化工学报 ›› 2019, Vol. 70 ›› Issue (11): 4428-4436.DOI: 10.11949/0438-1157.20190353
叶鹏1( ),全学军1(
),全学军1( ),秦险峰1,封承飞1,李纲1,鹿存房1,齐学强1,蒋丽2
),秦险峰1,封承飞1,李纲1,鹿存房1,齐学强1,蒋丽2
收稿日期:2019-04-04
修回日期:2019-07-10
出版日期:2019-11-05
发布日期:2019-11-05
通讯作者:
全学军
作者简介:叶鹏(1995—),男,硕士研究生,基金资助:
Peng YE1( ),Xuejun QUAN1(
),Xuejun QUAN1( ),Xianfeng QIN1,Chengfei FENG1,Gang LI1,Cunfang LU1,Xueqiang QI1,Li JIANG2
),Xianfeng QIN1,Chengfei FENG1,Gang LI1,Cunfang LU1,Xueqiang QI1,Li JIANG2
Received:2019-04-04
Revised:2019-07-10
Online:2019-11-05
Published:2019-11-05
Contact:
Xuejun QUAN
摘要:
铬渣是铬铁矿生产铬盐剩下的尾矿,因含有大量铬铁铝镁元素,也是一种二次资源。采用湿法冶金工艺回收铬渣中铬、铁、铝、镁,以浓盐酸作为浸提剂,考察了液固比、浸出温度以及时间对铬、铁、铝、镁浸出效果的影响。结果表明,最佳浸出条件为:盐酸浓度12 mol·L-1,液固比5.6 ml·g-1,浸出温度110℃,时间6 h,该条件下铬浸出率为67.76%,同时铁铝镁浸出率分别达到89.89%、93.99%和95.21%。铬、铁、铝、镁在铬渣中存在物相不同造成了其浸出率之间的差异。此外,铬、铁、铝、镁浸出过程均符合未反应缩核模型,且主要受界面化学反应控制,其表观活化能分别为102.31、78.10、66.44和81.66 kJ·mol-1。
中图分类号:
叶鹏, 全学军, 秦险峰, 封承飞, 李纲, 鹿存房, 齐学强, 蒋丽. 铬铁矿无钙焙烧渣盐酸浸出[J]. 化工学报, 2019, 70(11): 4428-4436.
Peng YE, Xuejun QUAN, Xianfeng QIN, Chengfei FENG, Gang LI, Cunfang LU, Xueqiang QI, Li JIANG. Leaching of chromite ore processing residue from non-calcium roasting with hydrochloric acid[J]. CIESC Journal, 2019, 70(11): 4428-4436.
| Cr3+ | Fe3+ | Al3+ | Mg2+ | HCl |
|---|---|---|---|---|
| 0.05 | 0.24 | 0.31 | 0.12 | 1.31 |
表1 酸浸液成分
Table 1 Composition of leaching solution/(mol·L-1)
| Cr3+ | Fe3+ | Al3+ | Mg2+ | HCl |
|---|---|---|---|---|
| 0.05 | 0.24 | 0.31 | 0.12 | 1.31 |
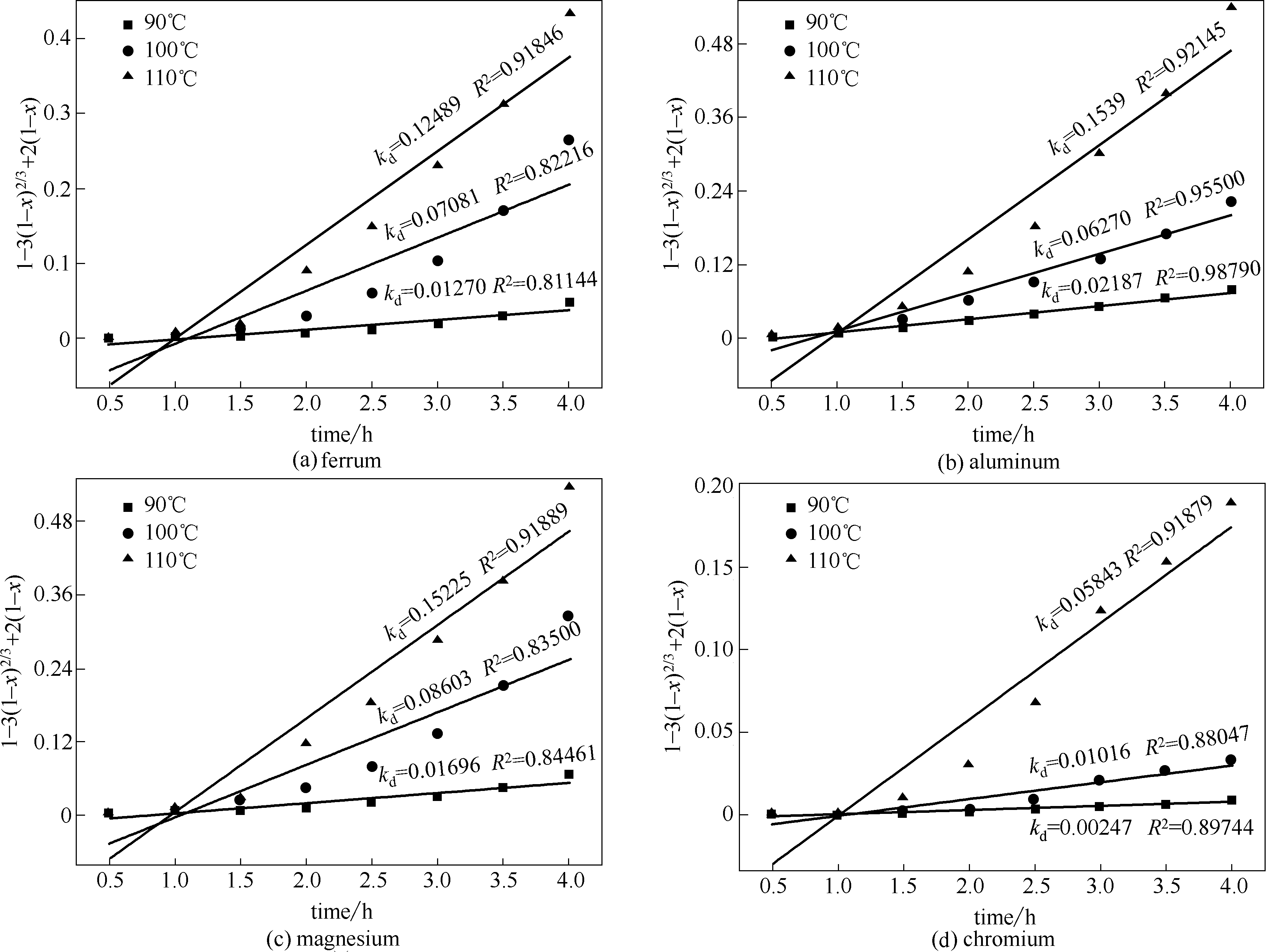
图4 铬、铁、铝、镁产物层扩散控制动力学模型
Fig.4 Diffusion through product layer controlled kinetics model of leaching of ferrum, aluminum, magnesium and chromium at various temperatures
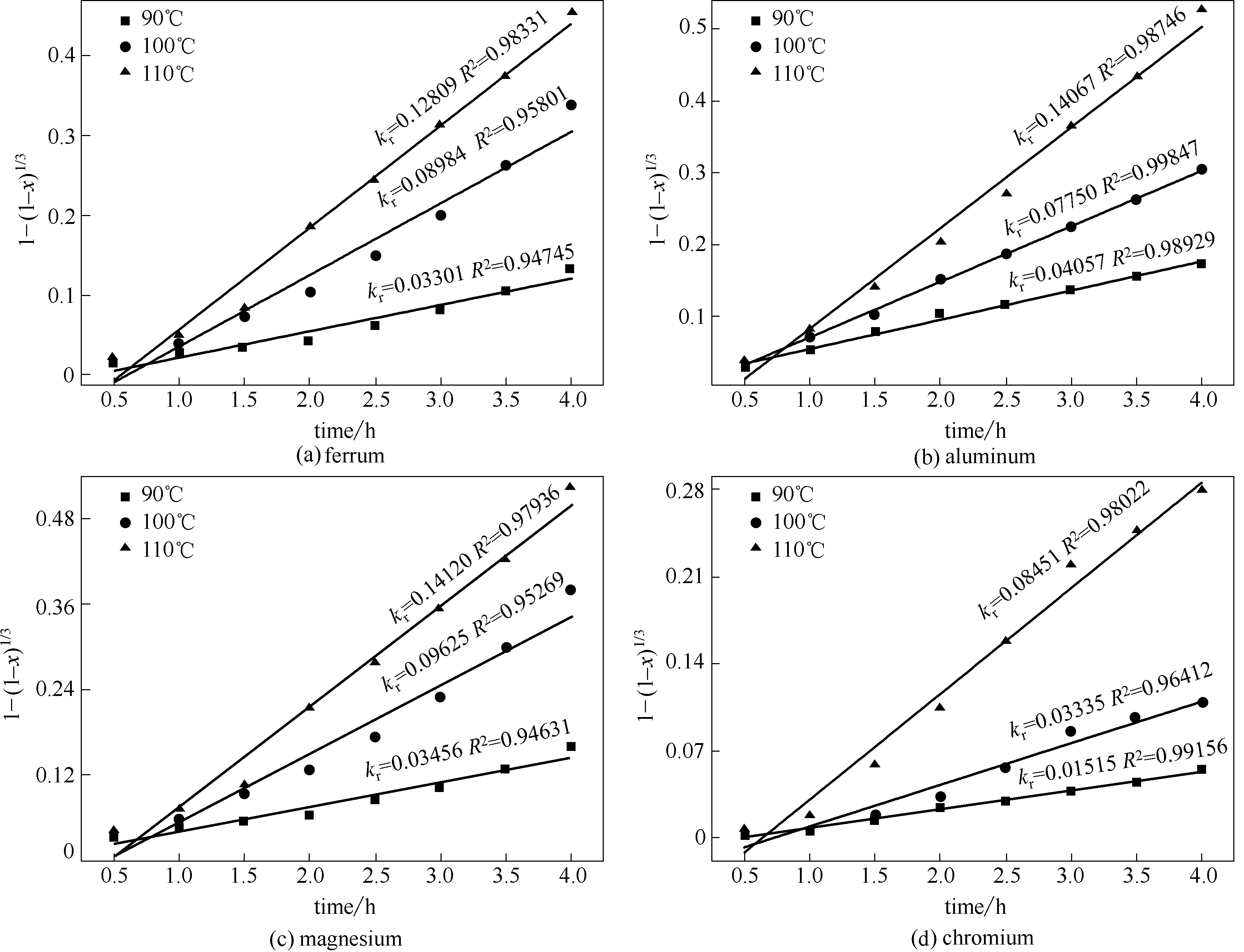
图5 铬、铁、铝、镁化学反应控制动力学模型
Fig.5 Chemical reaction controlled kinetic model of leaching of ferrum, aluminum, magnesium and chromium at various temperatures
| 样品 | Cr2O3 | Fe2O3 | Al2O3 | MgO | Na2O | SiO2 | TiO2 | CaO | Other |
|---|---|---|---|---|---|---|---|---|---|
| 铬渣 | 11.34 | 39.49 | 31.55 | 10 | 3.4 | 2.69 | 1.35 | 0.22 | 0.16 |
| 浸出渣 | 4.11 | 2.41 | 0.28 | 2.35 | 2.31 | 82.81 | 2.35 | 0.25 | 0.24 |
表2 铬渣及浸出渣组成
Table 2 Composition of COPR and leaching residue/%(mass)
| 样品 | Cr2O3 | Fe2O3 | Al2O3 | MgO | Na2O | SiO2 | TiO2 | CaO | Other |
|---|---|---|---|---|---|---|---|---|---|
| 铬渣 | 11.34 | 39.49 | 31.55 | 10 | 3.4 | 2.69 | 1.35 | 0.22 | 0.16 |
| 浸出渣 | 4.11 | 2.41 | 0.28 | 2.35 | 2.31 | 82.81 | 2.35 | 0.25 | 0.24 |
| 1 | Wang T , He M , Pan Q . A new method for the treatment of chromite ore processing residues[J]. Journal of Hazardous Materials, 2007, 149(2): 440. |
| 2 | 李小斌, 齐天贵, 彭志宏, 等 . 铬铁矿氧化焙烧动力学[J]. 中国有色金属学报, 2010, 20(9): 1822-1828. |
| Li X B , Qi T G , Peng Z H , et al . Kinetics of chromite ore in oxidation roasting process[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(9): 1822-1828. | |
| 3 | Matern K , Kletti H , Mansfeldt T . Chemical and mineralogical characterization of chromite ore processing residue from two recent Indian disposal sites[J]. Chemosphere, 2016, 155: 188-195. |
| 4 | Deakin D , West L J , Stewart D I , et al . The leaching characteristics of chromite ore processing residue[J]. Environmental Geochemistry & Health, 2001, 23(3): 201-206. |
| 5 | Thompson C M , Kirman C R , Proctor D M , et al . A chronic oral reference dose for hexavalent chromium-induced intestinal cancer[J]. Journal of Applied Toxicology, 2014, 34(5): 525-536. |
| 6 | Li Y , Cundy A B , Feng J , et al . Remediation of hexavalent chromium contamination in chromite ore processing residue by sodium dithionite and sodium phosphate addition and its mechanism[J]. Journal of Environmental Management, 2017, 192: 100. |
| 7 | Li J , Chen Z , Shen J , et al . The enhancement effect of pre-reduction using zero-valent iron on the solidification of chromite ore processing residue by blast furnace slag and calcium hydroxide[J]. Chemosphere, 2015, 134: 159-165. |
| 8 | Huang X , Zhuang R , Muhammad F , et al . Solidification·stabilization of chromite ore processing residue using alkali-activated composite cementitious materials[J]. Chemosphere, 2017, 168: 300-308. |
| 9 | 余学, 罗琳, 李巧巧 . 利用铬渣制备重铬酸钠[J]. 化工环保, 2012, 32(1): 49-52. |
| Yu X , Luo L , Li Q Q . Preparation of sodium dichromate from chromium slag[J]. Environment Protection of Chemical Industry, 2012, 32(1): 49-52. | |
| 10 | 郑敏, 李先荣, 孟艳艳, 等 . 氯化焙烧法回收铬渣中的铬[J]. 化工环保, 2010, 30(3): 61-64. |
| Zheng M , Li X R , Meng Y Y , et al . Recovery of chromium from chromium slag by chloridizing roasting process[J]. Environment Protection of Chemical Industry, 2010, 30(3): 61-64. | |
| 11 | 高怀友, 漆玉邦 . 浸提-交换法处理铬渣的研究[J]. 农业环境科学学报, 1998, (6): 260-262. |
| Gao H Y , Qi Y B . Study on chromium slag treatment by leaching-exchange method [J]. Agro-environmental Protection, 1998, (6): 260-262. | |
| 12 | 张忠诚, 王信东 . 利用铬渣制备铬酸铅的研究[J]. 山东大学学报(工学版), 2001, 31(6): 554-557. |
| Zhang Z C , Wang X D . Preparation of lead chromate by using chromate sludge[J]. Journal of Shandong University (Engineering Science), 2001, 31(6): 554-557. | |
| 13 | 赵备备, 王少娜, 郑诗礼, 等 . 铬盐无钙焙烧渣加压硫酸浸出[J]. 过程工程学报, 2014, 14(6): 915-922. |
| Zhao B B , Wang S N , Zheng S L , et al . Pressure leaching of chromium-containing slag from non-calcium roasting with sulfuric acid[J]. The Chinese Journal of Process Engineering, 2014, 14(6): 915-922. | |
| 14 | Luo M J , Liu C L , Xue J , et al . Leaching kinetics and mechanism of alunite from alunite tailings in highly concentrated KOH solution[J]. Hydrometallurgy, 2017, 174: 10-20. |
| 15 | Mamo S K , Elie M , Baron M G , et al . Leaching kinetics, separation, and recovery of rhenium and component metals from CMSX-4 superalloys using hydrometallurgical processes[J]. Separation and Purification Technology, 2019, 212: 150-160. |
| 16 | Liu C C , Liu S C , Qin Y H , et al . The intensified leaching behavior of potassium from phosphorus-potassium associated ore in HCl-CaF2 system with surfactant(PartⅠ): Kinetics and modelling[J]. Separation and Purification Technology, 2019, 212: 89-100. |
| 17 | 吴俊 . 铬铁矿无钙焙烧渣的解毒及浸出研究[D]. 重庆: 重庆理工大学, 2018. |
| Wu J . Detoxification and leaching behaviour of chromite ore processing residue (COPR)[D]. Chongqing: Chongqing University of Technology, 2018. | |
| 18 | Hillier S , Roe M J , Geelhoed J S , et al . Role of quantitative mineralogical analysis in the investigation of sites contaminated by chromite ore processing residue[J]. Science of the Total Environment, 2003, 308(1/2/3): 195-210. |
| 19 | Peng H , Guo J , Zheng X G , et al . Leaching kinetics of vanadium from calcification roasting converter vanadium slag in acidic medium[J]. Journal of Environmental Chemical Engineering, 2018, 6: 5119-5124. |
| 20 | Zhou X , Chen Y , Yin J , et al . Leaching kinetics of cobalt from the scraps of spent aerospace magnetic materials[J]. Waste Management, 2018, 76: 663-670. |
| 21 | Sohn H Y , Wadsworth M E . Rate Processes of Extractive Metallurgy[M]. New York: Plenum Press, 1979. |
| 22 | Tkáčová K , Baláž P , Mišura B , et al . Selective leaching of zinc from mechanically activated complex Cu Pb-Zn concentrate[J]. Hydrometallurgy, 1993, 33(3): 291-300. |
| 23 | Levenspiel O . Chemical Reaction Engineering[M]. New York: John Willey & Sons, 1999. |
| 24 | Habbache N , Alane N , Djerad S , et al . Leaching of copper oxide with different acid solutions[J]. Chemical Engineering Journal, 2009, 152(2/3): 503-508. |
| 25 | Li N , Zhang Y , Kong D , et al . Fluid particle group reaction model and experimental verification[J]. Advanced Powder Technology, 2013, 24(1): 200-206. |
| 26 | Wang H H , Li G Q , Zhao D , et al . Dephosphorization of high phosphorus oolitic haematite by acid leaching and the leaching kinetics[J]. Hydrometallurgy, 2017, 171: 61-68. |
| 27 | Qin S Y , Yin B W , Zhang Y F , et al . Leaching kinetics of szaibelyite ore in NaOH solution[J]. Hydrometallurgy, 2015, 157: 333-339. |
| 28 | 马荣骏 . 湿法冶金原理[M]. 北京: 冶金工业出版社, 2007: 110-113. |
| Ma R J . Principle on Hydrometallury[M]. Beijing: Metallurgical Industry Press, 2007: 110-113. | |
| 29 | 天津大学物理化学教研室 . 物理化学[M]. 北京: 高等教育出版社, 2009: 353-357. |
| Physical Chemistry Teaching and Research Department of Tianjin University . Physical Chemistry[M]. Beijing: Higher Education Press, 2009: 353-357. | |
| 30 | An J , Yang W Q , Yin J G , et al . Kinetics of phosphorus removal from high-phosphorus iron ores by HCl leaching[J]. Advanced Materials Research, 2014, 868: 455-458. |
| 31 | Chi R A , Zhu G C , Tian J . Leaching kinetics of rare earth from black weathering mud with hydrochloric acid[J]. Transactions of Nonferrous Metals Society of China, 2000, 10(4): 531-533. |
| 32 | Demirkıran N , Künkül A . Dissolution kinetics of ulexite in perchloric acid solutions[J]. International Journal of Mineral Processing, 2007, 83(1/2): 76-80. |
| 33 | Deventer J S J V . Kinetics of metallurgical processes[J]. Minerals Engineering, 1999, 13(3): 329-330. |
| 34 | Ashraf M , Zafar Z I , Ansari T M . Selective leaching kinetics and upgrading of low-grade calcareous phosphate rock in succinic acid[J]. Hydrometallurgy, 2005, 80(4): 286-29. |
| 35 | Li Y , Kawashima N , Li J , et al . A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite[J]. Adv. Colloid Interface Sci., 2013, 197/198(9): 1-32. |
| 36 | Vardar E , Eric R H , Letowski F K . Acid leaching of chromite[J]. Minerals Engineering, 1994, 7(5/6): 605-617. |
| 37 | Geveci A , Topkaya Y , Ayhan E . Sulfuric acid leaching of Turkish chromite concentrate[J]. Minerals Engineering, 2002, 15(11): 885-888. |
| 38 | Jiang M F , Zhao Q , Liu C J , et al . Sulfuric acid leaching of South African chromite(Part 2): Optimization of leaching conditions[J]. International Journal of Mineral Processing, 2014, 130(4): 102-107. |
| 39 | 冷启超 . 铬铁矿硫酸浸出过程及废渣循环利用研究[D]. 沈阳: 东北大学, 2016. |
| Leng Q C . Study on sulfuric acid leaching process of chromite and waste residue recycling[D]. Shenyang: Northeastern University, 2016. | |
| 40 | Kittipongpatana O S , Kittipongpatana N . Preparation and physicomechanical properties of co-precipitated rice starch-colloidal silicon dioxide[J]. Powder Technology, 2012, 217: 377-382. |
| 41 | Mashifana T , Ntuli F , Okonta F . Leaching kinetics on the removal of phosphorus from waste phosphogypsum by application of shrinking core model[J]. South African Journal of Chemical Engineering, 2019, 27: 1-6. |
| [1] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [2] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [3] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [4] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [5] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [6] | 肖明堃, 杨光, 黄永华, 吴静怡. 浸没孔液氧气泡动力学数值研究[J]. 化工学报, 2023, 74(S1): 87-95. |
| [7] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [8] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [9] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [10] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [11] | 曾如宾, 沈中杰, 梁钦锋, 许建良, 代正华, 刘海峰. 基于分子动力学模拟的Fe2O3纳米颗粒烧结机制研究[J]. 化工学报, 2023, 74(8): 3353-3365. |
| [12] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [13] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [14] | 杨越, 张丹, 郑巨淦, 涂茂萍, 杨庆忠. NaCl水溶液喷射闪蒸-掺混蒸发的实验研究[J]. 化工学报, 2023, 74(8): 3279-3291. |
| [15] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号