CIESC Journal ›› 2023, Vol. 74 ›› Issue (2): 843-860.DOI: 10.11949/0438-1157.20221063
• Energy and environmental engineering • Previous Articles Next Articles
Na ZHANG1( ), Helin PAN1, Bo NIU1, Yayun ZHANG1(
), Helin PAN1, Bo NIU1, Yayun ZHANG1( ), Donghui LONG1,2
), Donghui LONG1,2
Received:2022-07-29
Revised:2022-11-29
Online:2023-03-21
Published:2023-02-05
Contact:
Yayun ZHANG
张娜1( ), 潘鹤林1, 牛波1, 张亚运1(
), 潘鹤林1, 牛波1, 张亚运1( ), 龙东辉1,2
), 龙东辉1,2
通讯作者:
张亚运
作者简介:张娜(1997—),女,硕士研究生,zhna135472@163.com
基金资助:CLC Number:
Na ZHANG, Helin PAN, Bo NIU, Yayun ZHANG, Donghui LONG. Density functional theory study on thermal cracking reaction mechanism of phenolic resin[J]. CIESC Journal, 2023, 74(2): 843-860.
张娜, 潘鹤林, 牛波, 张亚运, 龙东辉. 酚醛树脂热裂解反应机理的密度泛函理论研究[J]. 化工学报, 2023, 74(2): 843-860.
Add to citation manager EndNote|Ris|BibTeX
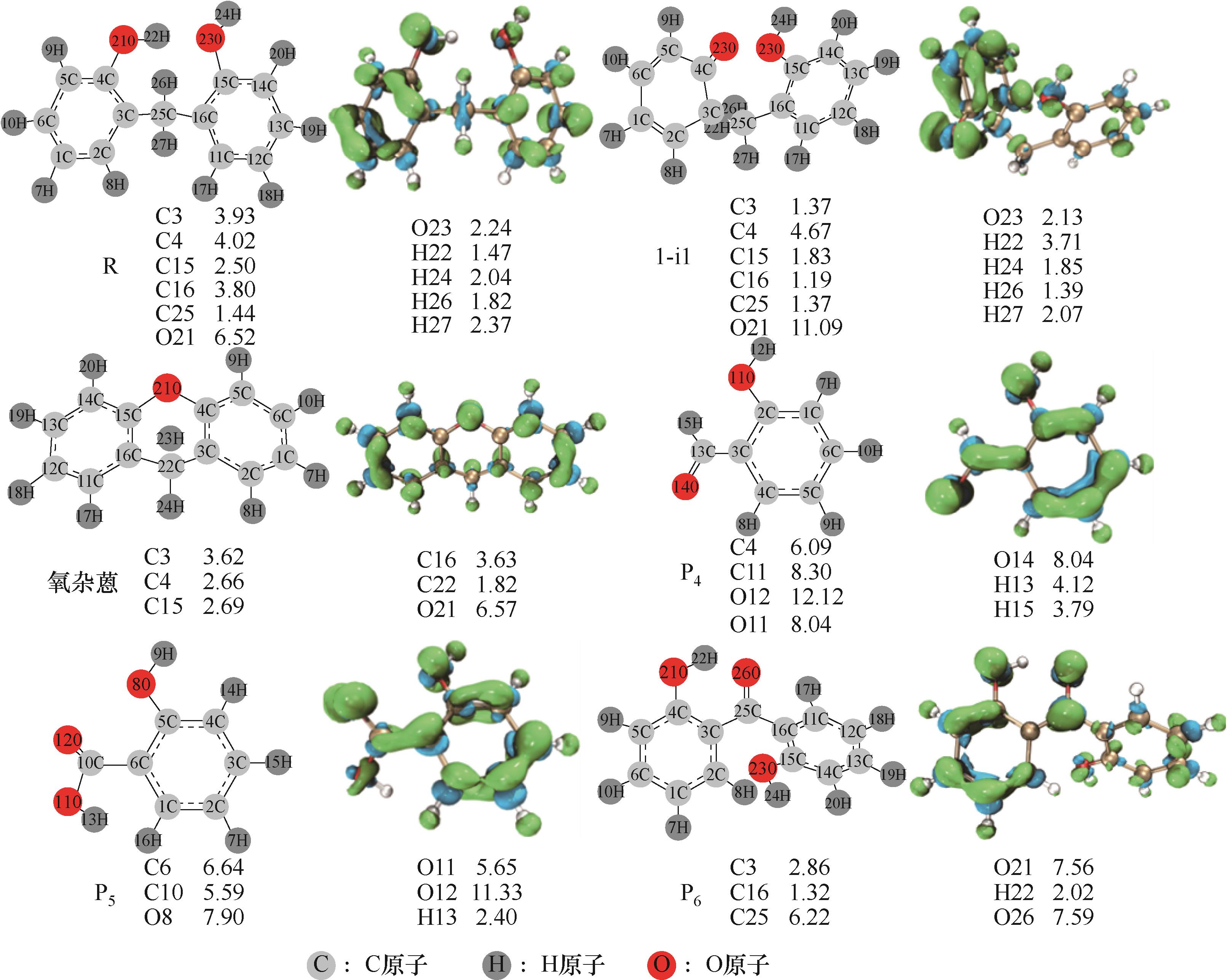
Fig.1 Optimized molecular structures and projection of Fukui function to electron density isosurfaces (PR) (on the left is the optimized molecular structures,the green areas in the corresponding isosurfaces on the right represents the electron-poor areas, and the blue areas represents the electron-rich areas, the values marked at the bottom of the figure are the Fukui function values of each reaction site, and the unit is e×100)

Fig.3 The Mayer bond order and the Laplacian bond order of the chemical bonds involved in the broken bonds in the reaction pathways of PR (the numbers “1, 2, 3, 4, 5, 6” in the abscissa represent the chemical bonds“C3—C25, C16—C25, C4—O21, C15—O23, O21—H22, O23—H24”,“7,8”represent the chemical bonds“C3—C13, C6—C10” in salicylaldehyde P4 and salicylic acid P5 respectively,“-1” represents the corresponding chemical bonds of isomerization product 1-i1,“-2” represents the corresponding chemical bonds of benzophenone)
| Species | Structure parameter | |||
|---|---|---|---|---|
| Bond length/Å | Bond angle/(°) | Dihedral angle/(°) | ||
| R |  | R(4,21) 1.3837 R(3,25) 1.5265 R(15,23) 1.4096 R(11,17) 1.0859 | A(3,4,21) 123.5698 A(16,15,23) 116.6298 A(3,25,16) 115.1569 A(26,25,27) 106.4195 | D(3,4,21,22) 17.5317 D(16,15,23,24) 177.6034 D(8,2,3,25) -0.5943 D(17,11,16,15) 179.8965 |
| TS1a |  | R(4,21) 1.3313 R(3,25) 1.5717 R(16,25) 1.5149 R(3,22) 1.4548 | A(3,4,21) 107.6645 A(3,25,26) 108.2772 A(15,16,25) 120.5249 A(26,25,27) 107.7724 | D(2,3,4,21) 146.3908 D(2,3,25,16) 57.0082 D(23,15,16,25) 0.7638 D(22,3,25,26) -31.9241 |
| 1-i1 |  | R(4,21) 1.2509 R(3,25) 1.5724 R(15,23) 1.3925 R(11,17) 1.0866 | A(3,4,21) 120.1579 A(16,15,23) 116.1152 A(3,25,16) 116.3922 A(26,25,27) 107.2023 | D(21,4,3,22) 69.4683 D(16,15,23,24) 178.8148 D(8,2,3,25) 42.8377 D(17,11,16,15) 179.6106 |
| 1-i2 |  | R(5,6) 1.433 R(6,13) 1.3991 R(13,14) 1.0799 R(13,15) 1.0825 | A(5,11,12) 109.1272 A(1,6,13) 122.1677 A(5,6,13) 121.3617 A(6,13,14) 121.2142 | D(6,5,11,12) -180.0119 D(7,1,6,13) -0.0014 D(1,6,13,14) -179.9998 D(1,6,13,15) -0.0012 |
| 1-i3 |  | R(3,4) 1.4523 R(4,5) 1.4523 R(5,6) 1.3748 R(4,11) 1.2514 | A(4,5,6) 120.9385 A(3,4,5) 116.9288 A(6,5,9) 122.1827 A(5,4,11) 121.5356 | D(9,5,6,10) -0.0001 D(9,5,4,11) 0.0001 D(2,3,4,11) 0.0001 D(6,5,4,11) -179.9999 |
| P1 |  | R(5,6) 1.4045 R(6,13) 1.5095 R(13,14) 1.0973 R(13,15) 1.0973 | A(5,11,12) 109.606 A(1,6,13) 121.6233 A(5,6,13) 120.4383 A(6,13,14) 112.0314 | D(6,5,11,12) -0.0037 D(7,1,6,13) 0.0006 D(1,6,13,14) 119.5144 D(1,6,13,15) -119.5242 |
| P3 |  | R(3,4) 1.3967 R(4,5) 1.3963 R(5,6) 1.3934 R(4,12) 1.3671 | A(4,5,6) 119.8367 A(3,4,5) 119.9397 A(6,5,10) 120.2222 A(4,12,13) 109.1344 | D(3,4,12,13) -179.9897 D(10,5,4,12) -0.001 D(3,4,12,13) 179.9582 D(5,4,12,13) -0.0447 |
| TS1c |  | R(3,4) 1.4079 R(2,8) 1.0837 R(13,15) 1.3486 R(17,18) 0.9812 | A(4,11,12) 111.8752 A(6,5,9) 120.3442 A(3,13,14) 115.9687 A(16,17,18) 122.7612 | D(8,2,3,4) -179.5535 D(5,4,11,12) 1.5985 D(2,3,13,14) -158.9475 D(13,16,17,18) 110.7506 |
Table 1 Optimized geometries of reactants, important transition states, intermediates and products in the pyrolysis reaction pathways of PR
| Species | Structure parameter | |||
|---|---|---|---|---|
| Bond length/Å | Bond angle/(°) | Dihedral angle/(°) | ||
| R |  | R(4,21) 1.3837 R(3,25) 1.5265 R(15,23) 1.4096 R(11,17) 1.0859 | A(3,4,21) 123.5698 A(16,15,23) 116.6298 A(3,25,16) 115.1569 A(26,25,27) 106.4195 | D(3,4,21,22) 17.5317 D(16,15,23,24) 177.6034 D(8,2,3,25) -0.5943 D(17,11,16,15) 179.8965 |
| TS1a |  | R(4,21) 1.3313 R(3,25) 1.5717 R(16,25) 1.5149 R(3,22) 1.4548 | A(3,4,21) 107.6645 A(3,25,26) 108.2772 A(15,16,25) 120.5249 A(26,25,27) 107.7724 | D(2,3,4,21) 146.3908 D(2,3,25,16) 57.0082 D(23,15,16,25) 0.7638 D(22,3,25,26) -31.9241 |
| 1-i1 |  | R(4,21) 1.2509 R(3,25) 1.5724 R(15,23) 1.3925 R(11,17) 1.0866 | A(3,4,21) 120.1579 A(16,15,23) 116.1152 A(3,25,16) 116.3922 A(26,25,27) 107.2023 | D(21,4,3,22) 69.4683 D(16,15,23,24) 178.8148 D(8,2,3,25) 42.8377 D(17,11,16,15) 179.6106 |
| 1-i2 |  | R(5,6) 1.433 R(6,13) 1.3991 R(13,14) 1.0799 R(13,15) 1.0825 | A(5,11,12) 109.1272 A(1,6,13) 122.1677 A(5,6,13) 121.3617 A(6,13,14) 121.2142 | D(6,5,11,12) -180.0119 D(7,1,6,13) -0.0014 D(1,6,13,14) -179.9998 D(1,6,13,15) -0.0012 |
| 1-i3 |  | R(3,4) 1.4523 R(4,5) 1.4523 R(5,6) 1.3748 R(4,11) 1.2514 | A(4,5,6) 120.9385 A(3,4,5) 116.9288 A(6,5,9) 122.1827 A(5,4,11) 121.5356 | D(9,5,6,10) -0.0001 D(9,5,4,11) 0.0001 D(2,3,4,11) 0.0001 D(6,5,4,11) -179.9999 |
| P1 |  | R(5,6) 1.4045 R(6,13) 1.5095 R(13,14) 1.0973 R(13,15) 1.0973 | A(5,11,12) 109.606 A(1,6,13) 121.6233 A(5,6,13) 120.4383 A(6,13,14) 112.0314 | D(6,5,11,12) -0.0037 D(7,1,6,13) 0.0006 D(1,6,13,14) 119.5144 D(1,6,13,15) -119.5242 |
| P3 |  | R(3,4) 1.3967 R(4,5) 1.3963 R(5,6) 1.3934 R(4,12) 1.3671 | A(4,5,6) 119.8367 A(3,4,5) 119.9397 A(6,5,10) 120.2222 A(4,12,13) 109.1344 | D(3,4,12,13) -179.9897 D(10,5,4,12) -0.001 D(3,4,12,13) 179.9582 D(5,4,12,13) -0.0447 |
| TS1c |  | R(3,4) 1.4079 R(2,8) 1.0837 R(13,15) 1.3486 R(17,18) 0.9812 | A(4,11,12) 111.8752 A(6,5,9) 120.3442 A(3,13,14) 115.9687 A(16,17,18) 122.7612 | D(8,2,3,4) -179.5535 D(5,4,11,12) 1.5985 D(2,3,13,14) -158.9475 D(13,16,17,18) 110.7506 |

Fig.7 Optimized molecular structures and projection of Fukui function to electron density isosurfaces (BPR) (on the left is the optimized molecular structures,the green areas in the corresponding isosurfaces on the right represents the electron-poor areas, and the blue areas represents the electron-rich areas, the values marked in the middle of the figure are the Fukui function values of each reaction site, and the unit is e×100)
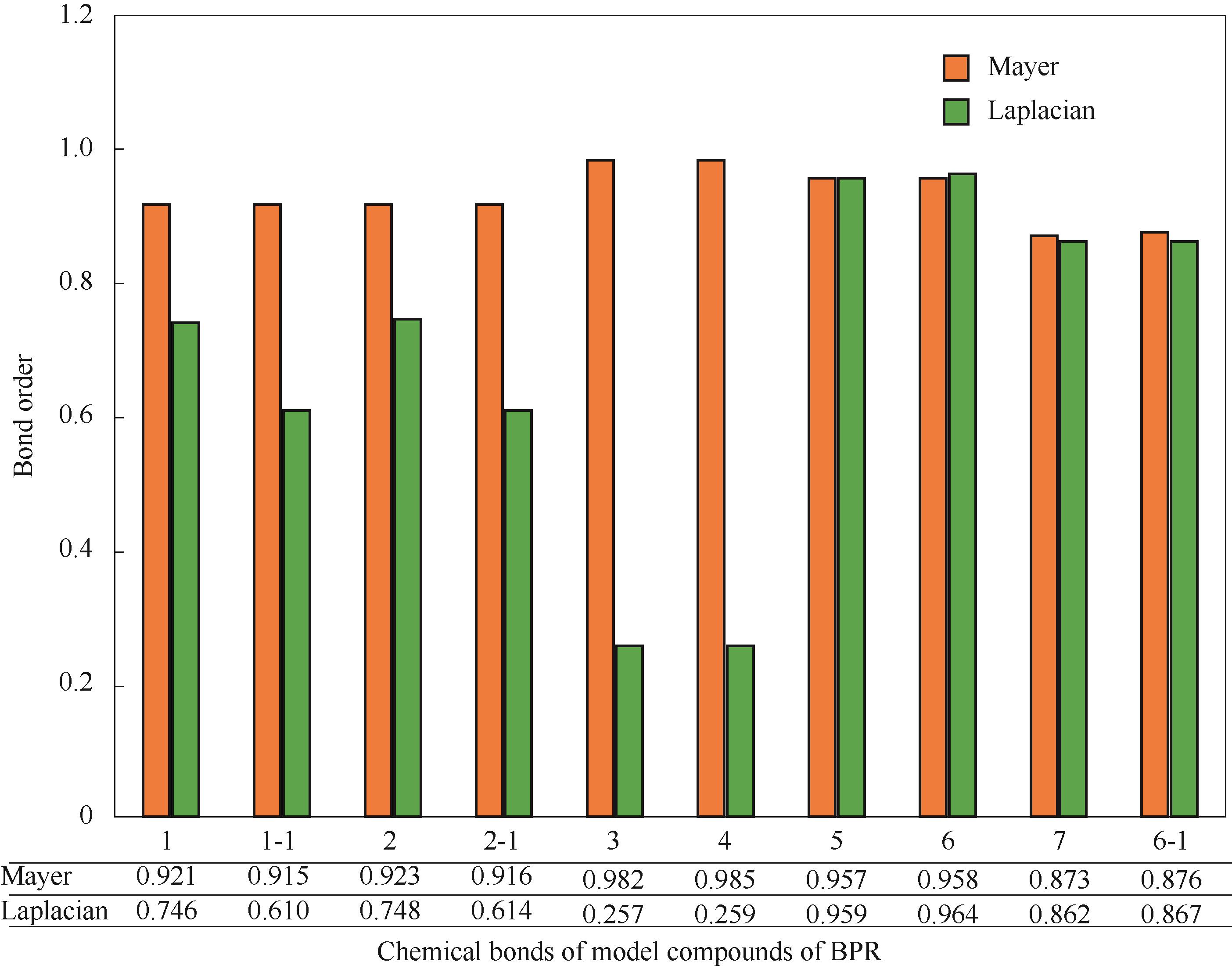
Fig.8 The Mayer bond order and the Laplacian bond order of the chemical bonds involved in the broken bonds in the reaction pathways of BPR (The numbers “1,3” in the abscissa represent the chemical bonds “C3—C25,C4—O21” of the reactant R (PR),“2,4”represent the chemical bonds “C3—C24,C4—O20” of the reactant R (BPR), and “5,7” represent the chemical bonds “C3—C13,C6—C10” of P4 and P5 in PR respectively, “-1” represents the corresponding chemical bonds of the isomerized product 1-i1,“6,6-1”represent the chemical bonds “C6—C28” of P4 and P5 in BPR respectively)
| Species | Structure parameter | |||
|---|---|---|---|---|
| Bond length/Å | Bond angle/(°) | Dihedral angle/(°) | ||
| R |  | R(3,4) 1.4129 R(3,24) 1.5263 R(6,10) 1.0856 R(11,16) 1.4053 | A(6,1,7) 120.5751 A(2,3,24) 119.9444 A(3,4,20) 123.7277 A(12,11,17) 119.7931 | D(7,1,6,10) -0.1032 D(8,2,3,4) -179.5135 D(24,3,4,5) -177.5334 D(4,3,24,25) 37.4906 |
| TS1a |  | R(1,7) 1.0847 R(2,8) 1.0872 R(3,21) 1.4603 R(11,16) 1.4039 | A(3,4,20) 108.8091 A(21,3,24) 89.6951 A(4,5,6) 116.4379 A(12,13,14) 118.4517 | D(2,3,4,20) -149.7406 D(4,3,24,26) 90.1564 D(21,4,5,9) -113.5019 D(15,16,24,3) 65.6825 |
| 1-i1 |  | R(2,8) 1.0868 R(3,21) 1.1056 R(3,24) 1.5676 R(6,10) 1.087 | A(3,2,8) 116.7196 A(4,3,21) 105.3531 A(21,3,24) 104.907 A(12,11,16) 121.5719 | D(7,1,2,3) 178.9128 D(8,2,3,4) -174.8452 D(21,3,4,20) -69.4398 D(21,3,24,26) 179.7412 |
| P4 |  | R(6,28) 1.4711 R(13,15) 1.3738 R(19,23) 1.0851 R(22,24) 1.3993 | A(6,1,7) 117.5481 A(2,3,9) 121.2674 A(13,14,17) 125.9009 A(17,18,20) 119.1459 | D(7,1,6,28) 0.018 D(9,3,4,5) -179.895 D(5,4,12,13) -179.9181 D(14,17,19,23) -0.5538 |
| P5 |  | R(4,12) 1.3987 R(6,28) 1.4911 R(13,14) 1.3956 R(22,24) 1.3993 | A(6,1,7) 119.0185 A(3,4,12) 125.829 A(17,18,20) 119.1356 A(18,20,25) 119.5079 | D(7,1,2,3) -177.9088 D(8,2,3,9) 0.5803 D(4,5,10,11) -1.8132 D(14,13,15,16) -2.0753 |
| P6 |  | R(3,24) 1.4668 R(11,16) 1.4076 R(14,25) 1.4006 R(26,27) 1.3968 | A(5,4,20) 117.4778 A(12,11,16) 121.0413 A(14,15,22) 119.9191 A(26,28,29) 119.5608 | D(8,2,3,24) 0.9432 D(5,4,20,21) 177.2876 D(17,11,12,13) 179.3928 D(22,15,16,11) -177.754 |
Table 2 Optimized geometries of reactants, important transition states, intermediates and products in the pyrolysis reaction pathways of BPR
| Species | Structure parameter | |||
|---|---|---|---|---|
| Bond length/Å | Bond angle/(°) | Dihedral angle/(°) | ||
| R |  | R(3,4) 1.4129 R(3,24) 1.5263 R(6,10) 1.0856 R(11,16) 1.4053 | A(6,1,7) 120.5751 A(2,3,24) 119.9444 A(3,4,20) 123.7277 A(12,11,17) 119.7931 | D(7,1,6,10) -0.1032 D(8,2,3,4) -179.5135 D(24,3,4,5) -177.5334 D(4,3,24,25) 37.4906 |
| TS1a |  | R(1,7) 1.0847 R(2,8) 1.0872 R(3,21) 1.4603 R(11,16) 1.4039 | A(3,4,20) 108.8091 A(21,3,24) 89.6951 A(4,5,6) 116.4379 A(12,13,14) 118.4517 | D(2,3,4,20) -149.7406 D(4,3,24,26) 90.1564 D(21,4,5,9) -113.5019 D(15,16,24,3) 65.6825 |
| 1-i1 |  | R(2,8) 1.0868 R(3,21) 1.1056 R(3,24) 1.5676 R(6,10) 1.087 | A(3,2,8) 116.7196 A(4,3,21) 105.3531 A(21,3,24) 104.907 A(12,11,16) 121.5719 | D(7,1,2,3) 178.9128 D(8,2,3,4) -174.8452 D(21,3,4,20) -69.4398 D(21,3,24,26) 179.7412 |
| P4 |  | R(6,28) 1.4711 R(13,15) 1.3738 R(19,23) 1.0851 R(22,24) 1.3993 | A(6,1,7) 117.5481 A(2,3,9) 121.2674 A(13,14,17) 125.9009 A(17,18,20) 119.1459 | D(7,1,6,28) 0.018 D(9,3,4,5) -179.895 D(5,4,12,13) -179.9181 D(14,17,19,23) -0.5538 |
| P5 |  | R(4,12) 1.3987 R(6,28) 1.4911 R(13,14) 1.3956 R(22,24) 1.3993 | A(6,1,7) 119.0185 A(3,4,12) 125.829 A(17,18,20) 119.1356 A(18,20,25) 119.5079 | D(7,1,2,3) -177.9088 D(8,2,3,9) 0.5803 D(4,5,10,11) -1.8132 D(14,13,15,16) -2.0753 |
| P6 |  | R(3,24) 1.4668 R(11,16) 1.4076 R(14,25) 1.4006 R(26,27) 1.3968 | A(5,4,20) 117.4778 A(12,11,16) 121.0413 A(14,15,22) 119.9191 A(26,28,29) 119.5608 | D(8,2,3,24) 0.9432 D(5,4,20,21) 177.2876 D(17,11,12,13) 179.3928 D(22,15,16,11) -177.754 |
| 1 | 闫联生, 姚冬梅, 杨学军. 新型耐烧蚀材料研究[J]. 宇航材料工艺, 2002, 32(2): 29-31. |
| Yan L S, Yao D M, Yang X J. A study of new ablation resistant materials[J]. Aerospace Materials & Technology, 2002, 32(2): 29-31. | |
| 2 | 王子龙, 邢素丽, 尹昌平, 等. 高残炭耐烧蚀树脂基体研究进展[J]. 工程塑料应用, 2018, 46(8): 131-137. |
| Wang Z L, Xing S L, Yin C P, et al. Research progress of ablative resistant resin matrix with high carbonization rate[J]. Engineering Plastics Application, 2018, 46(8): 131-137. | |
| 3 | 陈鸯飞, 陈智琴, 肖绍懿, 等. 酚醛树脂热降解过程中的结构变化[J]. 热固性树脂, 2008, 23(4): 4-8, 12. |
| Chen Y F, Chen Z Q, Xiao S Y, et al. The structural changes of phenolic resin during thermal degradation[J]. Thermosetting Resin, 2008, 23(4): 4-8, 12. | |
| 4 | 王亚楠, 李兆, 曹静, 等. 酚醛树脂及含硼酚醛树脂热裂解和碳化研究进展[J]. 当代化工, 2021, 50(9): 2235-2241. |
| Wang Y N, Li Z, Cao J, et al. Research progress of pyrolysis and carbonization of phenolic resin and boron-containing phenolic resin[J]. Contemporary Chemical Industry, 2021, 50(9): 2235-2241. | |
| 5 | 高南, 张亚峰, 邝健政, 等. 改性酚醛树脂及其在防腐涂料中的应用[J]. 新型建筑材料, 2011, 38(2): 11-14, 18. |
| Gao N, Zhang Y F, Kuang J Z, et al. Modified phenolic resin and its application in anticorrosive paint[J]. New Building Materials, 2011, 38(2): 11-14, 18. | |
| 6 | 张力, 张以河, 姚亚琳, 等. 硼酚醛树脂及其复合材料的研究进展[J]. 玻璃钢/复合材料, 2018(3): 107-120. |
| Zhang L, Zhang Y H, Yao Y L, et al. Research progress in boron modified phenolic resin and its composites[J]. Fiber Reinforced Plastics/Composites, 2018(3): 107-120. | |
| 7 | Jiang H Y, Wang J G, Wu S Q, et al. The pyrolysis mechanism of phenol formaldehyde resin[J]. Polymer Degradation and Stability, 2012, 97(8): 1527-1533. |
| 8 | 陈治宇, 胡宏林, 余瑞莲, 等. 一种酚醛树脂模型化合物热解反应的密度泛函理论研究及实验验证[J]. 宇航材料工艺, 2018, 48(1): 30-36. |
| Chen Z Y, Hu H L, Yu R L, et al. Pyrolysis reactions of one model compound of phenolic resin using density functional theory and experimental validation[J]. Aerospace Materials & Technology, 2018, 48(1): 30-36. | |
| 9 | Abdalla M O, Ludwick A, Mitchell T. Boron-modified phenolic resins for high performance applications[J]. Polymer, 2003, 44(24): 7353-7359. |
| 10 | Lu T, Chen F W. Multiwfn: a multifunctional wavefunction analyzer[J]. Journal of Computational Chemistry, 2012, 33(5): 580-592. |
| 11 | Trick K A, Saliba T E. Mechanisms of the pyrolysis of phenolic resin in a carbon/phenolic composite[J]. Carbon, 1995, 33(11): 1509-1515. |
| 12 | Chen Z Q, Chen Y F, Liu H B. Pyrolysis of phenolic resin by TG-MS and FTIR analysis[C]//Materials Engineering for Advanced Technologies. Trans Tech Publications, 2012:102-107. |
| 13 | Wong H W, Peck J, Bonomi R E, et al. Quantitative determination of species production from phenol-formaldehyde resin pyrolysis[J]. Polymer Degradation and Stability, 2015, 112: 122-131. |
| 14 | Bennett A, Payne D R, Court R W. Pyrolytic and elemental analysis of decomposition products from a phenolic resin[J]. Macromolecular Symposia, 2014, 339(1): 38-47. |
| 15 | Fitzer E, Schaefer W, Yamada S. The formation of glasslike carbon by pyrolysis of polyfurfuryl alcohol and phenolic resin[J]. Carbon, 1969, 7(6): 643-648. |
| 16 | 马伟. 酚醛树脂的热解研究[D]. 重庆: 重庆大学, 2007. |
| Ma W. Study on the pyrolysis of phenolic resin[D]. Chongqing: Chongqing University, 2007. | |
| 17 | Chattaraj P K, Sarkar U, Roy D R. Electrophilicity index[J]. Chemical Reviews, 2006, 106(6): 2065-2091. |
| 18 | 张晨. 类煤模型化合物热解过程中脱羧反应和C—C/C—O断键规律研究[D]. 徐州: 中国矿业大学, 2020. |
| Zhang C. Research on decarboxylation reaction and C—C/C—O breaking law of coal-like model compounds during pyrolysis[D]. Xuzhou: China University of Mining and Technology, 2020. | |
| 19 | Li M, Mo C H, Luo X, et al. Exploring key reaction sites and deep degradation mechanism of perfluorooctane sulfonate via peroxymonosulfate activation under electrocoagulation process[J]. Water Research, 2021, 207: 117849. |
| 20 | 渠艳飞, 关泽宇, 马邕文, 等. 基于硫酸自由基氧化降解邻苯二甲酸二丁酯的密度泛函理论研究[J]. 环境化学, 2017, 36(9): 1896-1905. |
| Qu Y F, Guan Z Y, Ma Y W, et al. A theoretical investigation on the degradation of dibutyl phthalate based on sulfate radicals oxidation: a DFT study[J]. Environmental Chemistry, 2017, 36(9): 1896-1905. | |
| 21 | Shen Q R, Fu Z W, Li R, et al. A study on the pyrolysis mechanism of a β-O-4 lignin dimer model compound using DFT combined with Py-GC/MS[J]. Journal of Thermal Analysis and Calorimetry, 2021, 146(4): 1751-1761. |
| 22 | 张洋, 陈世荣. 羟基自由基与苯酚反应机理的量子化学研究[J]. 陕西师范大学学报(自然科学版), 2008, 36(5): 58-61. |
| Zhang Y, Chen S R. Theoretical study on the reaction of hydroxyl radical with phenol[J]. Journal of Shaanxi Normal University (Natural Science Edition), 2008, 36(5): 58-61. | |
| 23 | Ouchi K, Honda H. Pyrolysis of coal(Ⅰ): Thermal cracking of phenolformaldehyde resins taken as coal models[J]. Fuel, 1959, 38:429-443. |
| 24 | Jackson W M, Conley R T. High temperature oxidative degradation of phenol-formaldehyde polycondensates[J]. Journal of Applied Polymer Science, 1964, 8(5): 2163-2193. |
| 25 | 姚灿, 田红, 黄章俊, 等. 基于量子化学的玉米秸秆热解机理的模拟计算[J]. 西北大学学报(自然科学版), 2019, 49(1): 122-131. |
| Yao C, Tian H, Huang Z J, et al. Theoretical study on pyrolysis mechanism of corn stalk based on quantum chemistry[J]. Journal of Northwest University (Natural Science Edition), 2019, 49(1): 122-131. | |
| 26 | Tang B, Wang Y T, Peng X L, et al. Efficient predictions of Gibbs free energy for the gases CO, BF, and gaseous BBr[J]. Journal of Molecular Structure, 2020, 1199: 126958. |
| 27 | 梁韬. 基于Py-GC/MS的半纤维素热裂解机理研究[D]. 杭州: 浙江大学, 2013. |
| Liang T. Mechanism research of hemicellulose pvrolvsis based on Py-GC/MS[D]. Hangzhou: Zhejiang University, 2013. | |
| 28 | 张亚运. 木质纤维素热化学转化机理及裂解气体CO2和H2吸附分离的分子模拟研究[D]. 重庆: 重庆大学, 2017. |
| Zhang Y Y. The mechanism of lignin cellulose thermal conversion and adsorption and separation of pyrolytic gases CO2 and H2 by molecular simulation[D]. Chongqing: Chongqing University, 2017. | |
| 29 | Costa L, di Montelera L R, Camino G, et al. Structure-charring relationship in phenol-formaldehyde type resins[J]. Polymer Degradation and Stability, 1997, 56(1): 23-35. |
| 30 | Wang S J, Wang Y, Bian C, et al. The thermal stability and pyrolysis mechanism of boron-containing phenolic resins: the effect of phenyl borates on the char formation[J]. Applied Surface Science, 2015, 331: 519-529. |
| [1] | Lei WU, Jiao LIU, Changcong LI, Jun ZHOU, Gan YE, Tiantian LIU, Ruiyu ZHU, Qiuli ZHANG, Yonghui SONG. Catalytic microwave pyrolysis of low-rank pulverized coal for preparation of high value-added modified bluecoke powders containing carbon nanotubes [J]. CIESC Journal, 2023, 74(9): 3956-3967. |
| [2] | Zhenghao YANG, Zhen HE, Yulong CHANG, Ziheng JIN, Xia JIANG. Research progress in downer fluidized bed reactor for biomass fast pyrolysis [J]. CIESC Journal, 2023, 74(6): 2249-2263. |
| [3] | Simin YI, Yali MA, Weiqiang LIU, Jinshuai ZHANG, Yan YUE, Qiang ZHENG, Songyan JIA, Xue LI. Study on ammonia evaporation and hydration kinetics of microcrystalline magnesite [J]. CIESC Journal, 2023, 74(4): 1578-1586. |
| [4] | Ruizhe CHEN, Leilei CHENG, Jing GU, Haoran YUAN, Yong CHEN. Research progress in chemical recovery technology of fiber-reinforced polymer composites [J]. CIESC Journal, 2023, 74(3): 981-994. |
| [5] | Chen CHEN, Qian YANG, Yun CHEN, Rui ZHANG, Dong LIU. Chemical kinetic study on coal volatiles combustion for various oxygen concentrations [J]. CIESC Journal, 2022, 73(9): 4133-4146. |
| [6] | Zeguang HAO, Qian ZHANG, Zenglin GAO, Hongwen ZHANG, Zeyu PENG, Kai YANG, Litong LIANG, Wei HUANG. Study on synergistic effect of biomass and FCC slurry co-pyrolysis [J]. CIESC Journal, 2022, 73(9): 4070-4078. |
| [7] | Jian SHAO, Junzong FENG, Fengqi LIU, Yonggang JIANG, Liangjun LI, Jian FENG. Research progress on structural modulation and functionalized preparation of phenolic resin-based carbon microspheres [J]. CIESC Journal, 2022, 73(9): 3787-3801. |
| [8] | Kaihong TANG, Xiaofeng HE, Guiqiu XU, Yang YU, Xiaofeng LIU, Tiejun GE, Ailing ZHANG. Review on combustion behavior and flame retardant research of phenolic foams [J]. CIESC Journal, 2022, 73(8): 3483-3500. |
| [9] | Haoyu XIAO, Haiping YANG, Xiong ZHANG, Yingquan CHEN, Xianhua WANG, Hanping CHEN. Recent progress of catalytic pyrolysis of plastics to produce high value-added products [J]. CIESC Journal, 2022, 73(8): 3461-3471. |
| [10] | Yugong CHEN, Hao CHEN, Yaosong HUANG. Study on pyrolysis mechanism of hexamethyldisiloxane using reactive molecular dynamics simulations [J]. CIESC Journal, 2022, 73(7): 2844-2857. |
| [11] | Xiaoya LIU, Jinchao WANG, Ying LIU, Jinghuan MA. Progress in modified preparation and catalytic mechanism of nanocatalysts for hydrogen production from hydrous hydrazine [J]. CIESC Journal, 2022, 73(7): 2819-2834. |
| [12] | Yong’an CHEN, Anning ZHOU, Yunlong LI, Zhiwei SHI, Xinfu HE, Weihong JIAO. Preparation and coal pyrolysis performance of magnetic MgFe2O4 and its core-shell catalysts [J]. CIESC Journal, 2022, 73(7): 3026-3037. |
| [13] | Mo ZHENG, Xiaoxia LI. Revealing reaction compromise in competition for volatile radicals during coal pryolysis via ReaxFF MD simulation [J]. CIESC Journal, 2022, 73(6): 2732-2741. |
| [14] | Guanyi CHEN, Tujun TONG, Rui LI, Yanshan WANG, Beibei YAN, Ning LI, Li'an HOU. Influence of pyrolysis time on sludge-derived biochar performance for peroxymonosulfate activation [J]. CIESC Journal, 2022, 73(5): 2111-2119. |
| [15] | Xiqiang ZHAO, Jian ZHANG, Shuang SUN, Wenlong WANG, Yanpeng MAO, Jing SUN, Jinglong LIU, Zhanlong SONG. Study on the performance of biochar modified microspheres to remove inorganic phosphorus from chemical wastewater [J]. CIESC Journal, 2022, 73(5): 2158-2173. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||