/L 柠檬酸浓度:0.1 mol/L | 酸浸时间 | 15、30、45、60、90、120、180、240、360、480、600、720 min |
Table 3 Single factor experimental design
| 酸类型 | 相关参数 | 单因素 | 变量 |
|---|---|---|---|
盐酸 柠檬酸 | 水热炭质量:0.4 g 液固比:50 ml/g 酸浸时间:720 min | 酸浓度 | 0.01、0.05、0.1、0.15、0.3、0.5、0.8、1 mol/L |
酸体积:20 ml 盐酸浓度:0.3 mol/L 柠檬酸浓度:0.1 mol/L 酸浸时间:720 min | 液固比 | 10、15、25、50、100 ml/g | |
液固比:50 ml/g 盐酸浓度:0.3 mol/L 柠檬酸浓度:0.1 mol/L | 酸浸时间 | 15、30、45、60、90、120、180、240、360、480、600、720 min |
| 样品 | 含量/(mg/g) | ||||
|---|---|---|---|---|---|
| TP | IP | NAIP | AP | 总磷回收率R/% | |
| 污泥 | 22.89±0.18 | 20.70±0.38 | 15.77±0.27 | 5.59±0.21 | — |
| 水热炭 | 30.31±0.28 | 28.29±0.17 | 20.39±0.10 | 7.85±0.01 | 98.81 |
Table 4 Contents of different forms of phosphorus in sludge and hydrochar
| 样品 | 含量/(mg/g) | ||||
|---|---|---|---|---|---|
| TP | IP | NAIP | AP | 总磷回收率R/% | |
| 污泥 | 22.89±0.18 | 20.70±0.38 | 15.77±0.27 | 5.59±0.21 | — |
| 水热炭 | 30.31±0.28 | 28.29±0.17 | 20.39±0.10 | 7.85±0.01 | 98.81 |
| 数学模型 | 酸浸体系 | 表达式 | 相关参数值 | R2 |
|---|---|---|---|---|
| Elovich模型 | 柠檬酸 | α=0.3264; β=4.2571 | 0.9856 | |
| 盐酸 | α=401.71; β=10.06 | 0.7775 | ||
| 准二级动力学模型 | 柠檬酸 | qe=32.89; k2=1.9839×10-4 | 0.9861 | |
| 盐酸 | qe=28.49; k2=2.6448×10-4 | 0.9999 |
Table 5 Fit parameters of phosphorus release kinetics model in hydrochar
| 数学模型 | 酸浸体系 | 表达式 | 相关参数值 | R2 |
|---|---|---|---|---|
| Elovich模型 | 柠檬酸 | α=0.3264; β=4.2571 | 0.9856 | |
| 盐酸 | α=401.71; β=10.06 | 0.7775 | ||
| 准二级动力学模型 | 柠檬酸 | qe=32.89; k2=1.9839×10-4 | 0.9861 | |
| 盐酸 | qe=28.49; k2=2.6448×10-4 | 0.9999 |

Fig.8 Influence of acid leaching time on the leaching capacity of phosphorus and metal elements(a) lemon acid leaching system; (b) hydrochloric acid leaching system
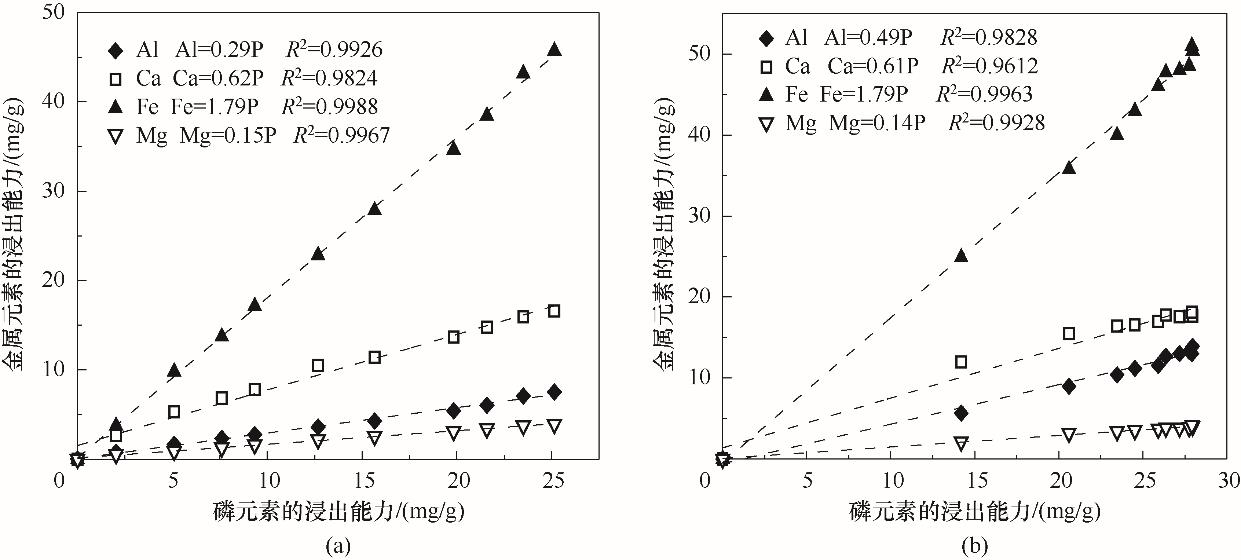
Fig.9 Relationship between leaching capacity of metal elements and phosphorus elements(a) lemon acid leaching system; (b) hydrochloric acid leaching system
| 酸浸体系 | 浸出浓度/(mg/L) | ||||
|---|---|---|---|---|---|
| Pb | Cu | Cr | Mn | Zn | |
| 柠檬酸 | 0.04 | 0.05 | 3.36 | 6.36 | 8.66 |
| 盐酸 | 0.41 | 1.46 | 3.60 | 6.43 | 12.02 |
Table 6 Leaching concentration of heavy metals under optimum acid leaching conditions
| 酸浸体系 | 浸出浓度/(mg/L) | ||||
|---|---|---|---|---|---|
| Pb | Cu | Cr | Mn | Zn | |
| 柠檬酸 | 0.04 | 0.05 | 3.36 | 6.36 | 8.66 |
| 盐酸 | 0.41 | 1.46 | 3.60 | 6.43 | 12.02 |

Fig.11 SEM-EDS analysis results of hydrothermal carbon (a), acid leaching residue of hydrochloric acid (b) and acid leaching residue of citric acid (c) respectively
| 1 | Cordell D, Drangert J O, White S. The story of phosphorus: global food security and food for thought[J]. Global Environmental Change, 2009, 19(2): 292-305. |
| 2 | Sørensen B L, Dall O L, Habib K. Environmental and resource implications of phosphorus recovery from waste activated sludge[J]. Waste Management, 2015, 45: 391-399. |
| 3 | Cieślik B, Konieczka P. A review of phosphorus recovery methods at various steps of wastewater treatment and sewage sludge management. The concept of “no solid waste generation” and analytical methods[J]. Journal of Cleaner Production, 2017, 142: 1728-1740. |
| 4 | Law K P, Pagilla K R. Reclaimed phosphorus commodity reserve from water resource recovery facilities—a strategic regional concept towards phosphorus recovery[J]. Resources, Conservation and Recycling, 2019, 150: 104429. |
| 5 | 崔荣国, 张艳飞, 郭娟, 等. 资源全球配置下的中国磷矿发展策略[J]. 中国工程科学, 2019, 21(1): 128-132. |
| Cui R G, Zhang Y F, Guo J, et al. Development strategy of phosphate rock in China under global allocation of resources[J]. Engineering Science, 2019, 21(1): 128-132. | |
| 6 | Tarayre C, De Clercq L, Charlier R, et al. New perspectives for the design of sustainable bioprocesses for phosphorus recovery from waste[J]. Bioresource Technology, 2016, 206: 264-274. |
| 7 | Cornel P, Schaum C. Phosphorus recovery from wastewater: needs, technologies and costs[J]. Water Science and Technology, 2009, 59(6): 1069-1076. |
| 8 | Havukainen J, Nguyen M T, Hermann L, et al. Potential of phosphorus recovery from sewage sludge and manure ash by thermochemical treatment[J]. Waste Management, 2016, 49: 221-229. |
| 9 | Egle L, Rechberger H, Zessner M. Overview and description of technologies for recovering phosphorus from municipal wastewater[J]. Resources, Conservation and Recycling, 2015, 105: 325-346. |
| 10 | Wang L P, Li A M, Chang Y Z. Relationship between enhanced dewaterability and structural properties of hydrothermal sludge after hydrothermal treatment of excess sludge[J]. Water Research, 2017, 112: 72-82. |
| 11 | Wang L P, Li A M. Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: the dewatering performance and the characteristics of products[J]. Water Research, 2015, 68: 291-303. |
| 12 | Wang L P, Zhang L, Li A M. Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: influence of operating conditions and the process energetics[J]. Water Research, 2014, 65: 85-97. |
| 26 | Liu H K, Xu F, Xie Y L, et al. Effect of modified coconut shell biochar on availability of heavy metals and biochemical characteristics of soil in multiple heavy metals contaminated soil[J]. Science of the Total Environment, 2018, 645: 702-709. |
| 27 | Peiris C, Nayanathara O, Navarathna C M, et al. The influence of three acid modifications on the physicochemical characteristics of tea-waste biochar pyrolyzed at different temperatures: a comparative study[J]. RSC Advances, 2019, 9(31): 17612-17622. |
| 28 | Rashid U S, Bezbaruah A N. Citric acid modified granular activated carbon for enhanced defluoridation[J]. Chemosphere, 2020, 252: 126639. |
| 13 | 郑晓园, 蒋正伟, 陈伟, 等. 污水污泥水热炭化过程中磷的迁移转化特性[J]. 化工进展, 2020, 39(5): 2017-2025. |
| Zheng X Y, Jiang Z W, Chen W, et al. Migration and transformation of phosphorus in sewage sludge during hydrothermal carbonization process[J]. Chemical Industry and Engineering Progress, 2020, 39(5): 2017-2025. | |
| 14 | 郝晓地, 于晶伦, 刘然彬, 等. 剩余污泥焚烧灰分磷回收及其技术进展[J]. 环境科学学报, 2020, 40(4): 1149-1159. |
| Hao X D, Yu J L, Liu R B, et al. Advances of phosphorus recovery from the incineration ashes of excess sludge and its associated technologies[J]. Acta Scientiae Circumstantiae, 2020, 40(4): 1149-1159. | |
| 15 | Yan W, Wu J Y, Chen Y, et al. Short reaction times coupled with alkalization improves the release of phosphorus from Al-waste activated sludge[J]. Bioresource Technology, 2021, 333: 125168. |
| 16 | Ruban V, López-Sánchez J F, Pardo P, et al. Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments — a synthesis of recent works[J]. Fresenius' Journal of Analytical Chemistry, 2001, 370(2/3): 224-228. |
| 17 | Pardo P, López-Sánchez J F, Rauret G. Relationships between phosphorus fractionation and major components in sediments using the SMT harmonised extraction procedure[J]. Analytical and Bioanalytical Chemistry, 2003, 376(2): 248-254. |
| 18 | García-Albacete M, Martín A, Cartagena M C. Fractionation of phosphorus biowastes: characterisation and environmental risk[J]. Waste Management, 2012, 32(6): 1061-1068. |
| 19 | 龙加洪, 谭菊, 吴银菊, 等. 土壤重金属含量测定不同消解方法比较研究[J]. 中国环境监测, 2013, 29(1): 123-126. |
| Long J H, Tan J, Wu Y J, et al. A comparative study on the detection of heavy metal in soil with different digestion methods[J]. Environmental Monitoring in China, 2013, 29(1): 123-126. | |
| 20 | 徐杰,黄群星,孟详东, 等. 钙基添加剂对污水污泥在水热炭化过程中磷形态及生物有效性的影响[J]. 化工进展, 2021, 40(6):3507-3514. |
| Xu J, Huang Q X, Meng X D, et al. Effect of calcium based additives on phosphorus form and bioavailability of sewage sludge during hydrothermal carbonization[J]. Chemical Industry and Engineering Progress, 2021, 40(6):3507-3514. | |
| 21 | Shi Y, Luo G, Rao Y, et al. Hydrothermal conversion of dewatered sewage sludge: focusing on the transformation mechanism and recovery of phosphorus[J]. Chemosphere, 2019, 228: 619-628. |
| 22 | 方俊华, 唐琦, 李杨, 等. 污泥水热碳化中磷的形态变化及金属浸出行为[J]. 化工学报, 2020, 71(7): 3288-3295. |
| Fang J H, Tang Q, Li Y, et al. Morphology of phosphorus and metal extraction behavior in sewage sludge during hydrothermal carbonization treatment[J]. CIESC Journal, 2020, 71(7): 3288-3295. | |
| 23 | 王涛. 城市污泥(水)热处理固体产物中磷的迁移转化及释放回收研究[D]. 长沙: 湖南大学, 2018. |
| Wang T. Hydrothermal carbonization of municipal sewage sludge: invesgation on the transformation release and recovery of phosphorus in hydrochar[D]. Changsha: Hunan University, 2018. | |
| 24 | Barca C, Martino M, Hennebert P, et al. Kinetics and capacity of phosphorus extraction from solid residues obtained from wet air oxidation of sewage sludge[J]. Waste Management, 2019, 89: 275-283. |
| 25 | Wang S Y, Ai S Y, Nzediegwu C, et al. Carboxyl and hydroxyl groups enhance ammonium adsorption capacity of iron (Ⅲ) chloride and hydrochloric acid modified biochars[J]. Bioresource Technology, 2020, 309: 123390. |
| 29 | Liu M M, Zhao Z Y, Yu W Z. Citric acid modified wood membranes for efficient adsorption of tetracycline: effect of alkali pretreatment concentration and adsorption mechanism[J]. Chemical Engineering Journal, 2020, 393: 124748. |
| 30 | Liu G F, Liao L, Dai Z M, et al. Organic adsorbents modified with citric acid and Fe3O4 enhance the removal of Cd and Pb in contaminated solutions[J]. Chemical Engineering Journal, 2020, 395: 125108. |
| [1] | Chunyu LIU, Huanyu ZHOU, Yue MA, Changtao YUE. Drying characteristics and mathematical model of CaO-conditioned oil sludge [J]. CIESC Journal, 2023, 74(7): 3018-3027. |
| [2] | Yuanhao QU, Wenyi DENG, Xiaodan XIE, Yaxin SU. Study on electro-osmotic dewatering of sludge assisted by activated carbon/graphite [J]. CIESC Journal, 2023, 74(7): 3038-3050. |
| [3] | Yuhao CHEN, Xiaoping CHEN, Jiliang MA, Cai LIANG. Gaseous pollutants emissions from rotary kiln combustion of municipal sewage sludge [J]. CIESC Journal, 2023, 74(5): 2170-2178. |
| [4] | Lanhe ZHANG, Qingyi LAI, Tiezheng WANG, Xiaozhuo GUAN, Mingshuang ZHANG, Xin CHENG, Xiaohui XU, Yanping JIA. Effect of H2O2 on nitrogen removal and sludge properties in SBR [J]. CIESC Journal, 2023, 74(5): 2186-2196. |
| [5] | Hao CHEN, Yijuan TIAN, Xuejun QUAN, Ziwen JIANG, Gang LI. Decomposition behaviour of chromite in the HCl-HF system [J]. CIESC Journal, 2023, 74(3): 1161-1174. |
| [6] | Jiahui SHEN, Kanhong WANG, Dawei YU, Dazhou HU, Yuansong WEI. Free ammonia conditioning promoted micro-molecule organics release and methanogenesis of thickened sludge [J]. CIESC Journal, 2022, 73(9): 4147-4155. |
| [7] | Xinyi LUO, Chao FENG, Jing LIU, Yu QIAO. Phosphorus recovery from products of sewage sludge via different thermal treatment processes [J]. CIESC Journal, 2022, 73(9): 4034-4044. |
| [8] | Jun ZHANG, Sheng HU, Jing GU, Haoran YUAN, Yong CHEN. Catalytic hydrogenation of furfural over magnetic polymetallic materials derived from electroplating sludge in methanol [J]. CIESC Journal, 2022, 73(7): 2996-3006. |
| [9] | Chaoyu SONG, Yaxuan XIONG, Jinhua ZHANG, Yuhe JIN, Chenhua YAO, Huixiang WANG, Yulong DING. Preparation and performance study of incinerated slag based shape-stable phase change composites [J]. CIESC Journal, 2022, 73(5): 2279-2287. |
| [10] | Guanyi CHEN, Tujun TONG, Rui LI, Yanshan WANG, Beibei YAN, Ning LI, Li'an HOU. Influence of pyrolysis time on sludge-derived biochar performance for peroxymonosulfate activation [J]. CIESC Journal, 2022, 73(5): 2111-2119. |
| [11] | Xiaoyang YANG, Baofeng WANG, Xutao SONG, Fengling YANG, Fangqin CHENG. Migration of sulfur and nitrogen during co-hydrothermal carbonization process of sewage sludge and high-sulfur coal [J]. CIESC Journal, 2022, 73(11): 5211-5219. |
| [12] | Li ZHANG, Jianhua WU, Shuhui CUI, Feng YAN, Hao SUN, Feiyue QIAN. Analysis of bacterial function in combined PN/A granular sludge and solid phase denitrification processes [J]. CIESC Journal, 2022, 73(11): 5128-5137. |
| [13] | Shan CHENG, Rui LUO, Hong TIAN, Zhenqi WANG, Jingchun HUANG, Yu QIAO. Effect of hydrothermal carbonization temperature on transformation path of organic nitrogen in sludge [J]. CIESC Journal, 2022, 73(11): 5220-5229. |
| [14] | Lanhe ZHANG, Lu WANG, Zimeng LI, Hong TANG, Jingbo GUO, Yanping JIA, Mingshuang ZHANG. The treatment of anionic surfactant wastewater using electrode ultrafiltration membrane bioreactor [J]. CIESC Journal, 2022, 73(10): 4679-4691. |
| [15] | Li YANG, Yundong SUN, Yong JIAO, Ye YANG, Jianbiao CHEN, Chuanhua LIAO. Synergistic catalytic mechanism of ash in pyrolysis and gasification of textile dyeing sludge [J]. CIESC Journal, 2021, 72(9): 4718-4729. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||