化工学报 ›› 2020, Vol. 71 ›› Issue (10): 4601-4610.DOI: 10.11949/0438-1157.20200359
李巧灵( ),吴晓宇,王学伟,谢智,于晓飞,杨晓婧,黄阳,李兰兰(
),吴晓宇,王学伟,谢智,于晓飞,杨晓婧,黄阳,李兰兰( )
)
收稿日期:2020-04-07
修回日期:2020-05-16
出版日期:2020-10-05
发布日期:2020-10-05
通讯作者:
李兰兰
作者简介:李巧灵(1992—),女,博士研究生,基金资助:
Qiaoling LI( ),Xiaoyu WU,Xuewei WANG,Zhi XIE,Xiaofei YU,Xiaojing YANG,Yang HUANG,Lanlan LI(
),Xiaoyu WU,Xuewei WANG,Zhi XIE,Xiaofei YU,Xiaojing YANG,Yang HUANG,Lanlan LI( )
)
Received:2020-04-07
Revised:2020-05-16
Online:2020-10-05
Published:2020-10-05
Contact:
Lanlan LI
摘要:
选择性去除燃油中的硫化物对环境和人类健康具有重要意义。采用基于密度泛函理论(DFT)和含色散矫正的密度泛函理论(D-DFT)的方法,研究了多孔氮化硼(p-BN)及其空位缺陷对燃油中噻吩类硫化物及非硫化物的吸附行为及吸附选择性。结果表明:B—N极性键与硫化物极性分子之间的分子间力使p-BN能选择性去除燃油中的二苯并噻吩(DBT);引入N、B空位缺陷后,缺陷能级与S原子形成化学相互作用并伴随电荷转移,进一步增强了p-BN对硫化物的吸附。通过对N、B空位缺陷形成能的计算,预测了合成含VN、VB的p-BN所需的化学条件:在富硼条件下,采用B2H4作为B源比采用B、α-B12和BH3等更有利于VN的形成;而在富氮环境下采用N2H4作为N源比采用N2、NH3等更有利于VB形成。为实验上有目的地合成高效吸附脱硫材料提供理论依据。
中图分类号:
李巧灵, 吴晓宇, 王学伟, 谢智, 于晓飞, 杨晓婧, 黄阳, 李兰兰. 多孔BN选择性去除燃油中硫化合物的密度泛函理论研究[J]. 化工学报, 2020, 71(10): 4601-4610.
Qiaoling LI, Xiaoyu WU, Xuewei WANG, Zhi XIE, Xiaofei YU, Xiaojing YANG, Yang HUANG, Lanlan LI. Porous BN for selective adsorption of sulfur-containing compounds from fuel oil: DFT study[J]. CIESC Journal, 2020, 71(10): 4601-4610.

图2 p-BN、VN和VB分别对DBT、正十六烷和正辛烷最佳吸附构型的俯视及侧视图
Fig.2 The geometric configurations of adsorbed DBT, n-hexadecane and n-octane on perfect p-BN, p-BN with VN defect and p-BN with VB defect, respectively

图3 完整的p-BN、VN和VB分别对DBT、正十六烷和正辛烷的吸附能 (Eads)
Fig.3 Calculated adsorption energies (Eads) for DBT, n-hexadecane and n-octane on the perfect p-BN, VN and VB, respectively
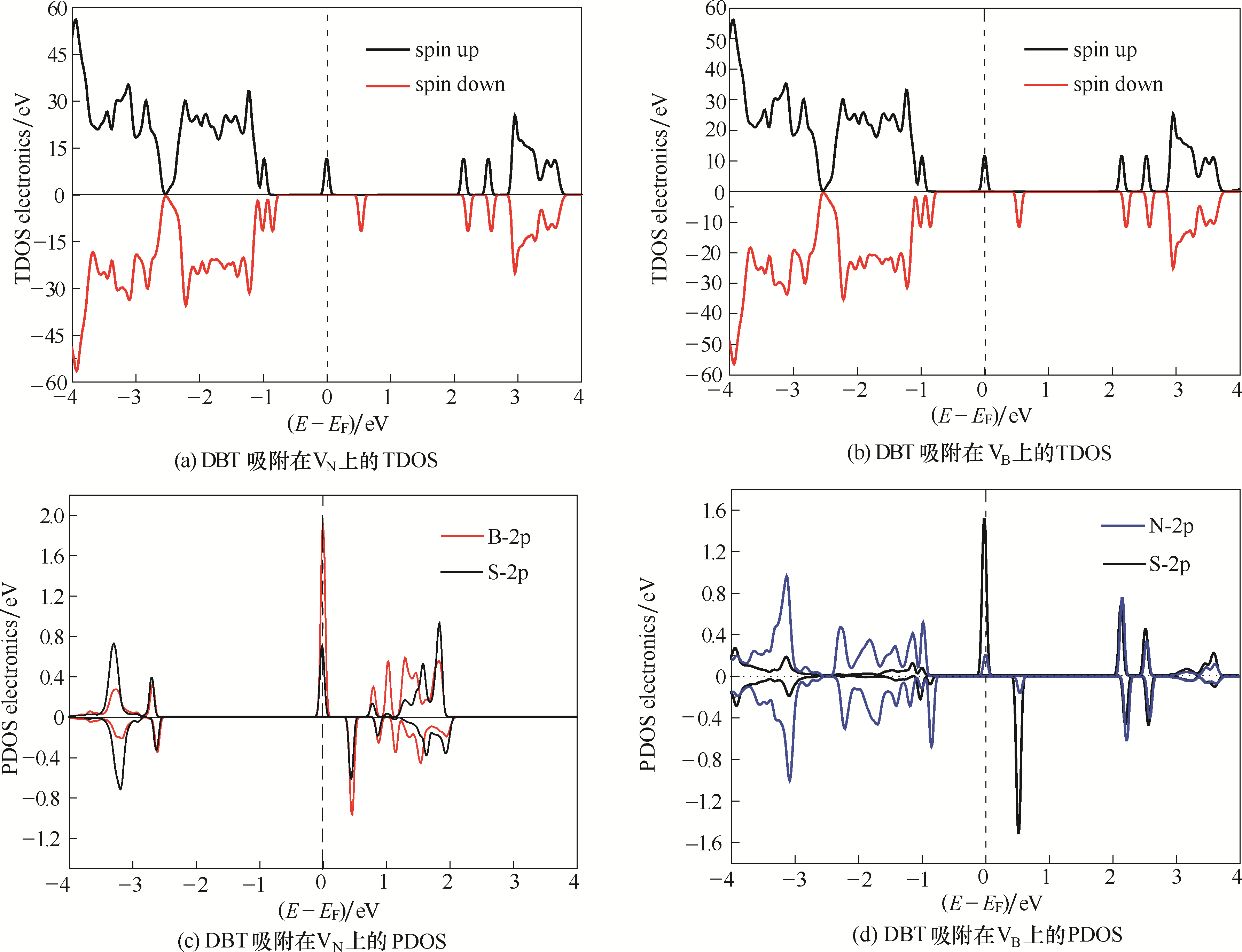
图5 DBT吸附在VN 和VB 上的总态密度 (TDOS) 和分态密度 (PDOS)(费米能级的位置在图中用虚线标出)adsorbed on VN and VB (the position of the Fermi level was marked in dashed line)
Fig.5 Total density of states (TDOS) and projected density of states (PDOS) for DBT
| Atom lable | Charge/e | |
|---|---|---|
| VN | VB | |
| B1/N1 | 0.119 | 0.24 |
| B2/N2 | 0.089 | 0.163 |
| B3/N3 | 0.116 | 0.249 |
| S | 0.205 | 0.404 |
| Call | -0.432 | -0.399 |
| Hall | 0.396 | 0.396 |
表1 DBT分别吸附在VN和VB上的Hirshfeld电荷分布
Table 1 Hirshfeld atomic charge of DBT adsorption on VN and VB defect configuration
| Atom lable | Charge/e | |
|---|---|---|
| VN | VB | |
| B1/N1 | 0.119 | 0.24 |
| B2/N2 | 0.089 | 0.163 |
| B3/N3 | 0.116 | 0.249 |
| S | 0.205 | 0.404 |
| Call | -0.432 | -0.399 |
| Hall | 0.396 | 0.396 |

图8 甲苯(toluene)、4,6-二甲基二苯并噻吩(4,6-DMDBT)、噻吩(T) 和 苯并噻吩(BT) 在VB上最优吸附构型
Fig.8 Optimized geometric configurations of adsorbed toluene, 4,6-DMDBT, T and BT on VB
| Item | N-rich condition | B-rich condition | ||||||
|---|---|---|---|---|---|---|---|---|
| N2H4 | N2 | NH3 | B2H6 | α-B12 | BH3 | B2H4 | ||
| VN | PBE | 6.44 | 6.31 | 5.54 | 5.26 | 4.66 | 3.71 | 3.36 |
| VB | PBE | 6.20 | 6.33 | 7.11 | 7.39 | 7.99 | 8.94 | 9.28 |
表2 在各种化学条件下VB和VN的缺陷形成能(Eform/eV)
Table 2 The defect formation energies (Eform/eV) of the VB and the VN under various chemical conditions
| Item | N-rich condition | B-rich condition | ||||||
|---|---|---|---|---|---|---|---|---|
| N2H4 | N2 | NH3 | B2H6 | α-B12 | BH3 | B2H4 | ||
| VN | PBE | 6.44 | 6.31 | 5.54 | 5.26 | 4.66 | 3.71 | 3.36 |
| VB | PBE | 6.20 | 6.33 | 7.11 | 7.39 | 7.99 | 8.94 | 9.28 |
| 1 | Liu P, Rodriguez J A, Muckerman J T. Sulfur adsorption and sulfidation of transition metal carbides as hydrotreating catalysts[J]. Journal of Molecular Catalysis A: Chemistry, 2005, 239(1/2): 116-124. |
| 2 | 岳源源, 郑晓桂, 康颖, 等. 基于镁铝水滑石的Mo/Al2O3-MgO催化剂制备及其加氢脱硫性能[J]. 化工学报, 2018, 69(1): 405-413. |
| Yue Y Y, Zheng X G, Kang Y, et al. Mo/Al2O3-MgO catalyst preparation from MgAl-hydrotalcite and their hydrogenation desulfurization performance[J]. CIESC Journal, 2018, 69(1): 405-413. | |
| 3 | Chen M, Cuib J, Wang Y, et al. Amine modified nano-sized hierarchical hollow system for highly effective and stable oxidative-adsorptive desulfurization[J]. Fuel, 2020, 266: 116960. |
| 4 | 于凤丽, 谢盼辉, 朱国强, 等. 有机-无机杂多酸类离子液体催化汽油超声氧化脱硫[J]. 高等学校化学学报, 2016, 37(12): 68-74. |
| Yu F L, Xie P H, Zhu G Q, et al. Oxidative desulfurization of gasoline catalyzed by organic-inorganic heteropoly acid ionic liquids under ultrasound [J]. Chemical Journal of Chinese Universities, 2016, 37(12): 68-74. | |
| 5 | Yu G X, Jin M, Sun J, et al. Oxidative modifications of rice hull-based carbons for dibenzothiophene adsorptive removal[J]. Catalysis Today, 2013, 212: 31-37. |
| 6 | Gao J, Meng H, Lu Y, et al. A carbonium pseudo ionic liquid with excellent extractive desulfurization performance[J]. AIChE Journal, 2013, 59(3): 948-958. |
| 7 | Zhang C, Song W, Sun G, et al. Synthesis, characterization, and evaluation of activated carbon spheres for removal of dibenzothiophene from model diesel fuel[J]. Industrial Engineering Chemistry Research, 2014, 53(11): 4271-4276. |
| 8 | Shi Y, Zhang X, Wang L, et al. MOF-derived porous carbon for adsorptive desulfurization[J]. AIChE Journal, 2014, 60(8): 2747-2751. |
| 9 | Song C. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel[J]. Catalysis Today, 2003, 86(1/2/3/4): 211-263. |
| 10 | 丁建伟, 王睿, 于美青, 等. MIL-101固载磷钨酸催化氧气氧化脱硫性能[J]. 化工学报, 2016, 67: 283-288. |
| Ding J W, Wang R, Yu M Q, et al. Catalytic performance of phosphotungstic acid encapsulated into MIL-101 for oxidative desulfurization with oxygen[J]. CIESC Journal, 2016, 67: 283-288. | |
| 11 | Zhang T, Guo C, Wei S, et al. Investigation on CH3SH desulfurization mechanism at the edge site of Co-doped MoS2 cluster[J]. Acta Chimca Sinica, 2018, 76(1): 62-67. |
| 12 | Wu L, Sitamraju S, Xiao J, et al. Effect of liquid-phase O3 oxidation of activated carbon on the adsorption of thiophene[J]. Chemical Engineering Journal, 2014, 242(8): 211-219. |
| 13 | Kampouraki Z C, Giannakoudakis D A, Nair V, et al. Metal organic frameworks as desulfurization adsorbents of DBT and 4, 6-DMDBT from fuels[J]. Molecules, 2019, 24: 4525. |
| 14 | Song L, Bu T, Zhu L, et al. Synthesis of organically-inorganically functionalized MCM-41 for adsorptive desulfurization of C4 hydrocarbons[J]. Journal of Physical Chemistry C, 2014, 118(18): 9468-9476. |
| 15 | Liu W, Liu X, Yang Y, et al. Selective removal of benzothiophene and dibenzothiophene from gasoline using double-template molecularly imprinted polymers on the surface of carbon microspheres[J]. Fuel, 2014, 117: 184-190. |
| 16 | Bazyari A, Khodadadi A A, Mamaghani A H, et al. Microporous titania–silica nanocomposite catalyst-adsorbent for ultra-deep oxidative desulfurization[J]. Applied Catalysis B-Environmental, 2016, 180: 65-77. |
| 17 | Xue Y, Li Y, Zhang J, et al. 2D graphdiyne materials: challenges and opportunities in energy field[J]. Science China-Chemistry, 2018, 61(7): 765-786. |
| 18 | Yan Z, Lin J, Yuan X, et al. Desulfurization of model oil by selective adsorption over porous boron nitride fibers with tailored microstructures[J]. Scientific Reports, 2017, 7(1): 3297-3304. |
| 19 | Portehault D, Giordano C, Gervais C, et al. High-surface-area nanoporous boron carbon nitrides for hydrogen storage[J]. Advanced Functional Materials, 2010, 20: 1827-1833. |
| 20 | 刘畅, 闫志义, 李巧灵, 等. 选择吸附脱硫研究进展[J]. 化工进展, 2019, 38(11): 5114-5126. |
| Liu C, Yan Z Y, Li Q L, et al. Research progress of selective adsorption desulfurization[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 5114-5126. | |
| 21 | Delley B J. An all-electron numerical method for solving the local density functional for polyatomic molecules[J]. The Journal of Chemical Physics, 1990, 92(1): 508-517. |
| 22 | Delley B J. From molecules to solids with the Dmol3 approach[J]. The Journal of Chemical Physics, 2000, 113(18): 7756-7764. |
| 23 | Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple[J]. Physical Review Letters, 1996, 77(18): 3865-3868. |
| 24 | Koelling D D, Harmon B N J. A technique for relativistic spin-polarised calculations[J]. Journal of Physics C: Solid State Physics, 2001, 10(16): 3107-3114. |
| 25 | Pulay P J. Improved SCF convergence acceleration[J]. Journal of Computational Chemistry, 1982, 3(4): 556-560. |
| 26 | Zhang H, Tong C J, Zhang Y, et al. Porous BN for hydrogen generation and storage[J]. Journal of Materials Chemistry A, 2015, 3(18): 9632-9637. |
| 27 | Li L, Yu X, Yang X, et al. Porous BN with vacancy defects for selective removal of CO from H2 feed gas in hydrogen fuel cells: a DFT study[J]. Journal of Materials Chemistry A, 2016, 4(40): 15631-15637. |
| 28 | Zhang S, Northrup J E. Chemical potential dependence of defect formation energies in GaAs: application to Ga self-diffusion[J]. Physical Review Letters, 1991, 67(17): 2339-2342. |
| 29 | Azevedo S, Kaschny J R, de Castilho C M C, et al. A theoretical investigation of defects in a boron nitride monolayer[J]. Nanotechnology, 2007, 18(49): 495707. |
| 30 | Ding S J, Zhou Y S, Wei Q, et al. Substituent effects of 4, 6-DMDBT on direct hydrodesulfurization routes catalyzed by Ni-Mo-S active nanocluster—a theoretical study[J]. Catalysis Today, 2018, 305: 28-39. |
| 31 | 王广建, 仙保震, 刘影. 等. 吸附法脱除柴油中噻吩类含硫化合物的研究进展[J]. 化工进展, 2014, 33(10): 2764-2770. |
| Wang G J, Xian B Z, Liu Y, et al. Advances on adsorptive desulfurization of diesel for thiophenic sulfur compounds[J]. Chemical Industry and Engineering Progress, 2014, 33(10): 2764-2770. | |
| 32 | Nag A, Raidongia K, Hembram K P, et al. Graphene analogues of BN: novel synthesis and properties[J]. ACS Nano, 2010, 4(3): 1539-1544. |
| [1] | 叶展羽, 山訸, 徐震原. 用于太阳能蒸发的折纸式蒸发器性能仿真[J]. 化工学报, 2023, 74(S1): 132-140. |
| [2] | 张龙, 宋孟杰, 邵苛苛, 张旋, 沈俊, 高润淼, 甄泽康, 江正勇. 管翅式换热器迎风侧翅片末端霜层生长模拟研究[J]. 化工学报, 2023, 74(S1): 179-182. |
| [3] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [4] | 王志国, 薛孟, 董芋双, 张田震, 秦晓凯, 韩强. 基于裂隙粗糙性表征方法的地热岩体热流耦合数值模拟与分析[J]. 化工学报, 2023, 74(S1): 223-234. |
| [5] | 宋嘉豪, 王文. 斯特林发动机与高温热管耦合运行特性研究[J]. 化工学报, 2023, 74(S1): 287-294. |
| [6] | 张思雨, 殷勇高, 贾鹏琦, 叶威. 双U型地埋管群跨季节蓄热特性研究[J]. 化工学报, 2023, 74(S1): 295-301. |
| [7] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [8] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [9] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [10] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [11] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [12] | 赵佳佳, 田世祥, 李鹏, 谢洪高. SiO2-H2O纳米流体强化煤尘润湿性的微观机理研究[J]. 化工学报, 2023, 74(9): 3931-3945. |
| [13] | 刘远超, 关斌, 钟建斌, 徐一帆, 蒋旭浩, 李耑. 单层XSe2(X=Zr/Hf)的热电输运特性研究[J]. 化工学报, 2023, 74(9): 3968-3978. |
| [14] | 何松, 刘乔迈, 谢广烁, 王斯民, 肖娟. 高浓度水煤浆管道气膜减阻两相流模拟及代理辅助优化[J]. 化工学报, 2023, 74(9): 3766-3774. |
| [15] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号