化工学报 ›› 2020, Vol. 71 ›› Issue (4): 1609-1617.DOI: 10.11949/0438-1157.20191177
朱林1( ),韩威1,李文松1,邬长城2,李芳1,2,薛伟1,2(
),韩威1,李文松1,邬长城2,李芳1,2,薛伟1,2( ),王延吉1,2
),王延吉1,2
收稿日期:2019-10-11
修回日期:2020-01-09
出版日期:2020-04-05
发布日期:2020-04-05
通讯作者:
薛伟
作者简介:朱林(1996—),女,硕士研究生, 基金资助:
Lin ZHU1( ),Wei HAN1,Wensong LI1,Changcheng WU2,Fang LI1,2,Wei XUE1,2(
),Wei HAN1,Wensong LI1,Changcheng WU2,Fang LI1,2,Wei XUE1,2( ),Yanji WANG1,2
),Yanji WANG1,2
Received:2019-10-11
Revised:2020-01-09
Online:2020-04-05
Published:2020-04-05
Contact:
Wei XUE
摘要:
为提高HZSM-5催化乙酸环己酯水解反应生成环己醇的选择性,筛选得到了离子液体[BMIm]Br助剂。发现[BMIm]Br可抑制乙酸环己酯热分解副反应的发生,大幅度提高环己醇选择性。并且,[BMIm]Br可与HZSM-5发生离子交换反应,释放出的H+可促进乙酸环己酯水解反应的发生。在优化的条件下,[BMIm]Br辅助HZSM-5催化乙酸环己酯水解反应转化率为87.5%,环己醇选择性为97.3%。考察了[BMIm]Br和HZSM-5的重复使用性。前者具有较好的稳定性,而HZSM-5活性随循环使用的次数增加而下降。这是由于HZSM-5的骨架铝含量在反应中逐渐降低,导致Br?nsted酸中心数量减少,因此其催化活性逐渐降低。
中图分类号:
朱林, 韩威, 李文松, 邬长城, 李芳, 薛伟, 王延吉. [BMIm]Br离子液体辅助HZSM-5催化乙酸环己酯高选择性水解反应[J]. 化工学报, 2020, 71(4): 1609-1617.
Lin ZHU, Wei HAN, Wensong LI, Changcheng WU, Fang LI, Wei XUE, Yanji WANG. Highly selective hydrolyzation of cyclohexyl acetate over HZSM-5 assisted by [BMIm]Br ionic liquid[J]. CIESC Journal, 2020, 71(4): 1609-1617.
| No. | Catalyst | Co-catalyst① | Cyclohexyl acetate conversion /% | Cyclohexanol selectivity/% |
|---|---|---|---|---|
| 1 | — | — | 1.0 | 32.0 |
| 2 | HZSM-5 | — | 56.9 | 27.9 |
| 3 | HZSM-5 | [BMIm]BF4 | 35.4 | 99.7 |
| 4 | HZSM-5 | [BMIm]Br | 74.8 | 98.4 |
| 5 | — | [BMIm]BF4 | 2.6 | 100 |
| 6 | — | [BMIm]Br | 1.3 | 100 |
| 7 | HZSM-5 | [HSO3BMIm]HSO4 [ | 81.6 | 79.7 |
| 8 | HZSM-5 | NaBr [ | 56.1 | 48.0 |
| 9 | HZSM-5 | ZnSO4 [ | 4.1 | 75.3 |
| 10 | HZSM-5 | NaHSO4 [ | 67.7 | 42.8 |
表1 不同助剂对HZSM-5催化乙酸环己酯水解反应的影响
Table 1 Effect of different co-catalyts on hydrolyzation of cyclohexyl acetate over HZSM-5 catalyst
| No. | Catalyst | Co-catalyst① | Cyclohexyl acetate conversion /% | Cyclohexanol selectivity/% |
|---|---|---|---|---|
| 1 | — | — | 1.0 | 32.0 |
| 2 | HZSM-5 | — | 56.9 | 27.9 |
| 3 | HZSM-5 | [BMIm]BF4 | 35.4 | 99.7 |
| 4 | HZSM-5 | [BMIm]Br | 74.8 | 98.4 |
| 5 | — | [BMIm]BF4 | 2.6 | 100 |
| 6 | — | [BMIm]Br | 1.3 | 100 |
| 7 | HZSM-5 | [HSO3BMIm]HSO4 [ | 81.6 | 79.7 |
| 8 | HZSM-5 | NaBr [ | 56.1 | 48.0 |
| 9 | HZSM-5 | ZnSO4 [ | 4.1 | 75.3 |
| 10 | HZSM-5 | NaHSO4 [ | 67.7 | 42.8 |

图2 反应温度对HZSM-5/[BMIm]Br催化乙酸环己酯水解反应的影响
Fig.2 Effect of reaction temperature on hydrolyzation of cyclohexyl acetate over HZSM-5/[BMIm]Br(m(HZSM-5)=1.2 g,V(cyclohexyl acetate)=8 ml (55 mmol), V(H2O)=24 ml (1333 mmol), m([BMIm]Br)=2 g,t= 5 h)
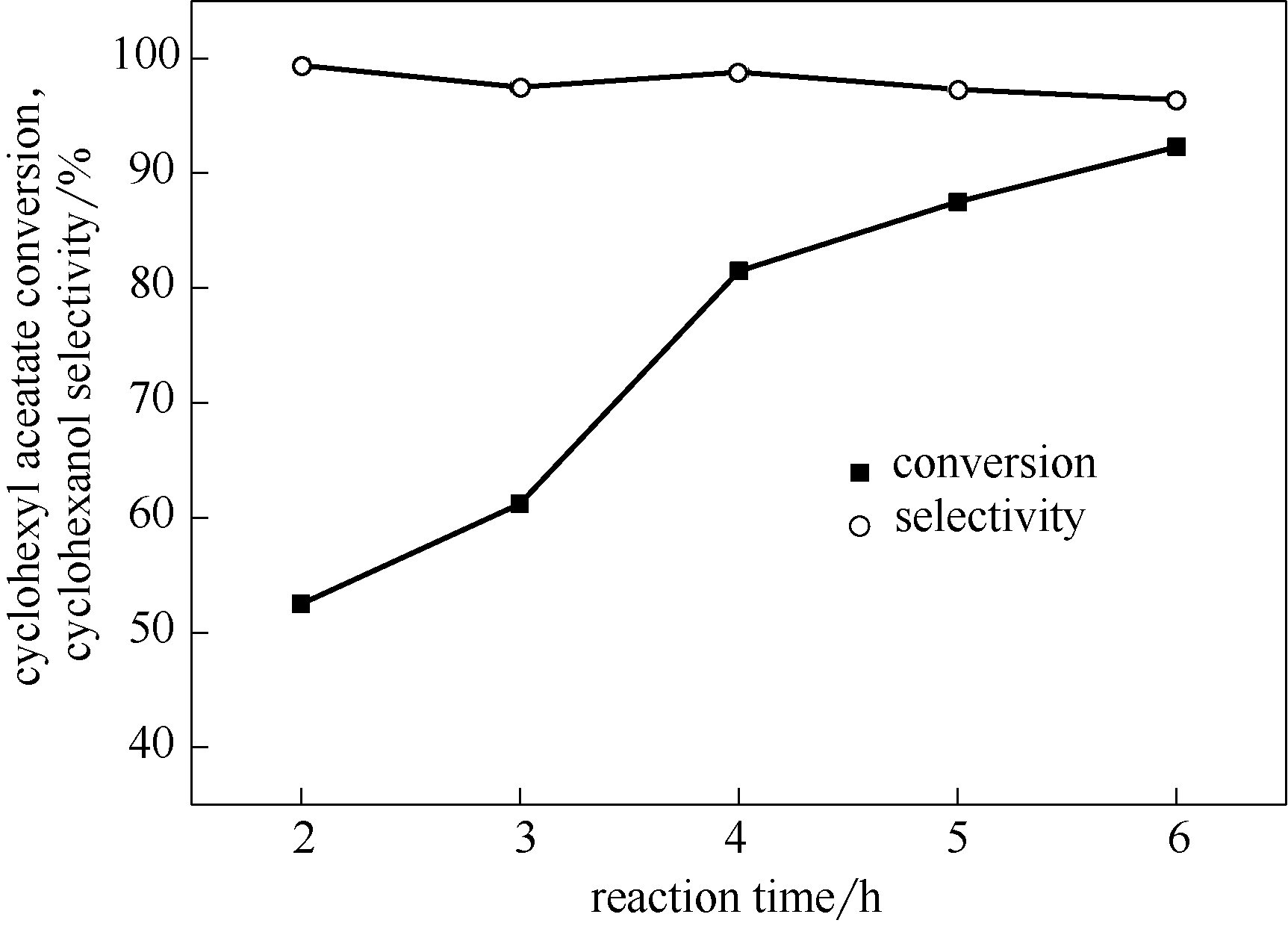
图3 反应时间对HZSM-5/[BMIm]Br催化乙酸环己酯水解反应的影响
Fig.3 Effect of reaction time on hydrolyzation of cyclohexyl acetate over HZSM-5/[BMIm]Br (m(HZSM-5)=1.2 g, V(cyclohexyl acetate)=8 ml (55 mmol),V(H2O)=24 ml (1333 mmol),m([BMIm]Br)=2 g,T= 140℃)
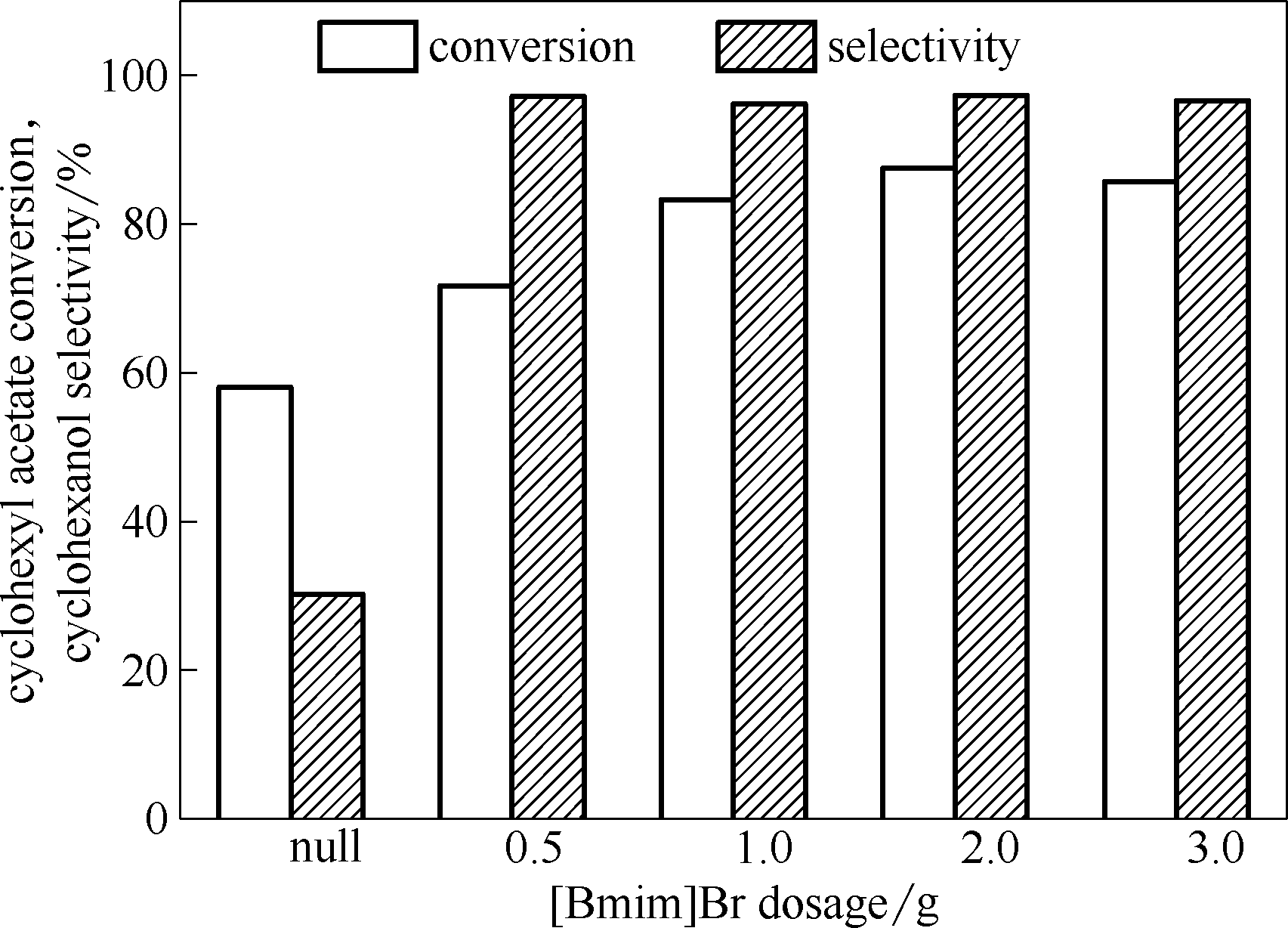
图4 [BMIm]Br用量对HZSM-5催化乙酸环己酯水解反应的影响
Fig.4 Effect of [BMIm]Br dosage on hydrolyzation of cyclohexyl acetate over HZSM-5 catalyst(m(HZSM-5)=1.2 g, V(cyclohexyl acetate)=8 ml (55 mmol),V(H2O)=24 ml (1333 mmol), T= 140℃,t=5 h)
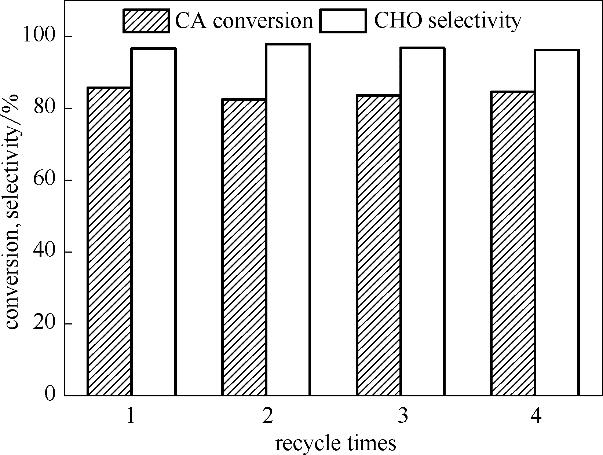
图6 HZSM-5催化乙酸环己酯水解反应中[BMIm]Br的重复使用性能
Fig.6 Reusability of [BMIm]Br for hydrolyzation of cyclohexyl acetate catalyzed by HZSM-5 (m([BMIm]Br)=3 g, m(HZSM-5)=1.2 g,V(cyclohexyl acetate) = 8 ml, V(H2O)=24 ml,T=140℃, t=5 h)
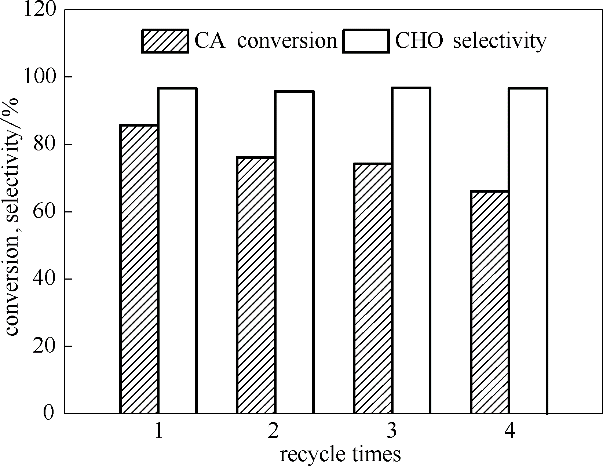
图7 乙酸环己酯水解反应中HZSM-5重复使用性能
Fig.7 Reusability of HZSM-5 for hydrolyzation of cyclohexyl acetate(m([BMIm]Br)=3 g,m(HZSM-5)=1.2 g, V(cyclohexyl acetate) = 8 ml,V(H2O)=24 ml, T=140℃, t=5 h)
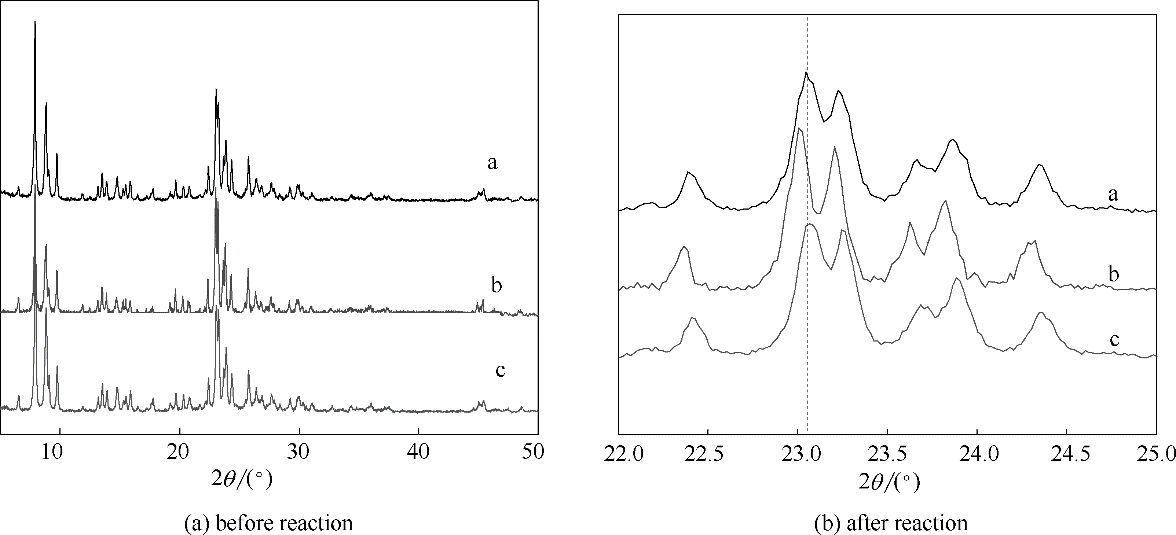
图10 使用前后HZSM-5分子筛的XRD谱图
Fig.10 XRD patterns of HZSM-5 before and after reactiona—fresh HZSM-5; b—recovered HZSM-5, washed with ethanol and dried at 120℃ for 12 h; c—recovered HZSM-5, sample b calcined at 550℃ for 5 h
| 1 | Zhou W J, Wischert R, Xue K, et al. Highly selective liquid-phase oxidation of cyclohexane to KA oil over Ti-MWW catalyst: evidence of formation of oxyl radicals[J]. ACS Catalysis, 2014, 4(1): 53-62. |
| 2 | 梁学博, 胡伯羽, 袁永军, 等. 金属卟啉催化空气氧化环己烷反应的工艺优化[J]. 化工学报, 2007, 58(3): 794-800. |
| Liang X B, Hu B Y, Yuan Y J, et al. Optimization of aerobic oxidation of cyclohexane catalyzed by metalloporphyrins[J]. Journal of Chemical Industry and Engineering (China), 2007, 58(3): 794-800. | |
| 3 | 纪红兵, 钱宇, 罗思睿, 等. 温和条件下环己烷液相选择性氧化改性VPO催化剂[J]. 化工学报, 2004, 55(12): 2027-2031. |
| Ji H B, Qian Y, Luo S R, et al. Liquid-phase selective oxidation of cyclohexane with modified-VPO catalysts under mild condition[J]. Journal of Chemical Industry and Engineering (China), 2004, 55(12): 2027-2031. | |
| 4 | Misono M, Inui T. New catalytic technologies in Japan[J]. Catalysis Today, 1999, 51(3): 369-375. |
| 5 | Ishida H. Liquid-phase hydration process of cyclohexene with zeolites[J]. Catalysis Surveys from Japan, 1997, 1: 241-246 |
| 6 | Nagahara H, Ono M, Konishi M, et al. Partial hydrogenation of benzene to cyclohexene[J]. Applied Surface Science, 1997, 121: 448-451. |
| 7 | Shan X, Cheng Z, Yuan P. Reaction kinetics and mechanism for hydration of cyclohexene over ion-exchange resin and H-ZSM-5[J]. Chemical Engineering Journal, 2011, 175: 423-432. |
| 8 | 娄舒洁, 肖超贤, 孙耿, 等. 由苯制备环己醇新途径[J]. 催化学报, 2013, 34(1): 251-256. |
| Lou S J, Xiao C X, Sun G, et al. A new method for preparation of cylohexanol from benzene[J]. Chines Journal of Catalysis, 2013, 34(1): 251-256. | |
| 9 | Li J, Yang L, Li F, et al. Hydration of cyclohexene to cyclohexanol over SO3H-functionalized imidazole ionic liquids[J]. Reaction Kinetics, Mechanisms and Catalysis, 2015, 114: 173-183. |
| 10 | Ahamed I R, Freund H, Guit R P M, et al. Evaluation of different process concepts for the indirect hydration of cyclohexene to cyclohexanol[J]. Organic Process Research & Development, 2013, 17(3): 343-358. |
| 11 | Steyer F, Sundmacher K. VLE and LLE dataset for the system cyclohexane + cyclohexene + water + cyclohexanol + formic acid + formic acid cyclohexanol ester[J]. Journal of Chemical & Engineering Data, 2005, 50(4): 1277-1282. |
| 12 | Steyer, F, Sundmacher K. Cyclohexanol production via esterification of cyclohexene with formic acid andsubsequent hydration of the esters reaction kinetics[J]. Industrial & Engineering Chemistry Research, 2007, 46(4): 1099-1104. |
| 13 | Katariya A, Freund H, Sundmacher K. Two-step reactive distillation process for cyclohexanol production from cyclohexene [J]. Industrial & Engineering Chemistry Research, 2009, 48(21): 9534-9545. |
| 14 | 杜文明, 薛伟, 李芳, 等.由环己烯经甲酸环己酯制备环己醇催化反应研究[J].河北工业大学学报, 2012, 41(4): 34-39. |
| Du W M, Xue W, Li F, et al. Study on the catalytic synthesis of cyclohexanol from cyclohexene via cyclohexyl formate[J]. Journal of Hebei University of Technology, 2012, 41(4): 34-39. | |
| 15 | Cao Z J, Zhao X, He F Q, et al. Highly efficient indirect hydration of olefins to alcohols using superacidic polyoxometalate-based ionic hybridscatalysts[J]. Industrial & Engineering Chemistry Research, 2018, 57(19): 6654-6663. |
| 16 | Chakrabarti A, Sharma M M. Cyclohexanol from cyclohexene via cyclohexyl acetate: catalysis by ion-exchange resin and acid-treated clay[J]. Reactive Polymers, 1992, 18(2): 107-115. |
| 17 | Xue W, Zhao H, Yao J, et al. Esterification of cyclohexene with formic acid over a peanut shell-derived carbon solid acid catalyst[J]. Chinese Journal of Catalysis, 2016, 37(5): 769-777. |
| 18 | Lu B, Wu Z W, Ma L J, Yuan X. Phosphotungstic acid immobilized on sulphonic-acid-functionalized SBA-15 as a stable catalyst for the esterification of cyclohexene with formic acid[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 88: 1-7. |
| 19 | Ma L J, Xu L, Jiang H R, et al. Comparative research on three types of MIL-101(Cr)-SO3H for esterification of cyclohexene with formic acid[J]. RSC Advances, 2019, 9(10): 5692-5700. |
| 20 | 靳敬敬, 李芳, 杨丽红, 等. HZSM-5催化乙酸环己酯水解反应[J]. 石油学报(石油加工), 2014, 30(1): 169-174. |
| Jin J J, Li F, Yang L H, et al. Hydrolyzation of cyclohexyl acetate over HZSM-5 catalyst[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2014, 30(1): 169-174. | |
| 21 | Yang F, Xue W, Zhang D, et al. Hydrolysis of cyclohexyl acetate to cyclohexanol with high selectivity over SO3H-functionalized ionic liquids[J]. Reaction Kinetics, Mechanisms and Catalysis, 2015, 117(1): 1-11. |
| 22 | Liu Y, Liu W H, Wang L, et al. Efficient hydrolysis of cyclohexyl acetate to cyclohexanol catalyzed by dual-SO3H functionalized heteropolyacid-based solid acids[J]. Industrial & Engineering Chemistry Research, 2018, 57(15): 5207-5214. |
| 23 | Xue W, Sun L J, Yang F, et al. Peanut shell-derived carbon solid acid with large surface area and its application for the catalytic hydrolysis of cyclohexyl acetate [J]. Materials, 2016, 9(10): 833. |
| 24 | 提飞, 李芳, 王志苗, 等. 无机盐在HZSM-5催化乙酸环己酯水解反应中的作用[J]. 石油学报(石油加工), 2017, 33(1): 85-90. |
| Ti F, Li F, Wang Z M, et al. The role of inorganic salts in hydrolyzation of cyclohexyl acetate over HZSM-5[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2017, 33(1): 85-90. | |
| 25 | Cai H L, Li C Z, Wang A Q, et al. Zeolite-promoted hydrolysis of cellulose in ionic liquid, insight into the mutual behavior of zeolite, cellulose and ionic liquid[J]. Applied Catalysis B Environmental, 2012, 123/124: 333-338. |
| 26 | Grzechowiak J R, Rynkowski J, Wereszczako-Zieliñska I. Catalytic hydrotreatment on allumina-titania supported Ni-Mo sulfides[J]. Catalysis Today, 2001, 65(2): 225-231. |
| 27 | 尹双凤, 林洁, 于中伟. 锌含量对Zn/HZSM-5催化剂性能的影响[J]. 催化学报, 2001, 22(1): 57-61. |
| Yin S F, Lin J, Yu Z W. Effect of zinc content on properties of Zn/HZSM-5 zeolitecatalyst[J]. Chinese Journal of Catalysis, 2001, 22(1): 57-61. | |
| 28 | 高秀枝, 张翊, 王秀梅, 等. 脱铝HY分子筛酸中心结构与酸性的固体NMR研究[J]. 石油学报(石油加工), 2012, 28(2): 180-187. |
| Gao X Z, Zhang Y, Wang X M, et al. State of acidic center andacidity of dealuminated HY zeolites investigated by solid-state NMR spectroscopy[J]. Acta Petrolei Sinica(Petroleum Processing Section), 2012, 28(2): 180-187. | |
| 29 | Costas S T, Athanasios G V, Lori N, et al. Effect of the degree and type of the dealumination method on the structural, compositional and acidic characteristics of H-ZSM-5 zeolites[J]. Microporous and Mesoporous Materials, 2001, 47(1): 369-388. |
| 30 | 黄朝晖, 刘乃旺, 姚佳佳, 等. USY分子筛表面酸性的调变及其在催化脱除芳烃中烯烃的应用[J]. 化工进展, 2016, 35(1): 138-144. |
| Huang Z H, Liu N W, Yao J J, et al. Surface acid modification of zeolite and its application in removal of olefins in aromatics[J]. Chemical Industry and Engineering Progress, 2016, 35(1): 138-144. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [4] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [5] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [6] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [7] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [8] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [9] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [10] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [11] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [12] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [13] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [14] | 董茂林, 陈李栋, 黄六莲, 吴伟兵, 戴红旗, 卞辉洋. 酸性助水溶剂制备木质纳米纤维素及功能应用研究进展[J]. 化工学报, 2023, 74(6): 2281-2295. |
| [15] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号