化工学报 ›› 2020, Vol. 71 ›› Issue (2): 777-787.DOI: 10.11949/0438-1157.20190782
收稿日期:2019-07-09
修回日期:2019-12-05
出版日期:2020-02-05
发布日期:2020-02-05
通讯作者:
张扬
作者简介:李扬(1995—),男,硕士研究生,基金资助:
Yang LI( ),Yang ZHANG(
),Yang ZHANG( ),Xuanlong CHEN,Xun GONG
),Xuanlong CHEN,Xun GONG
Received:2019-07-09
Revised:2019-12-05
Online:2020-02-05
Published:2020-02-05
Contact:
Yang ZHANG
摘要:
钙基吸附剂循环CO2吸附性能对增强式生物质气化连续高效制氢起重要作用。采用将CaO颗粒分散在惰性载体中的方法并结合挤压成型技术制备了合成吸附剂颗粒。为了筛选循环吸附性能较好的吸附剂,在热重分析仪上进行了循环吸附性能测试。基于热重测试结果开展了吸附剂循环利用条件下的增强式生物质气化制氢实验。结果表明:添加惰性载体能延缓CaO烧结,提高吸附剂的循环吸附能力;挤压成型过程会破坏吸附剂原有孔隙结构,导致吸附剂颗粒吸附性能不同程度降低,其中CaSi75p、CaAl75p和CaY75p三种吸附剂循环性能较好;添加以上三种吸附剂颗粒均可显著提高生物质气化合成气中H2浓度及产率,5次循环过程中气体成分和产率变化不大,表明吸附剂循环吸附能力和稳定性较好。
中图分类号:
李扬, 张扬, 陈宣龙, 龚勋. 钙基吸附剂循环吸附性能对增强式生物质气化制氢的影响研究[J]. 化工学报, 2020, 71(2): 777-787.
Yang LI, Yang ZHANG, Xuanlong CHEN, Xun GONG. Effect of cyclic adsorption performance of calcium-based sorbent on enhanced biomass gasification for hydrogen production[J]. CIESC Journal, 2020, 71(2): 777-787.
| 钙基前体 | 载体前体 | 吸附剂粉末 | 吸附剂颗粒 |
|---|---|---|---|
| CaCO3 | — | CaO | CaOp |
| Ca(CH3COO)2?H2O | Mg(CH3COO)2·4H2O | CaMg75 | CaMg75p |
| Al(NO3)3·9H2O | CaAl75 | CaAl75p | |
| La(CH3COO)3·xH2O | CaLa75 | CaLa75p | |
| Y(CH3COO)3·4H2O | CaY75 | CaY75p | |
| Nd(CH3COO)3·xH2O | CaNd75 | CaNd75p | |
| C8H20O4Si | CaSi75 | CaSi75p |
表1 实验制备的吸附剂粉末及颗粒
Table 1 Adsorbent powders and particulates prepared in experiments
| 钙基前体 | 载体前体 | 吸附剂粉末 | 吸附剂颗粒 |
|---|---|---|---|
| CaCO3 | — | CaO | CaOp |
| Ca(CH3COO)2?H2O | Mg(CH3COO)2·4H2O | CaMg75 | CaMg75p |
| Al(NO3)3·9H2O | CaAl75 | CaAl75p | |
| La(CH3COO)3·xH2O | CaLa75 | CaLa75p | |
| Y(CH3COO)3·4H2O | CaY75 | CaY75p | |
| Nd(CH3COO)3·xH2O | CaNd75 | CaNd75p | |
| C8H20O4Si | CaSi75 | CaSi75p |
| 样品 | 工业分析/%(质量,空干基) | 元素分析/%(质量,空干基) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 水分 | 挥发分 | 灰分 | 固定碳 | 碳 | 氢 | 氮 | 硫 | 氧① | |
| 烟筋原样 | 7.30 | 66.32 | 16.93 | 9.45 | 33.52 | 5.00 | 2.46 | 0.77 | 34.02 |
表2 生物质样品基础特性分析
Table 2 Analysis of basic characteristics of biomass sample
| 样品 | 工业分析/%(质量,空干基) | 元素分析/%(质量,空干基) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 水分 | 挥发分 | 灰分 | 固定碳 | 碳 | 氢 | 氮 | 硫 | 氧① | |
| 烟筋原样 | 7.30 | 66.32 | 16.93 | 9.45 | 33.52 | 5.00 | 2.46 | 0.77 | 34.02 |
| 吸附剂粉末 | 比表面积/(m2/g) | 吸附剂颗粒 | 比表面积/(m2/g) |
|---|---|---|---|
| CaO | 10.49 | CaOp | 9.13 |
| CaMg75 | 18.56 | CaMg75p | 14.90 |
| CaAl75 | 12.14 | CaAl75p | 8.54 |
| CaLa75 | 10.83 | CaLa75p | 5.72 |
| CaY75 | 10.51 | CaY75p | 8.72 |
| CaNd75 | 8.81 | CaNd75p | 6.06 |
| CaSi75 | 22.06 | CaSi75p | 15.82 |
表3 合成CaO吸附剂比表面积
Table 3 Specific surface area of synthetic CaO adsorbents
| 吸附剂粉末 | 比表面积/(m2/g) | 吸附剂颗粒 | 比表面积/(m2/g) |
|---|---|---|---|
| CaO | 10.49 | CaOp | 9.13 |
| CaMg75 | 18.56 | CaMg75p | 14.90 |
| CaAl75 | 12.14 | CaAl75p | 8.54 |
| CaLa75 | 10.83 | CaLa75p | 5.72 |
| CaY75 | 10.51 | CaY75p | 8.72 |
| CaNd75 | 8.81 | CaNd75p | 6.06 |
| CaSi75 | 22.06 | CaSi75p | 15.82 |
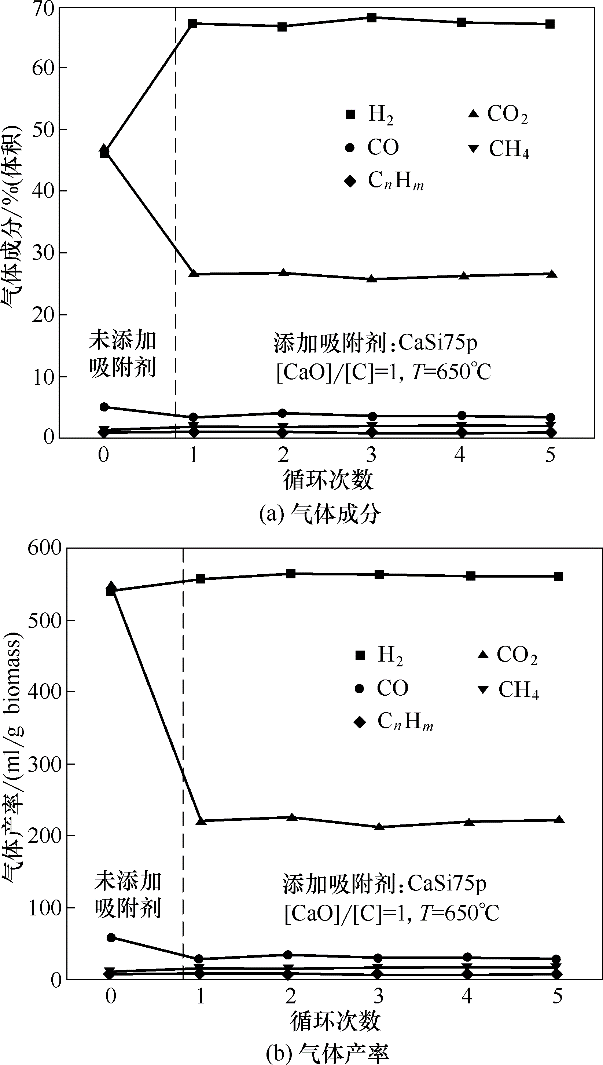
图6 添加CaSi75p在5次循环增强式生物质气化制氢中的气体产物成分及产率
Fig.6 Compositions and yields of gas products in process of 5 cycles enhanced biomass gasification for hydrogen production by adding CaSi75p
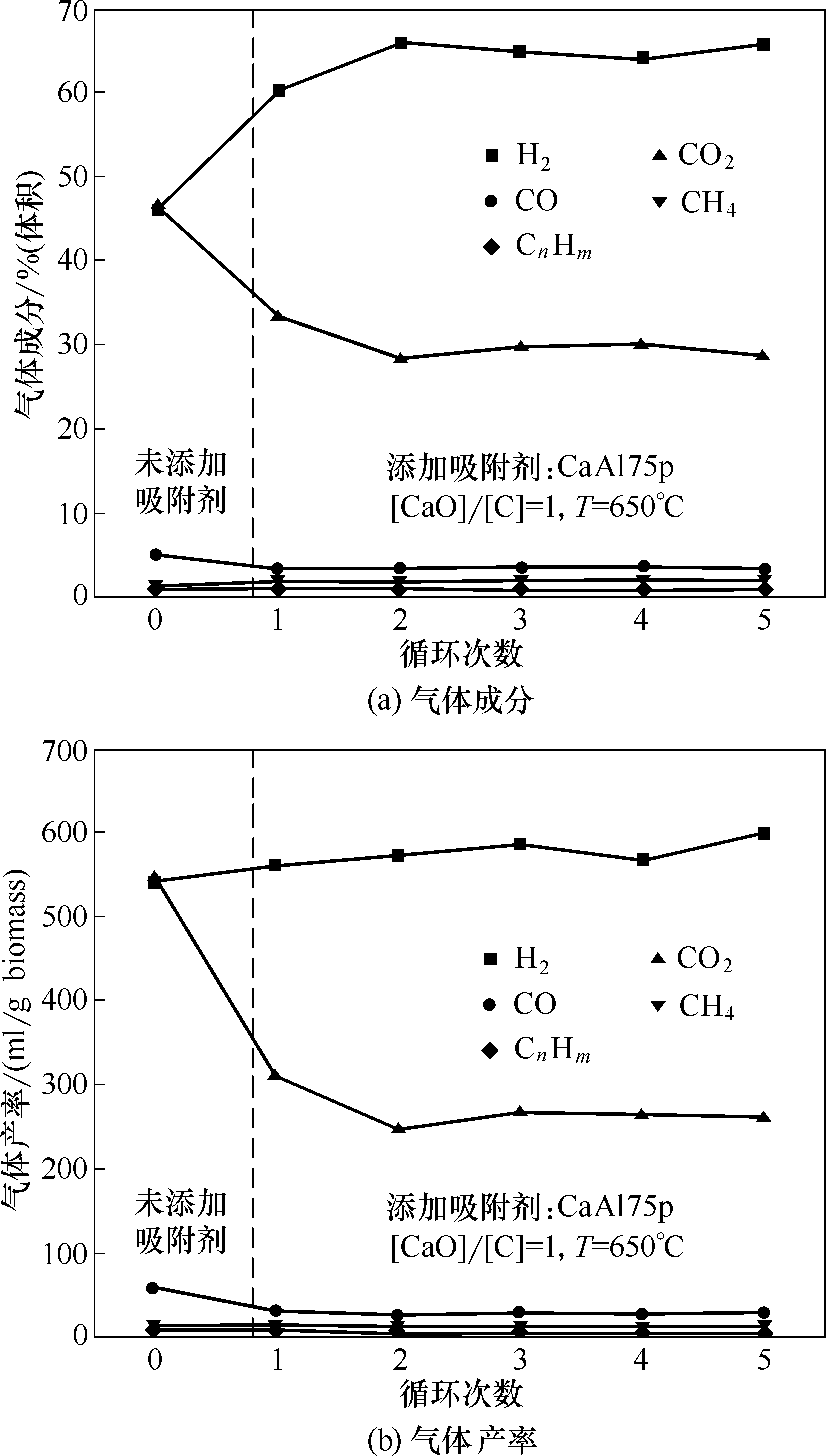
图7 添加CaAl75p在5次循环增强式生物质气化制氢中的气体产物成分及产率
Fig.7 Compositions and yields of gas products in process of 5 cycles enhanced biomass gasification for hydrogen production by adding CaAl75p
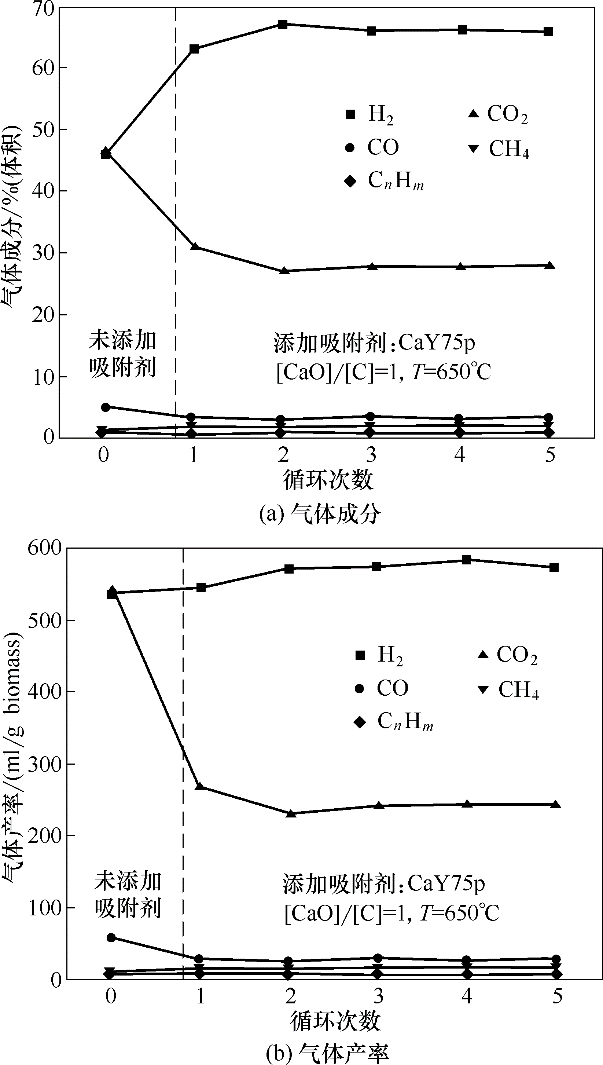
图8 添加CaY75p在5次循环增强式生物质气化制氢中的气体产物成分及产率
Fig.8 Compositions and yields of gas products in process of 5 cycles enhanced biomass gasification for hydrogen production by adding CaY75p
| 1 | Florin N H, Harris A T. Enhanced hydrogen production from biomass with in situ carbon dioxide capture using calcium oxide sorbents[J]. Chemical Engineering Science, 2008, 63(2): 287-316. |
| 2 | Zhang Y, Gong X, Zhang B, et al. Potassium catalytic hydrogen production in sorption enhanced gasification of biomass with steam[J]. International Journal of Hydrogen Energy, 2014, 39(9): 4234-4243. |
| 3 | Herce C, Stendardo S, Cortés C. Increasing CO2 carrying capacity of dolomite by means of thermal stabilization by triggered calcination[J]. Chemical Engineering Journal, 2015, 262: 18-28. |
| 4 | Kato Y, Harada N, Yoshizawa Y. Kinetic feasibility of a chemical heat pump for heat utilization of high-temperature processes[J]. Applied Thermal Engineering, 1999, 19(3): 239-254. |
| 5 | Grasa G, González, B, Alonso M, et al. Comparison of CaO-based synthetic CO2 sorbents under realistic calcination conditions[J]. Energy & Fuels, 2007, 21(6): 3560-3562. |
| 6 | Blamey J, Anthony E J, Wang J, et al. The calcium looping cycle for large-scale CO2 capture[J]. Progress in Energy & Combustion Science, 2010, 36(2): 260-279. |
| 7 | Liu W Q, An H, Qin C L, et al. Performance enhancement of calcium oxide sorbents for cyclic CO2 capture—a review[J]. Energy & Fuels, 2012, 26(5): 2751-2767. |
| 8 |
Barker R. The reversibility of the reaction CaCO3 CaO+CO2[J]. Journal of Applied Chemistry & Biotechnology, 1973, 23(10): 733–742. CaO+CO2[J]. Journal of Applied Chemistry & Biotechnology, 1973, 23(10): 733–742.
|
| 9 | Barker R. The reactivity of calcium oxide towards carbon dioxide and its use for energy storage[J]. Journal of Applied Chemistry & Biotechnology, 1974, 24(4/5): 221-227. |
| 10 | Wu S F, Zhu Y Q. Behavior of CaTiO3/nano-CaO as a CO2 reactive adsorbent[J]. Industrial & Engineering Chemistry Research, 2010, 49(6): 2701-2706. |
| 11 | Liu W Q, Feng B, Wu Y Q, et al. Synthesis of sintering-resistant sorbents for CO2 capture[J]. Environmental Science & Technology, 2010, 44(8): 3093-3097. |
| 12 | Silaban A, Narcida M, Harrison D P. Calcium acetate as a sorbent precursor for the removal of carbon dioxide from gas streams at high temperature[J]. Resources Conservation & Recycling, 1992, 7(1/2/3): 139-153. |
| 13 | Gupta H, Fan L S. Carbonation-calcination cycle using high reactivity calcium oxide for carbon dioxide separation from flue gas[J]. Industrial & Engineering Chemistry Research, 2002, 41(16): 4035-4042. |
| 14 | Florin N H, Harris A T. Preparation and characterization of a tailored carbon dioxide sorbent for enhanced hydrogen synthesis in biomass gasifiers[J]. Industrial & Engineering Chemistry Research, 2008, 47(7): 2191-2202. |
| 15 | Roesch A, Reddy E P, Smirniotis P G. Parametric study of Cs/CaO sorbents with respect to simulated flue gas at high temperatures[J]. Industrial & Engineering Chemistry Research, 2005, 44(16): 6485-6490. |
| 16 | Li Z S, Cai N S, Huang Y Y, et al. Synthesis, experimental studies, and analysis of a new calcium-based carbon dioxide absorbent[J]. Energy & Fuels, 2005, 19(4): 1447-1452. |
| 17 | Ma X T, Li Y J, Shi L, et al. Fabrication and CO2 capture performance of magnesia-stabilized carbide slag by by-product of biodiesel during calcium looping process[J]. Applied Energy, 2016, 168: 85-95. |
| 18 | Qin C L, Yin J J, An H, et al. Performance of extruded particles from calcium hydroxide and cement for CO2 capture[J]. Energy & Fuels, 2011, 26(1): 154-161. |
| 19 | Manovic V, Wu Y H, He I, et al. Spray water reactivation/pelletization of spent CaO-based sorbent from calcium looping cycles[J]. Environmental Science & Technology, 2012, 46(22): 12720-12725. |
| 20 | Sun J, Liu W Q, Hu Y C, et al. Enhanced performance of extruded-spheronized carbide slag pellets for high temperature CO2 capture[J]. Chemical Engineering Journal, 2016, 285: 293-303. |
| 21 | Acharya B, Dutta A, Basu P. An investigation into steam gasification of biomass for hydrogen enriched gas production in presence of CaO[J]. International Journal of Hydrogen Energy, 2010, 35(4): 1582-1589. |
| 22 | Mahishi M R, Goswami D Y. An experimental study of hydrogen production by gasification of biomass in the presence of a CO2 sorbent[J]. International Journal of Hydrogen Energy, 2007, 32(14): 2803-2808. |
| 23 | Han L, Wang Q H, Yang Y K, et al. Hydrogen production via CaO sorption enhanced anaerobic gasification of sawdust in a bubbling fluidized bed[J]. International Journal of Hydrogen Energy, 2011, 36(8): 4820-4829. |
| 24 | Florin N H, Harris A T. Hydrogen production from biomass coupled with carbon dioxide capture: The implications of thermodynamic equilibrium[J]. International Journal of Hydrogen Energy, 2007, 32(17): 4119-4134. |
| 25 | Broda M, Manovic V, Imtiaz Q, et al. High-purity hydrogen via the sorption-enhanced steam methane reforming reaction over a synthetic CaO-based sorbent and a Ni catalyst[J]. Environmental Science & Technology, 2013, 47(11): 6007-6014. |
| 26 | Dang C X, Yu H, Wang H J, et al. A bi-functional Co-CaO-Ca12Al14O33 catalyst for sorption-enhanced steam reforming of glycerol to high-purity hydrogen[J]. Chemical Engineering Journal, 2016, 286: 329-338. |
| 27 | Müller C R, Pacciani R, Bohn C D, et al. Investigation of the enhanced water gas shift reaction using natural and synthetic sorbents for the capture of CO2[J]. Industrial & Engineering Chemistry Research, 2009, 48(23): 10284-10291. |
| 28 | Zhang Y, Gong X, Cheng X L, et al. Performance of synthetic CaO-based sorbent pellets for CO2 capture and kinetic analysis[J]. Fuel, 2018, 232: 205-214. |
| 29 | Broda M, Manovic V, Anthony E J, et al. Effect of pelletization and addition of steam on the cyclic performance of carbon-templated, CaO-based CO2 sorbents[J]. Environmental Science & Technology, 2014, 48(9): 5322-5328. |
| 30 | Zhao M, Shi J, Zhong X, et al. A novel calcium looping absorbent incorporated with polymorphic spacers for hydrogen production and CO2 capture[J]. Energy & Environmental Science, 2014, 7(10): 3291-3295. |
| 31 | Udomsirichakorn J, Basu P, Salam P A, et al. CaO-based chemical looping gasification of biomass for hydrogen-enriched gas production with in situ CO2, capture and tar reduction[J]. Fuel Processing Technology, 2014, 127(11): 7-12. |
| 32 | Li B, Yang H P, Wei L Y, et al. Hydrogen production from agricultural biomass wastes gasification in a fluidized bed with calcium oxide enhancing[J]. International Journal of Hydrogen Energy, 2017, 42(8): 4832-4839. |
| 33 | Wei L Y, Yang H P, Li B, et al. Absorption-enhanced steam gasification of biomass for hydrogen production: effect of calcium oxide addition on steam gasification of pyrolytic volatiles[J].International Journal of Hydrogen Energy, 2014, 39(28): 15416-15423. |
| [1] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [2] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 韩晨, 司徒友珉, 朱斌, 许建良, 郭晓镭, 刘海峰. 协同处理废液的多喷嘴粉煤气化炉内反应流动研究[J]. 化工学报, 2023, 74(8): 3266-3278. |
| [5] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [6] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [7] | 郑玉圆, 葛志伟, 韩翔宇, 王亮, 陈海生. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192. |
| [8] | 吴文涛, 褚良永, 张玲洁, 谭伟民, 沈丽明, 暴宁钟. 腰果酚生物基自愈合微胶囊的高效制备工艺研究[J]. 化工学报, 2023, 74(7): 3103-3115. |
| [9] | 杨峥豪, 何臻, 常玉龙, 靳紫恒, 江霞. 生物质快速热解下行式流化床反应器研究进展[J]. 化工学报, 2023, 74(6): 2249-2263. |
| [10] | 董茂林, 陈李栋, 黄六莲, 吴伟兵, 戴红旗, 卞辉洋. 酸性助水溶剂制备木质纳米纤维素及功能应用研究进展[J]. 化工学报, 2023, 74(6): 2281-2295. |
| [11] | 周必茂, 许世森, 王肖肖, 刘刚, 李小宇, 任永强, 谭厚章. 烧嘴偏转角度对气化炉渣层分布特性的影响[J]. 化工学报, 2023, 74(5): 1939-1949. |
| [12] | 葛泽峰, 吴雨青, 曾名迅, 查振婷, 马宇娜, 侯增辉, 张会岩. 灰化学成分对生物质气化特性的影响规律[J]. 化工学报, 2023, 74(5): 2136-2146. |
| [13] | 肖川宝, 李林洋, 刘武锋, 钟年丙, 解泉华, 钟登杰, 常海星. 光催化与离子交换吸附耦合有效去除2,4,6-三氯苯酚[J]. 化工学报, 2023, 74(4): 1587-1597. |
| [14] | 祖凌鑫, 胡荣庭, 李鑫, 陈余道, 陈广林. 木质生物质化学组分的碳释放产物特征和反硝化利用程度[J]. 化工学报, 2023, 74(3): 1332-1342. |
| [15] | 刘海芹, 李博文, 凌喆, 刘亮, 俞娟, 范一民, 勇强. 羟基-炔点击化学改性半乳甘露聚糖薄膜的制备及性能研究[J]. 化工学报, 2023, 74(3): 1370-1378. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号