化工学报 ›› 2020, Vol. 71 ›› Issue (7): 3229-3237.DOI: 10.11949/0438-1157.20200011
吴玉玲( ),邵明龙,周武林,高惠芳,张显,徐美娟,杨套伟,饶志明(
),邵明龙,周武林,高惠芳,张显,徐美娟,杨套伟,饶志明( )
)
收稿日期:2020-01-03
修回日期:2020-03-07
出版日期:2020-07-05
发布日期:2020-07-05
通讯作者:
饶志明
作者简介:吴玉玲(1994—),女,硕士研究生,基金资助:
Yuling WU( ),Minglong SHAO,Wulin ZHOU,Huifang GAO,Xian ZHANG,Meijuan XU,Taowei YANG,Zhiming RAO(
),Minglong SHAO,Wulin ZHOU,Huifang GAO,Xian ZHANG,Meijuan XU,Taowei YANG,Zhiming RAO( )
)
Received:2020-01-03
Revised:2020-03-07
Online:2020-07-05
Published:2020-07-05
Contact:
Zhiming RAO
摘要:
宝丹酮作为一种重要的蛋白同化雄性激素类固醇,具有提升肌肉质量和耐力的功能。宝丹酮的传统合成方法是以1,4-雄烯二酮(ADD)为底物通过化学法合成,但过程复杂、污染严重。17β-羟基类固醇脱氢酶(17β-HSD)可催化甾体化合物C-17位点的氧化还原反应,实现ADD和宝丹酮的相互转化。本研究通过基因序列同源性分析,筛选到6种不同来源的17β-HSD基因并对其在大肠杆菌中进行异源表达。利用不同重组菌转化ADD合成宝丹酮,结果表明重组菌BL21/pET28a-HSDPy的ADD转化率最高,因此选择BL21/pET28a-HSDPy进行进一步研究。鉴定了重组菌的酶学性质并优化其全细胞转化条件。结果表明在生物量为36 g·L-1、底物浓度为5.40 g·L-1条件下,经过两次补料,获得了3.66 g·L-1宝丹酮,比优化前提高了4.1倍。而且在生物转化过程中未检测到副产物。为生物合成宝丹酮提供了可能。
中图分类号:
吴玉玲, 邵明龙, 周武林, 高惠芳, 张显, 徐美娟, 杨套伟, 饶志明. 重组大肠杆菌表达17β-羟基类固醇脱氢酶全细胞催化合成宝丹酮的研究[J]. 化工学报, 2020, 71(7): 3229-3237.
Yuling WU, Minglong SHAO, Wulin ZHOU, Huifang GAO, Xian ZHANG, Meijuan XU, Taowei YANG, Zhiming RAO. Study on catalytic synthesis of boldenone by recombinant E. coli expressing 17β-hydroxysteroid dehydrogenase[J]. CIESC Journal, 2020, 71(7): 3229-3237.
| 菌株、质粒或引物 | 性质 | 来源 |
|---|---|---|
| 菌株 | ||
| Escherichia coli | ||
| JM109 | 用于所有克隆实验 | Invitrogen |
| BL21 (DE3) | 重组蛋白表达宿主 | Promega |
| BL21/pET28a-HSDCl | 重组E. coli BL21 (DE3) ,携带pET28a- HSDCl | 本研究构建 |
| BL21/pET28a-HSDPy | 重组E. coli BL21 (DE3) ,携带pET28a- HSDPy | 本研究构建 |
| BL21/pET28a-HSDBb | 重组E. coli BL21 (DE3) ,携带 pET28a- HSDBb | 本研究构建 |
| BL21/pET28a-HSDAo | 重组E. coli BL21 (DE3) ,携带 pET28a- HSDAo | 本研究构建 |
| BL21/pET28a-HSDCt | 重组E. coli BL21 (DE3) ,携带pET28a- HSDCt | 本研究构建 |
| BL21/pET28a-HSDBu | 重组E. coli BL21 (DE3) ,携带pET28a- HSDBu | 本研究构建 |
| 质粒 | ||
| pUC57- HSDCl | pUC57携带来源于Cochliobolus lunatus经过密码子优化的17β-HSD基因,AmpR | 金唯智合成 |
| pUC57- HSDPy | pUC57携带来源于Pyrenochaeta sp.经过密码子优化的17β-HSD基因, AmpR | 金唯智合成 |
| pUC57- HSDBb | pUC57携带来源于Beauveria bassiana经过密码子优化的17β-HSD基因, AmpR | 金唯智合成 |
| pUC57- HSDAo | pUC57携带来源于Arthroderma otae经过密码子优化的17β-HSD基因, AmpR | 金唯智合成 |
| pUC57- HSDCt | pUC57携带来源于Comamonas testosterone经过密码子优化的17β-HSD基因,AmpR | 金唯智合成 |
| pUC57- HSDBu | pUC57携带来源于Burkholderia sp.经过密码子优化的17β-HSD基因, AmpR | 金唯智合成 |
| pET28a(+) | 大肠杆菌表达载体,KanR | Novagen |
| pET28a-HSDCl | pET28a携带来源于Cochliobolus lunatus的17β-HSD基因, KanR | 本研究构建 |
| pET28a-HSDPy | pET28a携带来源于Pyrenochaeta sp.的17β-HSD基因, KanR | 本研究构建 |
| pET28a-HSDBb | pET28a携带来源于Beauveria bassiana的17β-HSD基因,KanR | 本研究构建 |
| pET28a-HSDAo | pET28a携带来源于Arthroderma otae的17β-HSD基因,KanR | 本研究构建 |
| pET28a-HSDCt | pET28a携带来源于Comamonas testosterone的17β-HSD基因,KanR | 本研究构建 |
| pET28a-HSDBu | pET28a携带来源于Burkholderia sp.的17β-HSD基因,KanR | 本研究构建 |
| 引物 | 序列(5′-3′) | |
| HSDCl-F | CG | |
| HSDCl-R | CC | |
| HSDPy-F | CG | |
| HSDPy-R | CC | |
| HSDBb-F | CG | |
| HSDBb-R | CC | |
| HSDAo-F | CG | |
| HSDAo-R | CC | |
| HSDCt-F | CG | |
| HSDCt-R | CC | |
| HSDBu-F | CG | |
| HSDBu-R | CC | |
表1 本研究所用到的菌株、质粒及引物
Table 1 Strains, plasmids and primers used in this study
| 菌株、质粒或引物 | 性质 | 来源 |
|---|---|---|
| 菌株 | ||
| Escherichia coli | ||
| JM109 | 用于所有克隆实验 | Invitrogen |
| BL21 (DE3) | 重组蛋白表达宿主 | Promega |
| BL21/pET28a-HSDCl | 重组E. coli BL21 (DE3) ,携带pET28a- HSDCl | 本研究构建 |
| BL21/pET28a-HSDPy | 重组E. coli BL21 (DE3) ,携带pET28a- HSDPy | 本研究构建 |
| BL21/pET28a-HSDBb | 重组E. coli BL21 (DE3) ,携带 pET28a- HSDBb | 本研究构建 |
| BL21/pET28a-HSDAo | 重组E. coli BL21 (DE3) ,携带 pET28a- HSDAo | 本研究构建 |
| BL21/pET28a-HSDCt | 重组E. coli BL21 (DE3) ,携带pET28a- HSDCt | 本研究构建 |
| BL21/pET28a-HSDBu | 重组E. coli BL21 (DE3) ,携带pET28a- HSDBu | 本研究构建 |
| 质粒 | ||
| pUC57- HSDCl | pUC57携带来源于Cochliobolus lunatus经过密码子优化的17β-HSD基因,AmpR | 金唯智合成 |
| pUC57- HSDPy | pUC57携带来源于Pyrenochaeta sp.经过密码子优化的17β-HSD基因, AmpR | 金唯智合成 |
| pUC57- HSDBb | pUC57携带来源于Beauveria bassiana经过密码子优化的17β-HSD基因, AmpR | 金唯智合成 |
| pUC57- HSDAo | pUC57携带来源于Arthroderma otae经过密码子优化的17β-HSD基因, AmpR | 金唯智合成 |
| pUC57- HSDCt | pUC57携带来源于Comamonas testosterone经过密码子优化的17β-HSD基因,AmpR | 金唯智合成 |
| pUC57- HSDBu | pUC57携带来源于Burkholderia sp.经过密码子优化的17β-HSD基因, AmpR | 金唯智合成 |
| pET28a(+) | 大肠杆菌表达载体,KanR | Novagen |
| pET28a-HSDCl | pET28a携带来源于Cochliobolus lunatus的17β-HSD基因, KanR | 本研究构建 |
| pET28a-HSDPy | pET28a携带来源于Pyrenochaeta sp.的17β-HSD基因, KanR | 本研究构建 |
| pET28a-HSDBb | pET28a携带来源于Beauveria bassiana的17β-HSD基因,KanR | 本研究构建 |
| pET28a-HSDAo | pET28a携带来源于Arthroderma otae的17β-HSD基因,KanR | 本研究构建 |
| pET28a-HSDCt | pET28a携带来源于Comamonas testosterone的17β-HSD基因,KanR | 本研究构建 |
| pET28a-HSDBu | pET28a携带来源于Burkholderia sp.的17β-HSD基因,KanR | 本研究构建 |
| 引物 | 序列(5′-3′) | |
| HSDCl-F | CG | |
| HSDCl-R | CC | |
| HSDPy-F | CG | |
| HSDPy-R | CC | |
| HSDBb-F | CG | |
| HSDBb-R | CC | |
| HSDAo-F | CG | |
| HSDAo-R | CC | |
| HSDCt-F | CG | |
| HSDCt-R | CC | |
| HSDBu-F | CG | |
| HSDBu-R | CC | |
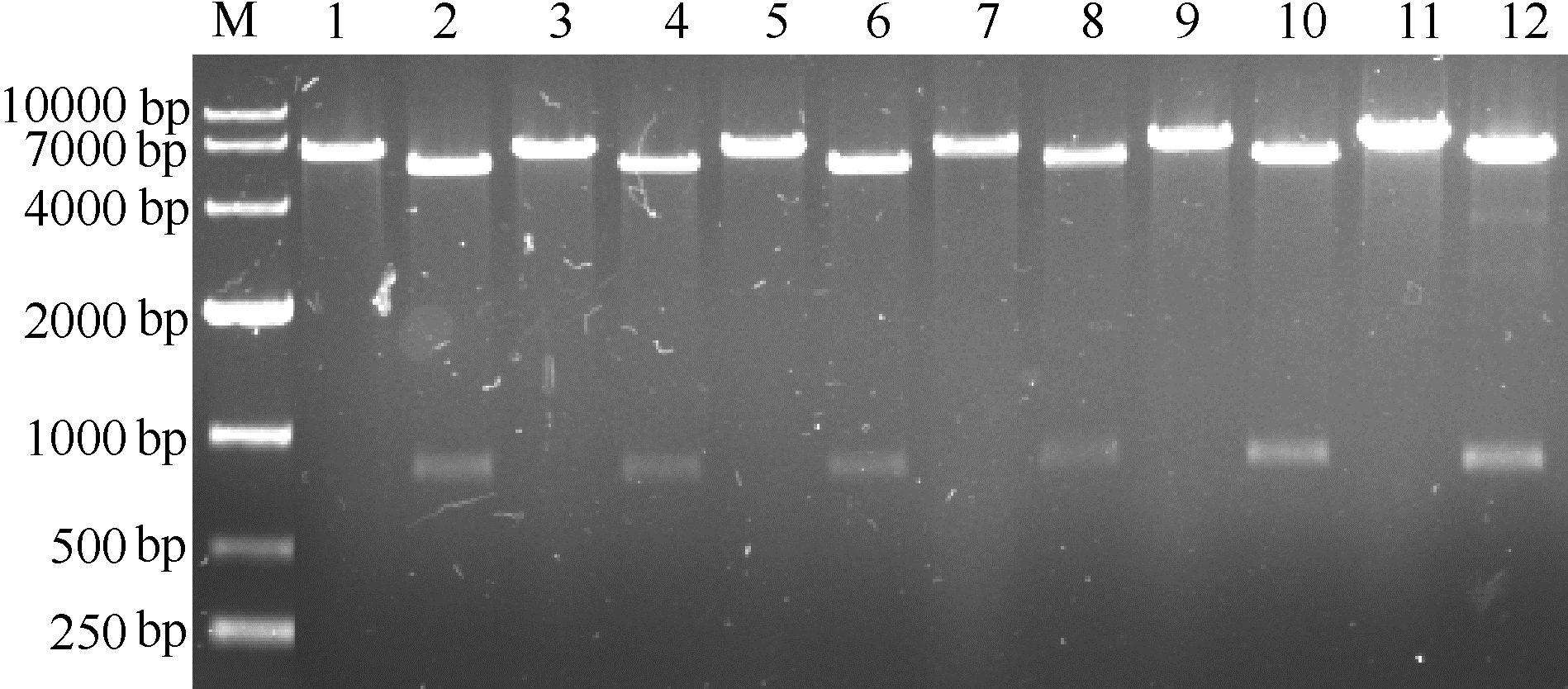
图3 重组质粒pET28a-HSD酶切验证M—DNA Marker;1,3,5,7,9,11—pET28a-HSDCl,pET28a-HSDPy,pET28a-HSDBb,pET28a-HSDAo,pET28a-HSDCt,pET28a-HSDBu通过BamH I酶切;2,4,6,8,10,12—pET28a-HSDCl,pET28a-HSDPy,pET28a-HSDBb,pET28a-HSDAo,pET28a-HSDCt,pET28a-HSDBu通过BamHI和 Hind III酶切
Fig.3 Identification of recombinant pET28a-HSD by enzyme digestion
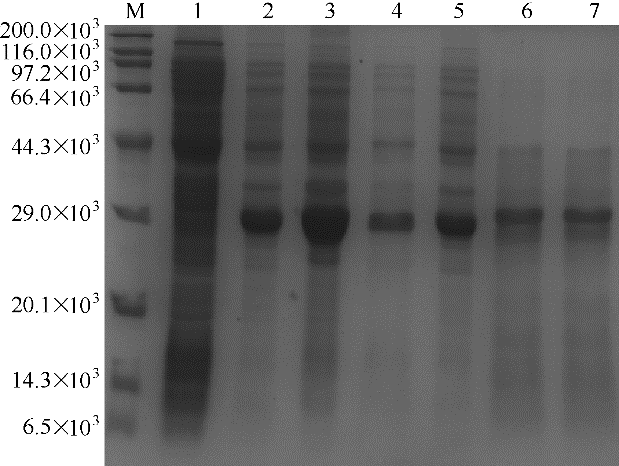
图4 重组大肠杆菌17β-HSD SDS-PAGE分析M—蛋白Marker;1—重组大肠杆菌BL21/pET28a粗酶液;2—重组大肠杆菌BL21/pET28a-HSDCl粗酶液;3—重组大肠杆菌BL21/pET28a-HSDPy粗酶液;4—重组大肠杆菌BL21/pET28a-HSDBb粗酶液;5—重组大肠杆菌BL21/pET28a-HSDAo粗酶液;6—重组大肠杆菌BL21/pET28a-HSDCt粗酶液;7—重组大肠杆菌BL21/pET28a-HSDBu粗酶液
Fig.4 SDS-PAGE analysis of 17β-HSD expression in recombinant E. coli
| 菌株 | 12 h转化率/% | 来源 |
|---|---|---|
| BL21/pET28a | — | — |
| BL21/pET28a-HSDCl | 54.90 | Cochliobolus lunatus |
| BL21/pET28a-HSDPy | 72.53 | Pyrenochaeta sp. |
| BL21/pET28a-HSDBb | 23.12 | Beauveria bassiana |
| BL21/pET28a-HSDAo | 10.11 | Arthroderma otae |
| BL21/pET28a-HSDCt | 9.39 | Comamonas testosterone |
| BL21/pET28a-HSDBu | 9.03 | Burkholderia sp. |
表2 重组大肠杆菌BL21/pET28a-HSD转化ADD
Table 2 Conversion of ADD by recombinant E. coli BL21/pET28a-HSD
| 菌株 | 12 h转化率/% | 来源 |
|---|---|---|
| BL21/pET28a | — | — |
| BL21/pET28a-HSDCl | 54.90 | Cochliobolus lunatus |
| BL21/pET28a-HSDPy | 72.53 | Pyrenochaeta sp. |
| BL21/pET28a-HSDBb | 23.12 | Beauveria bassiana |
| BL21/pET28a-HSDAo | 10.11 | Arthroderma otae |
| BL21/pET28a-HSDCt | 9.39 | Comamonas testosterone |
| BL21/pET28a-HSDBu | 9.03 | Burkholderia sp. |

图7 大肠杆菌BL21/pET28a-HSDPy的不同生物量(a)和不同底物浓度(b)对宝丹酮生产的影响
Fig.7 Effects of different biomasses of E. coli BL21/pET28a-HSDPy (a) and substrate concentration (b) on BD production
| 1 | Eisa M, El-Refai H, Amin M. Single step biotransformation of corn oil phytosterols to boldenone by a newly isolated Pseudomonas aeruginosa [J]. Biotechnol. Rep., 2016, 11: 36-43. |
| 2 | 系祖斌, 薛文武, 刘卫东, 等. 一种高收率的宝丹酮的合成方法:103030677 A [P]. 2012-09-26. |
| Xi Z B, Xue W W, Liu W D, et al. A high-yield method for the synthesis of boldenone:103030677 A [P]. 2012-09-26. | |
| 3 | Chen M M, Wang F Q, Lin L C, et al. Characterization and application of fusidane antibiotic biosynethsis enzyme 3-ketosteroid-1-dehydrogenase in steroid transformation [J]. Appl. Microbiol. Biotechnol., 2012, 96(1): 133-142. |
| 4 | Tang R, Shen Y, Xia M, et al. A highly efficient step-wise biotransformation strategy for direct conversion of phytosterol to boldenone [J]. Bioresour. Technol., 2019, 283: 242-250. |
| 5 | Moeller G, Adamski J. Multifunctionality of human 17beta-hydroxysteroid dehydrogenases [J]. Mol. Cell. Endocrinol., 2006, 248(1): 47-55. |
| 6 | Moeller G, Adamski J. Integrated view on 17beta-hydroxysteroid dehydrogenases [J]. Mol. Cell. Endocrinol., 2009, 301(1/2): 7-19. |
| 7 | Marchais-Oberwinkler S, Henn C, Moeller, et al. 17beta-Hydroxysteroid dehydrogenases (17beta-HSDs) as therapeutic targets: protein structures, functions, and recent progress in inhibitor development [J]. J. Steroid. Biochem. Mol. Biol., 2011, 125(1): 66-82. |
| 8 | Xu L Q, Liu Y J, Yao K, et al. Unraveling and engineering the production of 23, 24-bisnorcholenic steroids in sterol metabolism [J]. Sci. Rep., 2016, 6: 21928. |
| 9 | Kumar R, Dahiya J S, Singh D, et al. Biotransformation of cholesterol using Lactobacillus bulgaricus in a glucose-controlled bioreactor [J]. Bioresour. Technol., 2001, 78(2): 209-211. |
| 10 | Lanišnik Rižner T, Žakelj-Mavrič M. Characterization of fungal 17β-hydroxysteroid dehydrogenases [J]. Comp. Biochem. Physiol. B Biochem. Mol. Biol., 2000, 127(1): 53-63. |
| 11 | Donova M V, Egorova O V, Nikolayeva V M. Steroid 17β-reduction by microorganisms—a review [J]. Process Biochem., 2005, 40(7): 2253-2262. |
| 12 | Fernández-Cabezón L, Galán B, García J L. Engineering Mycobacterium smegmatis for testosterone production [J]. Microb. Biotechnol., 2017, 10(1): 151-161. |
| 13 | Xu X, Jin F, Yu X, et al. Expression and purification of a recombinant antibacterial peptide, cecropin, from Escherichia coli [J]. Protein Expr. Purif., 2007, 53(2): 293-301. |
| 14 | Bradfod M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding [J]. Anal. Biochem., 1976, 72: 248-254. |
| 15 | Shao M, Zhang X, Rao Z, et al. Efficient testosterone production by engineered Pichia pastoris co-expressing human 17β-hydroxysteroid dehydrogenase type 3 and Saccharomyces cerevisiae glucose 6-phosphate dehydrogenase with NADPH regeneration [J]. Green Chem., 2016, 18(6): 1774-1784. |
| 16 | Zhang W, Shao M, Rao Z, et al. Bioconversion of 4-androstene-3, 17-dione to androst-1, 4-diene-3, 17-dione by recombinant Bacillus subtilis expressing ksdd gene encoding 3-ketosteroid-Δ1-dehydrogenase from Mycobacterium neoaurum JC-12 [J]. J. Steroid. Biochem. Mol. Biol., 2013, 135: 36-42. |
| 17 | Egorova O V, Nikolayeva V M, Sukhodolskaya G V, et al. Transformation of C19-steroids and testosterone production by sterol-transforming strains of Mycobacterium spp. [J]. J. Mol. Catal., B Enzym., 2009, 57(1): 198-203. |
| 18 | Egorova O V, Nikolayeva V M, Suzina N E, et al. Localization of 17beta-hydroxysteroid dehydrogenase in Mycobacterium sp. VKM Ac-1815D mutant strain [J]. J. Steroid. Biochem. Mol. Biol., 2005, 94(5): 519-525. |
| 19 | Shao M, Chen Y, Zhang X, et al. Enhanced intracellular soluble production of 3-ketosteroid-Δ1-dehydrogenase from Mycobacterium neoaurum in Escherichia coli and its application in the androst-1, 4-diene-3, 17-dione production [J]. J. Chem. Technol. Biotechnol., 2017, 92(2): 350-357. |
| 20 | Letunic I, Bork P. Interactive tree of life (iTOL) v4: recent updates and new developments [J]. Nucleic Acids Res., 2019, 47(1): 256-259. |
| 21 | Marchler-Bauer A, Bo Y, Han L, et al. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures [J]. Nucleic Acids Res., 2017, 45(1): 200-203. |
| 22 | 戎晶晶, 刁振宇, 周国华. 大肠杆菌表达系统的研究进展 [J]. 药物生物技术, 2005, (6): 416-420. |
| Rong J J, Diao Z Y, Zhou G H. Research progress of E. coli expression system [J]. Pharm. Biotechnol., 2005, (6): 416-420. | |
| 23 | Kagawa N, Hori H, Waterman M R, et al. Characterization of stable human aromatase expressed in E. coli [J]. Steroids, 2004, 69(4): 235-243. |
| 24 | Agematu H, Matsumoto N, Fujii Y, et al. Hydroxylation of testosterone by bacterial cytochromes P450 using the Escherichia coli expression system [J]. Biosci. Biotechnol. Biochem., 2006, 70(1): 307-311. |
| 25 | Neunzig J, Milhim M, Schiffer L, et al. The steroid metabolite 16(beta)-OH-androstenedione generated by CYP21A2 serves as a substrate for CYP19A1 [J]. J. Steroid. Biochem. Mol. Biol., 2017, 167: 182-191. |
| 26 | Zhao Y, Shen Y, Ma S, et al. Production of 5α-androstene-3, 17-dione from phytosterols by co-expression of 5α-reductase and glucose-6-phosphate dehydrogenase in engineered Mycobacterium neoaurum [J]. Green Chem., 2019, 21(7): 1809-1815. |
| 27 | Borrego S, Niub E, Ancheta O, et al. Study of the microbial aggregation inmycobacterium using image analysis and electron microscopy [J]. Tissue Cell, 2000, 32(6): 494-500. |
| 28 | Zhang D, Zhang R, Zhang J, et al. Engineering a hydroxysteroid dehydrogenase to improve its soluble expression for the asymmetric reduction of cortisone to 11beta-hydrocortisone [J]. Appl. Microbiol. Biotechnol., 2014, 98(21): 8879-8886. |
| 29 | Zehentgruber D, Dragan C A, Bureik M, et al. Challenges of steroid biotransformation with human cytochrome P450 monooxygenase CYP21 using resting cells of recombinant Schizosaccharomyces pombe [J]. J. Biotechnol., 2010, 146(4): 179-185. |
| 30 | Naumann J M, Zollner A, Dragan C A, et al. Biotechnological production of 20-alpha-dihydrodydrogesterone at pilot scale [J]. Appl. Biochem. Biotechnol., 2011, 165(1): 190-203. |
| 31 | 姜佳伟, 张荣珍, 周晓天, 等. (S)-羰基还原酶Ⅱ与葡萄糖脱氢酶共催化高效合成(S)-苯乙二醇 [J]. 微生物学报, 2016, 56(10): 1647-1655. |
| Jiang J W, Zhang R Z, Zhou X T, et al. Efficient synthesis of (S) -phenylethylene glycol by (S) -carbonyl reductase Ⅱ and glucose dehydrogenase [J]. Acta Microbiologica Sinica, 2016, 56(10): 1647-1655. | |
| 32 | Szczebara F M, Chandelier C, Villeret C, et al. Total biosynthesis of hydrocortisone from a simple carbon source in yeast [J]. Nat. Biotechnol., 2003, 21(2): 143-149. |
| 33 | 陈天华, 张若思, 姜国珍, 等. 产蒎烯人工酵母细胞的构建 [J]. 化工学报, 2019, 70(1): 179-188. |
| Chen T H, Zhang R S, Jiang G Z, et al. Construction of artificial yeast cells producing limonene [J]. CIESC Journal, 2019, 70(1): 179-188. | |
| 34 | 王金鹤, 王冬, 李畏娴, 等. 酿酒酵母工程菌UDP-葡萄糖供给模块的优化与人参皂苷F_1生产 [J]. 中国中药杂志, 2019, 44(21): 4596-1604. |
| Wang J H, Wang D, Li W X, et al. Optimization of UDP-glucose supply module of Saccharomyces cerevisiae and production of ginsenoside F_1 [J]. China Journal of Chinese Materia Medica, 2019, 44(21): 4596-1604. |
| [1] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [2] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [3] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [4] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [5] | 胡阳, 孙彦. 酶分子的自驱动及其介导的微纳马达[J]. 化工学报, 2023, 74(1): 116-132. |
| [6] | 刘昕, 戈钧, 李春. 光驱动微生物杂合系统提高生物制造水平[J]. 化工学报, 2023, 74(1): 330-341. |
| [7] | 谭卓涛, 齐思雨, 许梦蛟, 戴杰, 朱晨杰, 应汉杰. 辅酶自循环的氧化还原级联体系在生物催化过程中的应用:机遇与挑战[J]. 化工学报, 2023, 74(1): 45-59. |
| [8] | 安绍杰, 许洪峰, 李思, 许远航, 李佳锡. 利用分子机器的组装与分解构建pH敏感性谷胱甘肽过氧化物人工酶[J]. 化工学报, 2022, 73(8): 3669-3678. |
| [9] | 张劢, 田瑶, 郭之旗, 王叶, 窦广进, 宋浩. 光催化-生物杂合系统设计优化用于燃料和化学品绿色合成[J]. 化工学报, 2022, 73(7): 2774-2789. |
| [10] | 孙甲琛, 孙文涛, 孙慧, 吕波, 李春. 甘草黄酮合酶Ⅱ催化甘草素特异性合成7,4′-二羟基黄酮[J]. 化工学报, 2022, 73(7): 3202-3211. |
| [11] | 王淋, 付乾, 肖帅, 李卓, 李俊, 张亮, 朱恂, 廖强. 高效可见光响应微生物/光电化学耦合人工光合作用系统[J]. 化工学报, 2022, 73(2): 887-893. |
| [12] | 宋伟, 王金辉, 胡贵鹏, 陈修来, 刘立明, 吴静. 多酶级联催化合成(R)-β-酪氨酸[J]. 化工学报, 2022, 73(1): 352-361. |
| [13] | 刘姝睿, 吴雪娥, 王远鹏. 纳米材料介导微生物胞外电子传递过程的研究进展[J]. 化工学报, 2021, 72(7): 3576-3589. |
| [14] | 段凌暄, 姚光晓, 江亮, 王世珍. 耐有机溶剂氨基酸脱氢酶基因挖掘与非天然氨基酸的非水相合成[J]. 化工学报, 2021, 72(7): 3757-3767. |
| [15] | 潘禹, 王华生, 詹鸿峰, 孙缓缓, 范超, 刘祖文, 闫海. 微囊藻毒素降解酶MlrA的结构功能分析[J]. 化工学报, 2021, 72(3): 1643-1653. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号