化工学报 ›› 2021, Vol. 72 ›› Issue (5): 2638-2646.DOI: 10.11949/0438-1157.20201245
朱倩倩( ),靳海波(
),靳海波( ),郭晓燕,何广湘,马磊,张荣月,谷庆阳,杨索和
),郭晓燕,何广湘,马磊,张荣月,谷庆阳,杨索和
收稿日期:2020-08-31
修回日期:2020-11-03
出版日期:2021-05-05
发布日期:2021-05-05
通讯作者:
靳海波
作者简介:朱倩倩(1995—),女,硕士研究生,基金资助:
ZHU Qianqian( ),JIN Haibo(
),JIN Haibo( ),GUO Xiaoyan,HE Guangxiang,MA Lei,ZHANG Rongyue,GU Qingyang,YANG Suohe
),GUO Xiaoyan,HE Guangxiang,MA Lei,ZHANG Rongyue,GU Qingyang,YANG Suohe
Received:2020-08-31
Revised:2020-11-03
Online:2021-05-05
Published:2021-05-05
Contact:
JIN Haibo
摘要:
在H2O2/乙腈体系下以沉淀法制备的MgO为催化剂催化Baeyer-Villiger(B-V)氧化环己酮合成ε-己内酯,考察了制备条件和反应条件对环己酮转化率和己内酯收率的影响。根据实验结果,Mg(NO3)2·6H2O为前体,在煅烧温度为600℃、煅烧时间为2 h时制备MgO氧化性能最佳,由X射线衍射(XRD)、扫描电镜(SEM)进行了分析,可知随温度升高MgO粒径逐渐增大,500~800℃范围内,MgO晶粒尺寸由9.53 nm增大到29.49 nm。在n(催化剂)∶n(环己酮)=0.45∶1、n(乙腈)∶n(环己酮)=12∶1、n(双氧水)∶n(环己酮)=10∶1、70℃、6 h时获得环己酮转化率95.2%及ε-己内酯收率83.1%。对双氧水B-V氧化环己酮机理进行了深入的研究,采用在线原位红外光谱对反应进行实时监测与分析,验证了其过氧缩酰胺反应路径。
中图分类号:
朱倩倩, 靳海波, 郭晓燕, 何广湘, 马磊, 张荣月, 谷庆阳, 杨索和. H2O2/乙腈体系下MgO催化环己酮Baeyer-Villiger绿色氧化合成ε-己内酯的研究[J]. 化工学报, 2021, 72(5): 2638-2646.
ZHU Qianqian, JIN Haibo, GUO Xiaoyan, HE Guangxiang, MA Lei, ZHANG Rongyue, GU Qingyang, YANG Suohe. Study on synthesis of ε-caprolactone with MgO catalysis by Baeyer-Villiger green oxidation of cyclohexanone in H2O2/acetonitrile system[J]. CIESC Journal, 2021, 72(5): 2638-2646.

图3 不同煅烧温度下MgO的SEM图(a)~(d)放大倍数为5千倍,(e)~(h)放大倍数为10万倍;(a)~(d)和(e)~(h)的煅烧温度都依次为500、600、700、800℃
Fig.3 SEM images of MgO at different calcination temperatures
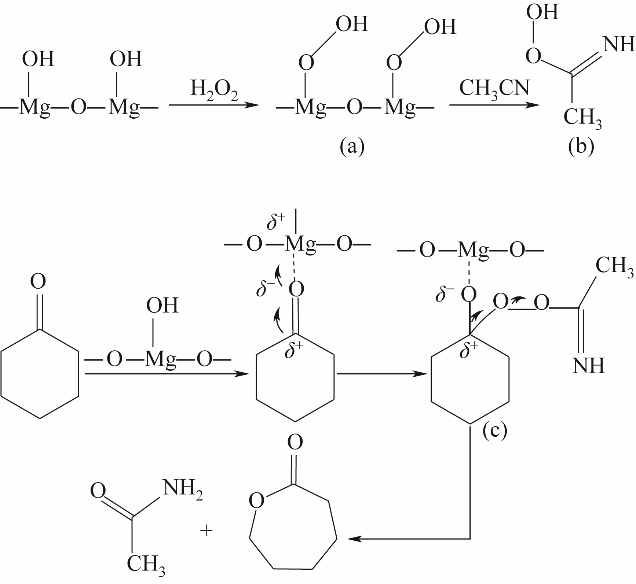
图11 路线Ⅱ:H2O2/乙腈体系下MgO催化环己酮B-V氧化反应机理(酮羰基与H2O2分子均被活化)
Fig.11 Route Ⅱ: MgO-catalyzed oxidation mechanism of cyclohexanone B-V in H2O2/acetonitrile system
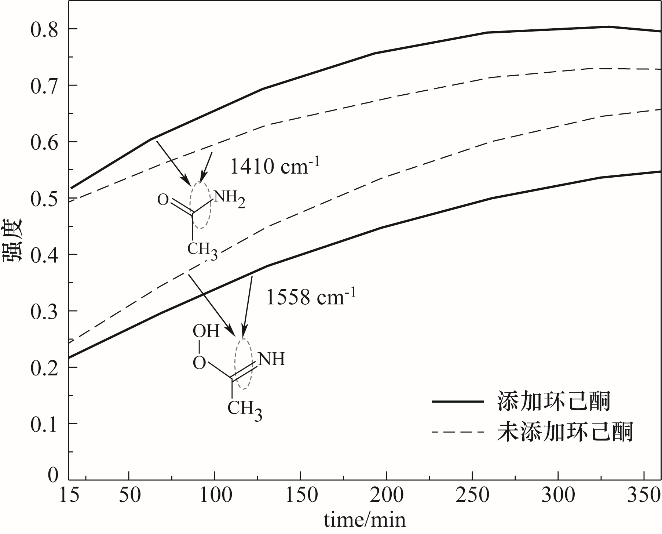
图13 MgO催化B-V氧化合成ε-己内酯中乙酰胺与过氧缩酰胺原位红外光谱峰强变化
Fig.13 MgO catalyzed oxidation of B-V to synthesize ε-caprolactone acetamide and peroxyacetal in-situ infrared spectrum peak intensity changes
| 1 | Ciardelli G, Chiono V, Vozzi G, et al. Blends of poly-(ε-caprolactone) and polysaccharides in tissue engineering applications[J]. Biomacromolecules, 2005, 6(4): 1961-1976. |
| 2 | Han Y, Li S, Ding R, et al. Baeyer-Villiger oxidation of cyclohexanone catalyzed by cordierite honeycomb washcoated with Mg-Sn-W composite oxides[J]. Chinese Journal of Chemical Engineering, 2018, 27(3): 91-101. |
| 3 | Lince F, Marchisio D L, Barresi A A. Strategies to control the particle size distribution of poly-ε-caprolactone nanoparticles for pharmaceutical applications[J]. Journal of Colloid & Interface Science, 2008, 322(2): 505-515. |
| 4 | 李子辉, 蒋晶, 金章勇, 等. 生物可降解 PCL/PLA 开孔发泡材料制备及吸油性能[J].化工学报, 2020, 71(12): 5840-5851. |
| Li Z H, Jiang J, Jin Z Y, et al. Preparation and oil absorption performance of biodegradable PCL/PLA open-cell foam material[J]. CIESC Journal, 2020, 71(12): 5840-5851. | |
| 5 | Houtchens G R, Foster M D, Desai T A, et al. Combined effects of microtopography and cyclic strain on vascular smooth muscle cell orientation[J]. Journal of Biomechanics, 2008, 41(4): 762-769. |
| 6 | Wang Z B, Mizusaki T, Sano T, et al. Baeyer-Villiger oxidation over HZSM-5 type zeolites[J]. Bulletin of the Chemical Society of Japan, 1997, 70(10): 2567-2570. |
| 7 | 严生虎, 韩玲玲, 沈卫, 等. 微通道中环己酮氧化合成ε-己内酯的连续流工艺[J]. 化工进展, 2014, 33(11): 3061-3066. |
| Yan S H, Han L L, Shen W, et al. Continuous synthesis process of ε-caprolactone from oxidization of cyclohexanone in micro-channel reactor[J]. Chemical Industry and Engineering Progress, 2014, 33(11): 3061-3066. | |
| 8 | Renz M, Meunier B. 100 years of Baeyer-Villiger oxidations[J]. European Journal of Organic Chemistry, 1999, (4): 737-750. |
| 9 | Zhang G X, Ren X C, Zhang H B, et al. MgO/SnO2/WO3 as catalysts for synthesis of ε-caprolactone over oxidation of cyclohexanone with peracetic acid[J]. Catalysis Communications, 2015, 58: 59-63. |
| 10 | Chen J, Zhao X, Zhang G, et al. Synthesis of ε-caprolactone by oxidation of cyclohexanone with monoperoxysuccinic acid[J]. Chinese Journal of Chemical Engineering, 2013, 21(12): 1404-1409. |
| 11 | Hazra S, Martins N M R, Mahmudov K, et al. A tetranuclear diphenyltin(Ⅳ) complex and its catalytic activity in the aerobic Baeyer-Villiger oxidation of cyclohexanone[J]. Journal of Organometallic Chemistry, 2018, 867: 193-200. |
| 12 | Zhang X, Yang H, Yang G, et al. Metal-free mesoporous SiO2 nanorods as a highly efficient catalyst for the Baeyer-Villiger oxidation under mild conditions[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(5): 5868-5876. |
| 13 | Sinhamahapatra A, Sinha A, Pahari S K, et al. Room temperature Baeyer-Villiger oxidation using molecular oxygen over mesoporous zirconium phosphate[J]. Catalysis Science & Technology, 2012, 2(11): 2375-2382. |
| 14 | Romero E, Ruben G C, Mattevi A, et al. Characterization and crystal structure of a robust cyclohexanone monooxygenase[J]. Angewandte Chemie International Edition, 2016, 55(51): 15852-15855. |
| 15 | Yachnin B J, McEvoy M B, MacCuish R J D, et al. Lactone-bound structures of cyclohexanone monooxygenase provide insight into the stereochemistry of catalysis[J]. ACS Chemical Biology, 2014, 9(12): 2843-2851. |
| 16 | Olszówka J E, Karcz R, Napruszewska B D, et al. Effect of Mg-Al hydrotalcite crystallinity on catalytic Baeyer-Villiger oxidation of cyclohexanone with H2O2/acetonitrile[J]. Catalysis Communications, 2018, 107: 48-52. |
| 17 | Olszówka J, Karcz R, Napruszewska B D, et al. Baeyer-Villiger oxidation of cyclohexanone with H2O2/acetonitrile over hydrotalcite-like catalysts: effect of Mg/Al ratio on the ε-caprolactone yield[J]. Catalysis Communications, 2017, 100: 196-201. |
| 18 | Pillai U R, Sahle-Demessie E. Sn-exchanged hydrotalcites as catalysts for clean and selective Baeyer-Villiger oxidation of ketones using hydrogen peroxide[J]. Journal of Molecular Catalysis A: Chemical, 2003, 191(1): 93-100. |
| 19 | Jiménez-Sanchidrián C, Hidalgo J M, Llamas R, et al. Baeyer-Villiger oxidation of cyclohexanone with hydrogen peroxide/benzonitrile over hydrotalcites as catalysts[J]. Applied Catalysis A: General, 2006, 312: 86-94. |
| 20 | Yang Z, Niu L, Ma Z, et al. Fabrication of highly active Sn/W mixed transition-metal oxides as solid acid catalysts[J]. Transition Metal Chemistry, 2011, 36(3): 269-274. |
| 21 | 李静霞, 黄靓, 戴维林, 等. 高活性MgO/SnO2复合金属氧化物催化剂的合成及其在双氧水选择氧化环己酮制ε-己内酯反应中的应用[J]. 化学学报, 2008, 66(1): 5-9. |
| Li J X, Huang L, Dai W L, et al. Baeyer-Villiger oxidation of cyclohexanone to caprolactone over highly efficient MgO/SnO2 catalyst using H2O2 as oxidant[J]. Acta Chimica Sinica, 2008, 66(1): 5-9. | |
| 22 | Ruiz J R, Jiménez-Sanchidrián C, Llamas R. Hydrotalcites as catalysts for the Baeyer-Villiger oxidation of cyclic ketones with hydrogen peroxide/benzonitrile[J]. Tetrahedron, 2006, 62(50): 11697-11703. |
| 23 | Ma Q G, Xing W Z, Xu J H, et al. Baeyer-Villiger oxidation of cyclic ketones with aqueous hydrogen peroxide catalyzed by transition metal oxides[J]. Catalysis Communications, 2014, 53: 5-8. |
| 24 | Corma A, Nemeth L T, Renz M, et al. Sn-zeolite beta as a heterogeneous chemoselective catalyst for Baeyer-Villiger oxidations[J]. Nature, 2001, 412(6845): 423-425. |
| 25 | Good R J, van Oss C J. The modern theory of contact angles and the hydrogen bond components of surface energies[M]//Schrader M E, Loeb G I. Modern Approaches to Wettability. New York: Springer, 1992: 1-27. |
| 26 | Olszówka J E, Karcz R, Michalik-Zym A, et al. Effect of grinding on the physico-chemical properties of Mg-Al hydrotalcite and its performance as a catalyst for Baeyer-Villiger oxidation of cyclohexanone[J]. Catalysis Today, 2019, 333: 147-153. |
| 27 | Xia C J, Lin M, Zhu B, et al. Study on the mechanisms for Baeyer-Villiger oxidation of cyclohexanone with hydrogen peroxide in different systems[J]. China Pet. Process. Pe. Technol., 2012, 14(2): 7-17. |
| 28 | 李萍. 球形纳米氧化镁的制备及其粒度影响因素[J]. 化学研究与应用, 2019, 31(4): 747-752. |
| Li P. Preparation of spherical nano-MgO and its influencing factors on particle size[J]. Chemical Research and Application, 2019, 31(4): 747-752. | |
| 29 | 王艳荣. 沉淀法制备纳米二氧化铈[D]. 成都: 成都理工大学, 2004. |
| Wang Y R. Preparation of CeO2 nanometer powder by precipitation method[D]. Chengdu: ChengduUniversity of Technology, 2004. | |
| 30 | 蒙慧芹. 有机化学中的溶剂化效应: 溶剂对反应历程和立体化学的影响[J]. 赤峰学院学报(自然科学版), 2011, 27(6): 1-3. |
| Meng H Q. Solvent effects in organic chemistry: effects of solvent on reaction process and stereochemistry[J]. Journal of Chifeng University(Natural Science Edition), 2011, 27(6): 1-3. |
| [1] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [2] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [3] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [4] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [5] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [6] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [7] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [8] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [9] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [10] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [11] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [12] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [13] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [14] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [15] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号