化工学报 ›› 2020, Vol. 71 ›› Issue (4): 1618-1626.DOI: 10.11949/0438-1157.20191332
刘帅1( ),李学雷2,王烁天1,李旭贺1,王彦娟1,苑兴洲1,张健1(
),李学雷2,王烁天1,李旭贺1,王彦娟1,苑兴洲1,张健1( ),封瑞江1
),封瑞江1
收稿日期:2019-11-05
修回日期:2020-01-21
出版日期:2020-04-05
发布日期:2020-04-05
通讯作者:
张健
作者简介:刘帅(1996—),男,硕士研究生,基金资助:
Shuai LIU1( ),Xuelei LI2,Shuotian WANG1,Xuhe LI1,Yanjuan WANG1,Xingzhou YUAN1,Jian ZHANG1(
),Xuelei LI2,Shuotian WANG1,Xuhe LI1,Yanjuan WANG1,Xingzhou YUAN1,Jian ZHANG1( ),Ruijiang FENG1
),Ruijiang FENG1
Received:2019-11-05
Revised:2020-01-21
Online:2020-04-05
Published:2020-04-05
Contact:
Jian ZHANG
摘要:
以钨酸铵、六水硝酸铈、尿素为原料,采用熔融法制备CeO2-WO3/g-C3N4催化剂,并对样品进行XRD、UV-Vis、TEM、PL和XPS表征。结果表明:CeO2的引入可以提高WO3在g-C3N4上的分散度,抑制光生电子空穴对的复合,同时Ce4+/Ce3+良好的储氧放氧能力有利于氧空位和活性氧的生成,从而有利于WO3/g-C3N4催化剂的催化性能。在以高压钠灯模拟可见光源的条件下,以过氧化羟基异丙苯为氧化剂,考察了CeO2的加入量对催化剂氧化二苯并噻吩(DBT)性能的影响,结果表明:最佳的CeO2引入量为5%(质量分数),在80℃、氧硫摩尔比(O/S)为5.0的反应条件下,反应180 min时DBT在WO3/g-C3N4和CeO2-WO3/g-C3N4催化剂的作用下转化率分别为72.9%和86.4%,且改性后的催化剂可以循环使用8次而催化活性没有明显降低。
中图分类号:
刘帅, 李学雷, 王烁天, 李旭贺, 王彦娟, 苑兴洲, 张健, 封瑞江. CeO2改性WO3/g-C3N4光催化氧化脱硫性能[J]. 化工学报, 2020, 71(4): 1618-1626.
Shuai LIU, Xuelei LI, Shuotian WANG, Xuhe LI, Yanjuan WANG, Xingzhou YUAN, Jian ZHANG, Ruijiang FENG. WO3/g-C3N4 modified by CeO2 and its oxidation and desulfurization properties[J]. CIESC Journal, 2020, 71(4): 1618-1626.

图1 g-C3N4、WO3、CeO2、WO3/g-C3N4和CeO2-WO3/g-C3N4样品的XRD谱图
Fig.1 XRD patterns of g-C3N4,WO3,CeO2,WO3/g-C3N4 and CeO2-WO3/g-C3N4a—g-C3N4; b—WO3; c—CeO2; d—WO3(20% mass fraction)/g-C3N4; e—CeO2(2% mass fraction)-WO3(20% mass fraction)/g-C3N4; f—CeO2(5% mass fraction)-WO3(20% mass fraction)/g-C3N4; g—CeO2(8% mass fraction)-WO3(20% mass fraction)/g-C3N4; h—CeO2(10% mass fraction)-WO3(20% mass fraction)/g-C3N4
| 催化剂 | WO3结晶 度/% | WO3平均晶粒尺寸 / nm |
|---|---|---|
| WO3(20%质量分数)/g-C3N4 | 76.39 | 66.3 |
| CeO2(2%质量分数)-WO3(20%质量分数)/g-C3N4 | 46.92 | 46.4 |
| CeO2(5%质量分数)-WO3(20%质量分数)/g-C3N4 | 20.63 | 30.3 |
| CeO2(8%质量分数)-WO3(20%质量分数)/g-C3N4 | 10.49 | 27.5 |
| CeO2(10%质量分数)-WO3(20%质量分数)/g-C3N4 | 2.71 | 18.6 |
表1 催化剂WO3组分晶粒数据
Table 1 Crystal data of WO3 components of catalyst
| 催化剂 | WO3结晶 度/% | WO3平均晶粒尺寸 / nm |
|---|---|---|
| WO3(20%质量分数)/g-C3N4 | 76.39 | 66.3 |
| CeO2(2%质量分数)-WO3(20%质量分数)/g-C3N4 | 46.92 | 46.4 |
| CeO2(5%质量分数)-WO3(20%质量分数)/g-C3N4 | 20.63 | 30.3 |
| CeO2(8%质量分数)-WO3(20%质量分数)/g-C3N4 | 10.49 | 27.5 |
| CeO2(10%质量分数)-WO3(20%质量分数)/g-C3N4 | 2.71 | 18.6 |
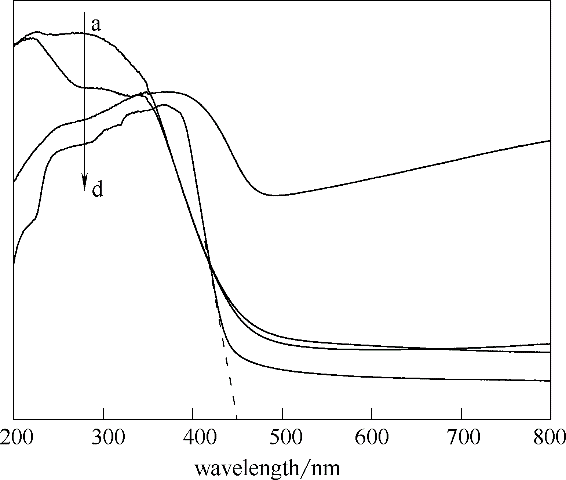
图3 g-C3N4、WO3、WO3/g-C3N4和CeO2-WO3/g-C3N4样品的UV-Vis谱图
Fig.3 UV-Vis spectra of g-C3N4,WO3,WO3/g-C3N4 and CeO2-WO3/g-C3N4a—WO3/g-C3N4; b—CeO2-WO3/g-C3N4; c—WO3; d—g-C3N4
| 催化剂 | Oβ/(Oα+Oβ) 浓度比/% | Oβ/Oα浓度比/% | Ce3+/(Ce3++Ce4+)摩尔比/% |
|---|---|---|---|
| CeO2 | 5.67 | 6.3 | 42.11 |
| WO3/g-C3N4 | 14.09 | 16.4 | — |
| CeO2-WO3/g-C3N4 | 54.49 | 84.1 | 63.78 |
表2 催化剂XPS峰面积数据
Table 2 XPS peak area data of catalyst
| 催化剂 | Oβ/(Oα+Oβ) 浓度比/% | Oβ/Oα浓度比/% | Ce3+/(Ce3++Ce4+)摩尔比/% |
|---|---|---|---|
| CeO2 | 5.67 | 6.3 | 42.11 |
| WO3/g-C3N4 | 14.09 | 16.4 | — |
| CeO2-WO3/g-C3N4 | 54.49 | 84.1 | 63.78 |
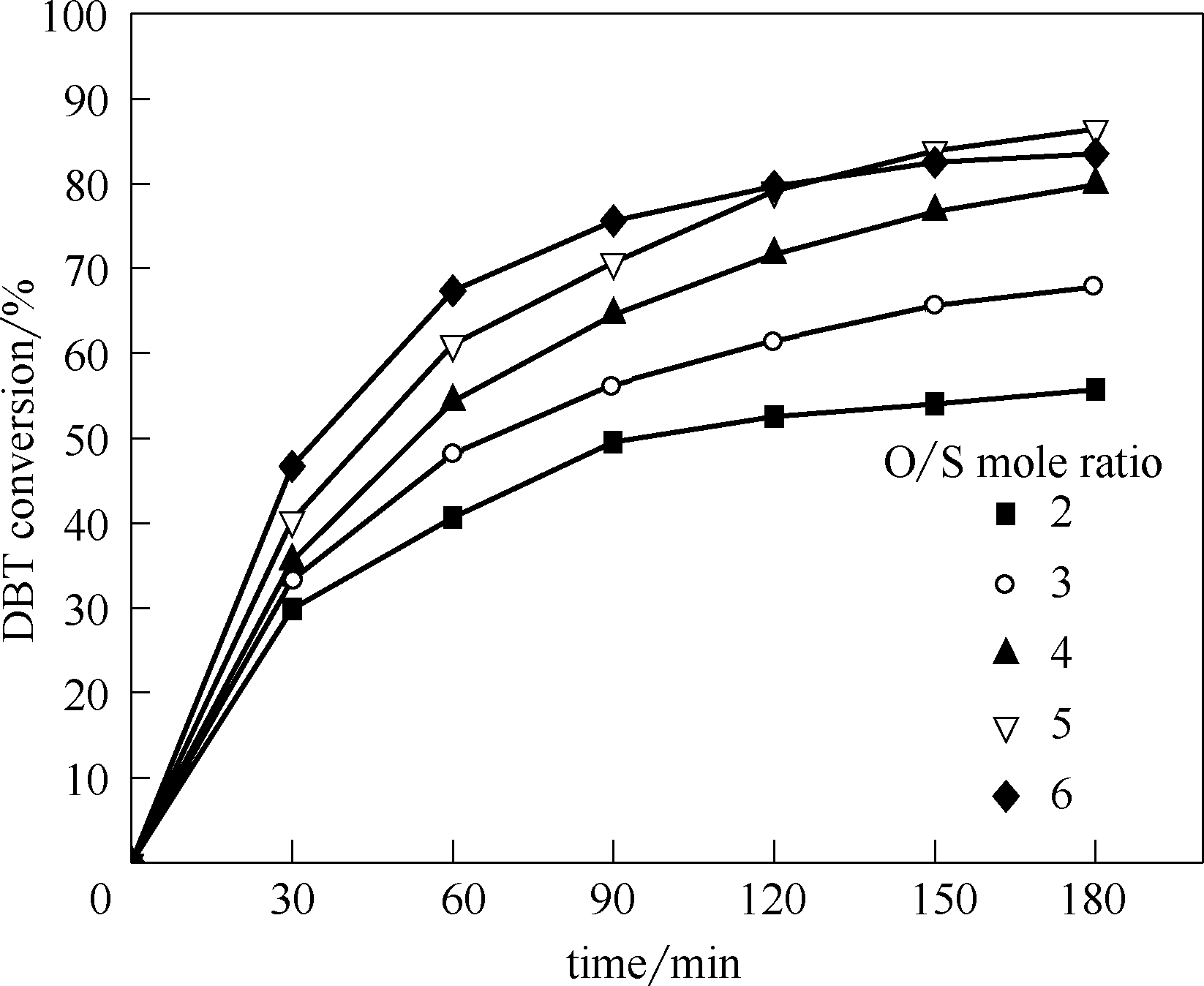
图9 氧硫摩尔比对DBT在反应体系中的影响
Fig.9 Influence of oxygen-sulfur mole ratio on DBT in reaction system(reaction conditions: 80℃, 180 min, payload of CeO2 5% (mass))

图10 CeO2(5%质量分数)-WO3(20%质量分数)/g-C3N4催化剂的循环使用性能
Fig.10 Catalytic performances of fresh and reused CeO2(5%,mass fraction)-WO3(20% ,mass fraction)/g-C3N4(reaction conditions: 80℃, O/S=5, 180 min, payload of CeO2 5% (mass))
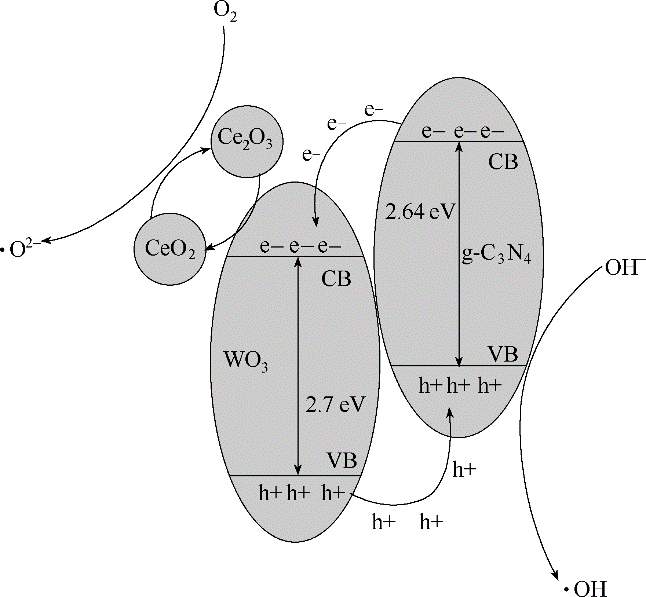
图11 CeO2(5%质量分数)-WO3(20%质量分数)/g-C3N4催化剂光催化机理示意图
Fig.11 Schematic diagram of photocatalytic mechanism of CeO2(5% mass fraction)-WO3(20% mass fraction)/g-C3N4 catalyst
| 1 | Hong Y Z, Jiang Y H, Li C S, et al. In-situ synthesis of direct solid-state Z-scheme V2O5/g-C3N4 heterojunctions with enhanced visible light efficiency in photocatalytic degradation of pollutants[J]. Appl. Catal. B: Environ. , 2016, 180: 663-673. |
| 2 | Katsumata H, Sakai T, Suzuki T, et al. Highly efficient photocatalytic activity of g-C3N4/Ag3PO4 hybrid photocatalysts through Z-scheme photocatalytic mechanism under visible light[J]. Ind. Eng. Chem. Res. , 2014, 53(19): 8018-8025. |
| 3 | Yan S C, Li Z S, Zou Z G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine[J]. Langmuir, 2009, 25(17): 10397-10401. |
| 4 | Miao X L, Shen X P, Wu J J, et al. Fabrication of an all solid Z-scheme photocatalyst g-C3N4/GO/AgBr with enhanced visible light photocatalystic activity[J]. Appl. Catal. A-Gen. , 2017, 539: 104-113. |
| 5 | Huang L Y, Xu H, Li Y P, et al. Visible-light-induced WO3/g-C3N4 composites with enhanced photocatalystic activity[J]. Dalton Trans. , 2013, 42(24): 8606-8616. |
| 6 | Zhao Z G, Miyauchi M. Nanoporous-walled tungsten oxide nanotubes as highly active visible-light-driven photocatalysts[J]. Angew. Chem.-Int. Edit. , 2008, 47(37): 7051-7055. |
| 7 | Aslam I, Cao C, Tanveer M, et al. The synergistic effect between WO3 and g-C3N4 towards efficient visible-light-driven photocatalytic performance[J]. New. J. Chem. , 2014, 38(11): 5462-5469. |
| 8 | Wang F, Li W, Feng X, et al. Decoration of Pt on Cu/Co double-doped CeO2 nanospheres and their greatly enhanced catalytic activity[J]. Chem. Sci. , 2016, 7(3): 1867-1873. |
| 9 | 曹国强. 助剂修饰半导体光催化材料的制备及其研究性能[D]. 武汉: 武汉理工大学, 2015. |
| Cao G Q. Preparation and properties of semiconductor photocatalytic materials modified by auxiliaries[D]. Wuhan: Wuhan University of Technology, 2015. | |
| 10 | Li R G, Han H X, Zhang F X, et al. Highly efficient photocatalysts constructed by rational assembly of dual-cocatalysts separately on different facets of BiVO4[J]. Energy. Sci. , 2014, 7(4): 1369-1376. |
| 11 | 刘锐. 银修饰型纳米复合材料的制备、表征与可见光光催化性能[D]. 武汉: 武汉理工大学, 2013. |
| Liu R. Preparation, characterization and photocatalytic performance of silver modified nanocomposites[D]. Wuhan: Wuhan University of Technology, 2013. | |
| 12 | 刘帅, 刘进博, 李旭贺, 等. WO3/g-C3N4异质结催化剂的制备及其氧化脱硫性能[J]. 燃料化学学报, 2019, 47(7): 852-862. |
| Liu S, Liu J B, Li X H, et al. Preparation of WO3/g-C3N4 heterojunction catalyst and its oxidation and desulfurization performance[J]. J. Fuel. Chem. Techno. , 2019, 47(7): 852-862. | |
| 13 | 桂明生, 王鹏飞, 袁东, 等. Bi2WO6/g-C3N4复合型催化剂的制备及其可见光光催化性[J]. 无机化学学报, 2013, 29(10): 2057-2064. |
| Gui M S, Wang P F, Yuan D, et al. Preparation of Bi2WO6/g-C3N4 composite catalyst and its visible photocatalytic performance[J]. J. Inorg. Chem. , 2013, 29(10): 2057-2064. | |
| 14 | Amoozadeh A, Rahmani S. Nano-WO3-supported sulfonic acid: new efficient and high reusable heterogeneous nanocatalyst[J]. J. Mol. Catal. A: Chem. , 2015, 396: 96-107. |
| 15 | Cai W D, Chen F, Shen X X, et al. Enhanced catalytic degradation of AO7 in the CeO2-H2O2 system with Fe3+ doping[J]. Appl. Catal. B: Environ. , 2010, 101(1/2): 160-168. |
| 16 | Lei W W, Portenhault D, Dimova R, et al. Boron carbon nitride nanostructures from salt melts: tunable water-soluble phosphors[J]. J. Chem. Soc. , 2011, 133(18): 5300-5303. |
| 17 | 王丽, 赵辉. WO3/N-TiO2异质节可见光催化降解亚甲基蓝[J]. 工业水处理, 2016, 36(11): 78-81. |
| Wang L, Zhao H. Visible light catalytic degradation of methylene blue by WO3/N-TiO2 heterogeneous[J]. Ind. Water Treat., 2016, 36(11): 78-81. | |
| 18 | Claude B, Ahmed B, Jearr C. A spectroscopic characterization of the reduction of ceria from electronic transitions of intrinsic point defects [J]. J. Phys. Chem. , 1994, 98(25): 6392-6398. |
| 19 | 张启涛. 高效g-C3N4基纳米复合异质结光催化剂的制备及其光催化性能的研究[D]. 扬州: 扬州大学, 2017. |
| Zhang Q T. Preparation and photocatalytic performance of g-C3N4-based nanocomposite heterojunction photocatalyst[D]. Yangzhou: Yangzhou University, 2017. | |
| 20 | Katsumata H, Tachi Y, Suzuki T, et al. Z-scheme photocatalytic hydrogen production over WO3/g-C3N4 composite photocatalysts[J]. RSC. Adv. , 2014, 4(41): 21405-21409. |
| 21 | Luo J, Zhou X S, Ma L, et al. Enhancing visible-light photocatalytic activity of g-C3N4 by doping phosphorus and coupling with CeO2 for the degradation of methyl orange under visible light irradiation[J]. RSC Adv. , 2015, 5(84): 68728-68735. |
| 22 | Li C H, Li K Z, Wang H, et al. Soot combustion over Ce1-xFexO2-δand CeO2/Fe2O3 catalysts: roles of solid solution and interfacial interactions in the mixed oxides[J]. Appl. Surf. Sci. , 2016, 390: 513-525. |
| 23 | Li H, Wang G F, Zhang F, et al. Surfactant-assisted synthesis of CeO2 nanoparticles and their application in wastewater treatment[J]. RSC Adv. , 2012, 2(32): 12413-12423. |
| 24 | 王青春. 纳米氧化铈/碳复合载体的制备、结构及催化性能研究[D]. 北京: 北京科技大学, 2016. |
| Wang Q C. Preparation, structure and catalytic performance of nanometer cerium oxide/carbon composite carrier[D]. Beijing: University of Science and Technology Beijing, 2016. | |
| 25 | Zhang R, Zhong Q, Zhao W L, et al. Promotional effect of fluorine on the selective catalytic reduction of NO with NH3 over CeO2-TiO2 catalyst at low temperature[J]. Appl. Surf. Sci. , 2014, 289: 237-244. |
| 26 | Chen L, Li J H, Ge M F. Promotional effect of Ce-doped V2O5-WO3/TiO2 with low vanadium loadings for selective catalytic reduction of NOx by NH3[J]. J. Phys. Chem. C, 2009, 113(50): 21177-21184. |
| 27 | Shan W P, Liu F D, He H, et al. A superior Ce-W-Ti mixed oxide catalyst for the selective catalytic reduction of NOx with NH3[J]. Appl. Catal. B: Environ. , 2012, 115/116: 100-106. |
| 28 | Zeng L P, Li K Z, Wang H, et al. CO oxidation on Au / α-Fe2O3-hollow catalysts: general synthesis and structural dependence[J]. J. Phys. Chem. C, 2017, 121(23): 12696-12710. |
| 29 | 牛微, 王刚, 董颖男, 等. Ce掺杂六方相WO3光催化剂的制备及其光解水制氢性能[J]. 人工晶体学报, 2016, 45(1): 187-191. |
| Niu W, Wang G, Dong Y N, et al. Preparation of Ce doped hexagonal-phase WO3 photocatalyst and its photolysis performance for hydrogen production[J]. J. Synth. Cryst. , 2016, 45(1): 187-191. | |
| 30 | Senanayake S D, Rodriguez J A, Stacchiola D. Electronic metal-support interactions and the production of hydrogen through the water-gas shift reaction and ethanol steam reforming: fundamental studies with well-defined model catalysts[J]. Top. Catal. , 2013, 56(15): 1488-1498. |
| 31 | Xiao X, Zhong H, Zheng C X, et al. Deep oxidative desulfurization of dibenzothiophene using a flower-like WO3·H2O catalyst in an organic biphasic system[J]. Chem. Eng. J. , 2016, 304: 908-916. |
| 32 | 卞振锋, 阮大明, 李和兴. Pt负载对TiO2光催化氧化还原的影响[J]. 中国科技论文, 2016, 11(18): 2091-2095. |
| Bian Z F, Ruan D M, Li H X. Effect of Pt loading on photocatalytic REDOX of TiO2[J]. Chin. J. Sci. Technol. , 2016, 11(18): 2091-2095. |
| [1] | 连梦雅, 谈莹莹, 王林, 陈枫, 曹艺飞. 地下水预热新风一体化热泵空调系统制热性能研究[J]. 化工学报, 2023, 74(S1): 311-319. |
| [2] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [3] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [4] | 邵苛苛, 宋孟杰, 江正勇, 张旋, 张龙, 高润淼, 甄泽康. 水平方向上冰中受陷气泡形成和分布实验研究[J]. 化工学报, 2023, 74(S1): 161-164. |
| [5] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [6] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [7] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [8] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [9] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [10] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [11] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [12] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [13] | 王浩, 王振雷. 基于自适应谱方法的裂解炉烧焦模型化简策略[J]. 化工学报, 2023, 74(9): 3855-3864. |
| [14] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [15] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号