化工学报 ›› 2025, Vol. 76 ›› Issue (5): 2011-2025.DOI: 10.11949/0438-1157.20240819
收稿日期:2024-07-18
修回日期:2024-08-28
出版日期:2025-05-25
发布日期:2025-06-13
通讯作者:
周阿武,李建荣
作者简介:安昊天(1997—),男,博士研究生,anhaotian@emails.bjut.edu.cn
基金资助:
Haotian AN( ), Zhangye HAN, Muyao LU, Awu ZHOU(
), Zhangye HAN, Muyao LU, Awu ZHOU( ), Jianrong LI(
), Jianrong LI( )
)
Received:2024-07-18
Revised:2024-08-28
Online:2025-05-25
Published:2025-06-13
Contact:
Awu ZHOU, Jianrong LI
摘要:
金属-有机框架(MOF)是一类由金属离子或金属簇与有机配体通过配位键形成的新型多孔材料。它们展现出结构多样、高比表面积、高孔隙率、结构与性质多样化调控等优点。近年来,MOF在吸附、分离、催化和传感等不同领域的应用逐渐受到关注。然而,MOF宏量制备面临着合成条件严苛、反应时间延长、产量低以及成本高等化学工程领域的挑战。因此,研究人员正在探索各种新的合成方法,如水热/溶剂热法、常温快速合成法、溶剂回流法、碱辅助法、机械化学法、电化学法、微波辅助法、超声波辅助法、喷雾干燥法、干凝胶转化法、加速老化法等。以此为主题,综述了宏量制备MOF的方法,并对成本、环境影响、安全性和可行性进行了评价,也简要讨论了MOF成型加工技术的最新进展,展望了未来MOF材料在宏量制备与成型方面的挑战与机遇。
中图分类号:
安昊天, 韩章烨, 陆慕瑶, 周阿武, 李建荣. 推进MOF产业化应用:宏量制备与成型[J]. 化工学报, 2025, 76(5): 2011-2025.
Haotian AN, Zhangye HAN, Muyao LU, Awu ZHOU, Jianrong LI. Promoting industrial application of MOF: scale-up preparation and shaping[J]. CIESC Journal, 2025, 76(5): 2011-2025.
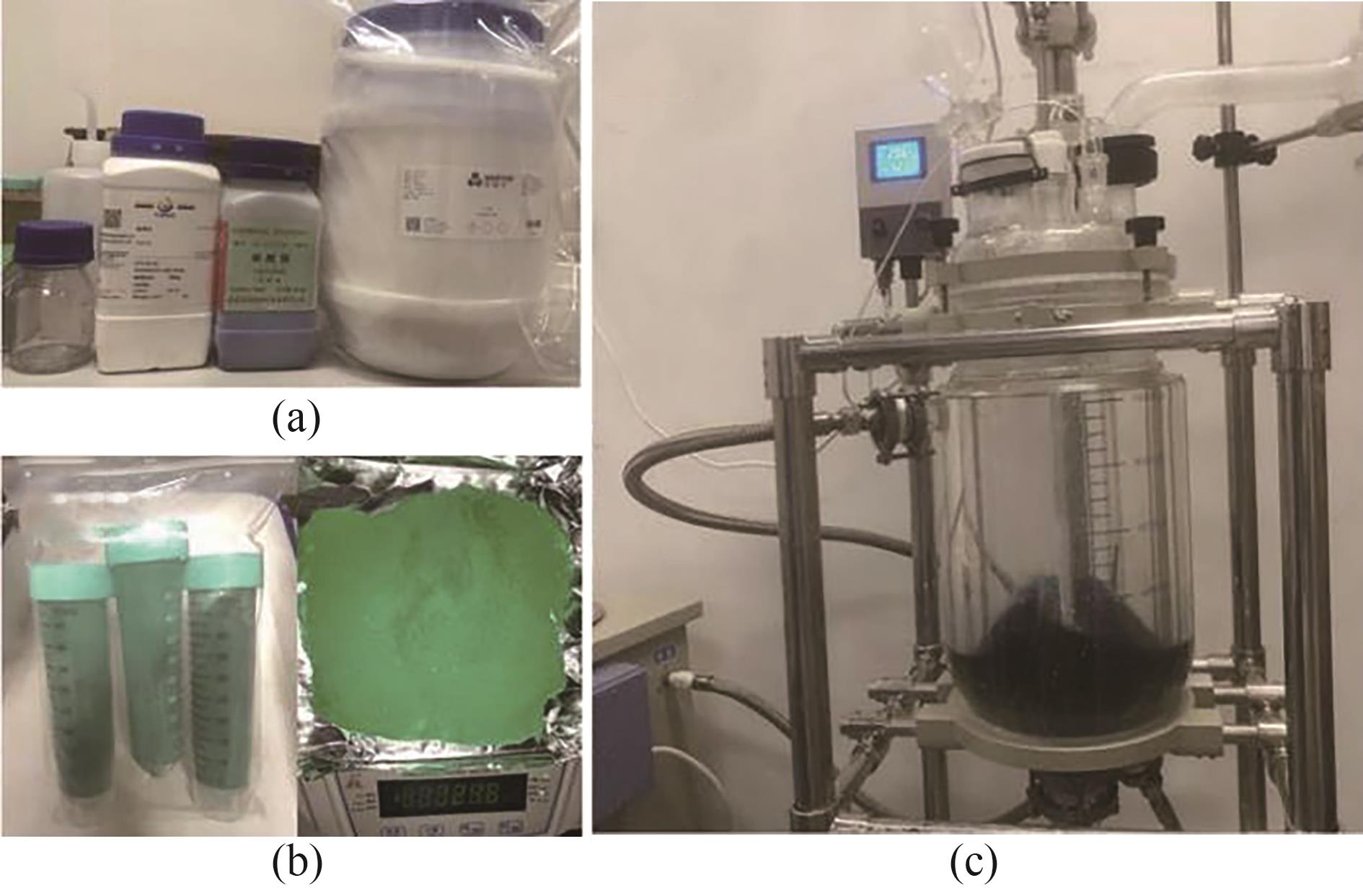
图3 宏量制备Cu-AD-SA:(a)原料;(b)产物;(c)反应装置[25]
Fig.3 Large-scale synthesis of Cu-AD-SA: (a) the starting materials; (b) the products; (c) the reaction setup[25]
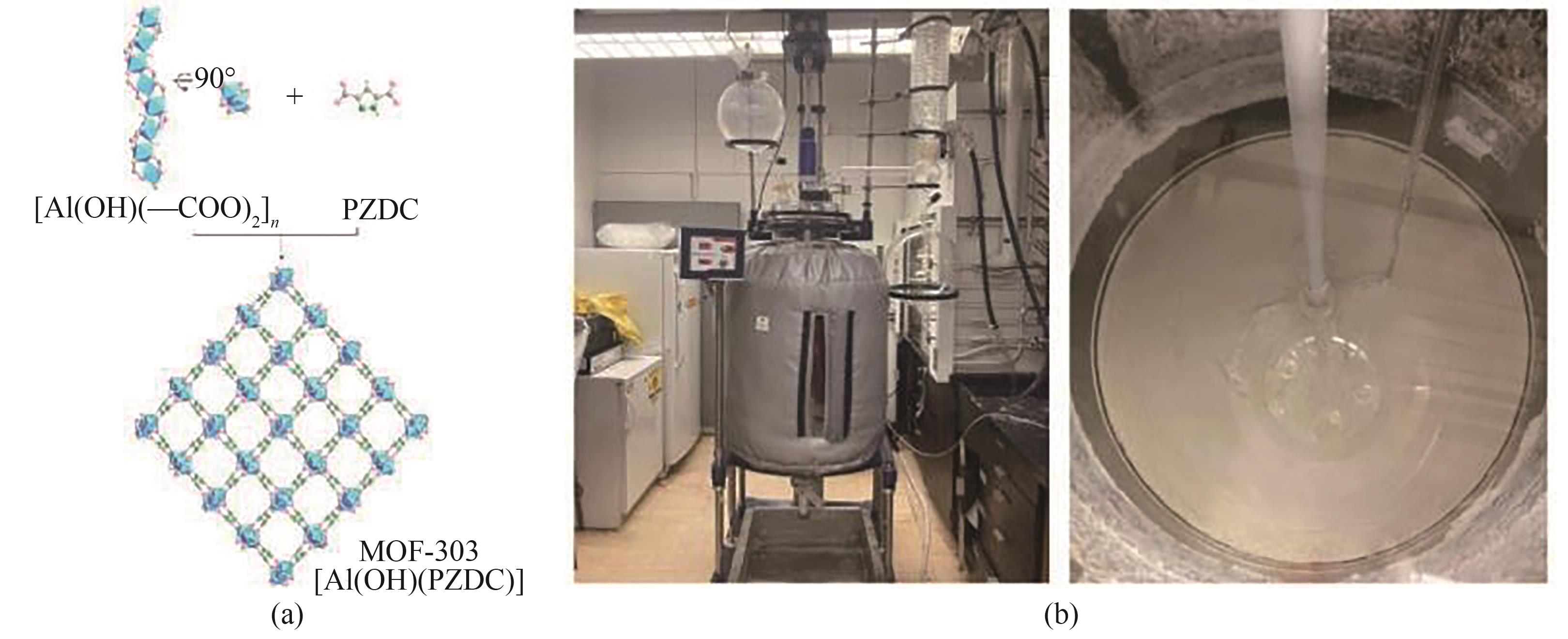
图4 (a) MOF-303结构示意图;(b) 在200 L反应容器中合成3.5 kg规模的MOF-303[28]
Fig.4 (a) Schematic of MOF-303 structure; (b) Synthesis of 3.5 kg scale MOF-303 in a 200 L reaction vessel[28]
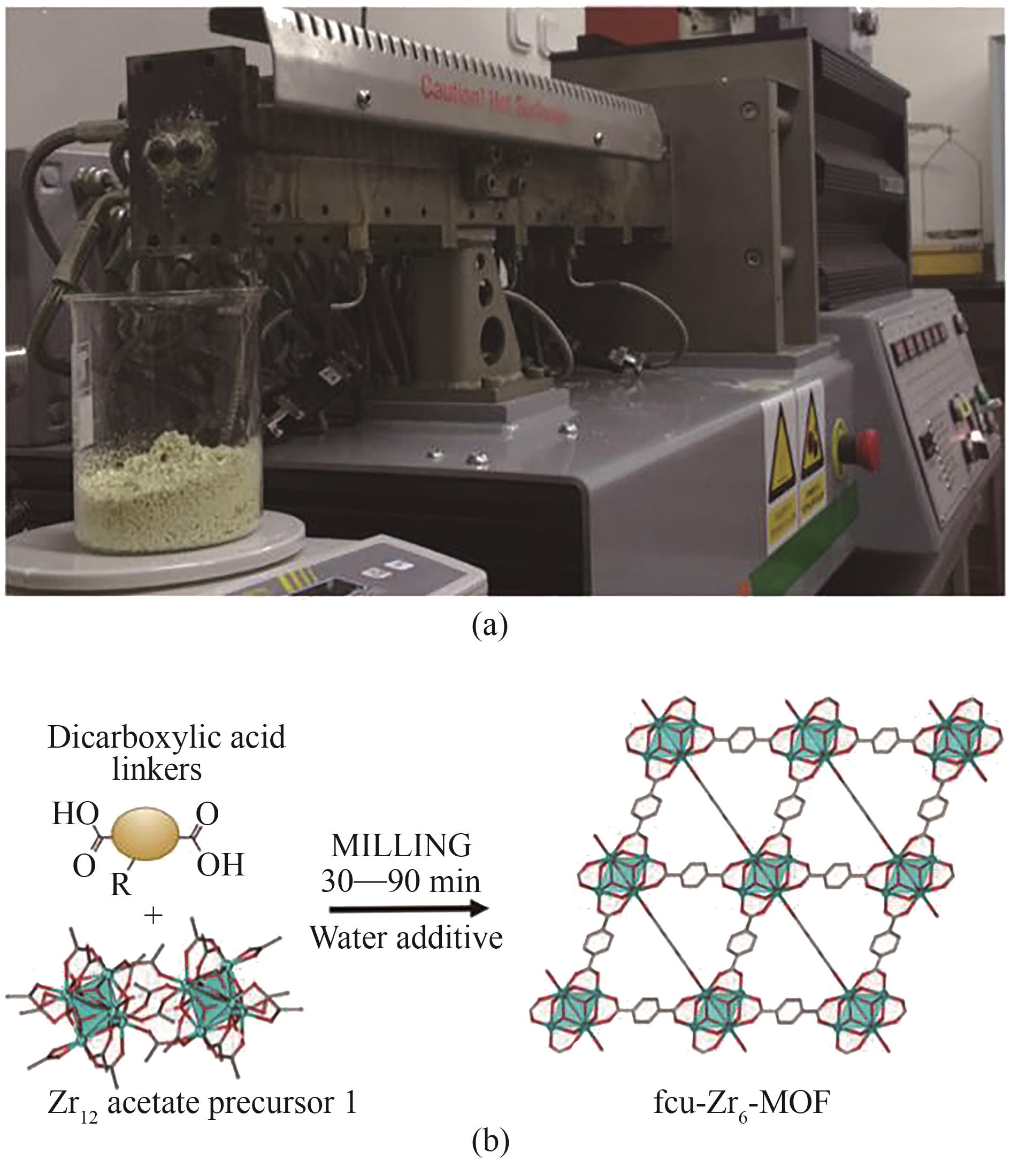
图5 (a)用于UiO-66-NH2连续生产的双螺杆挤出装置示意图; (b)水辅助机械化学绿色合成Zr-MOF示意图[38]
Fig.5 (a) TSE setup used for continuous mechanochemical synthesis of UiO-66-NH2; (b) Water-assisted mechanochemical procedure for green synthesis of zirconium MOF[38]
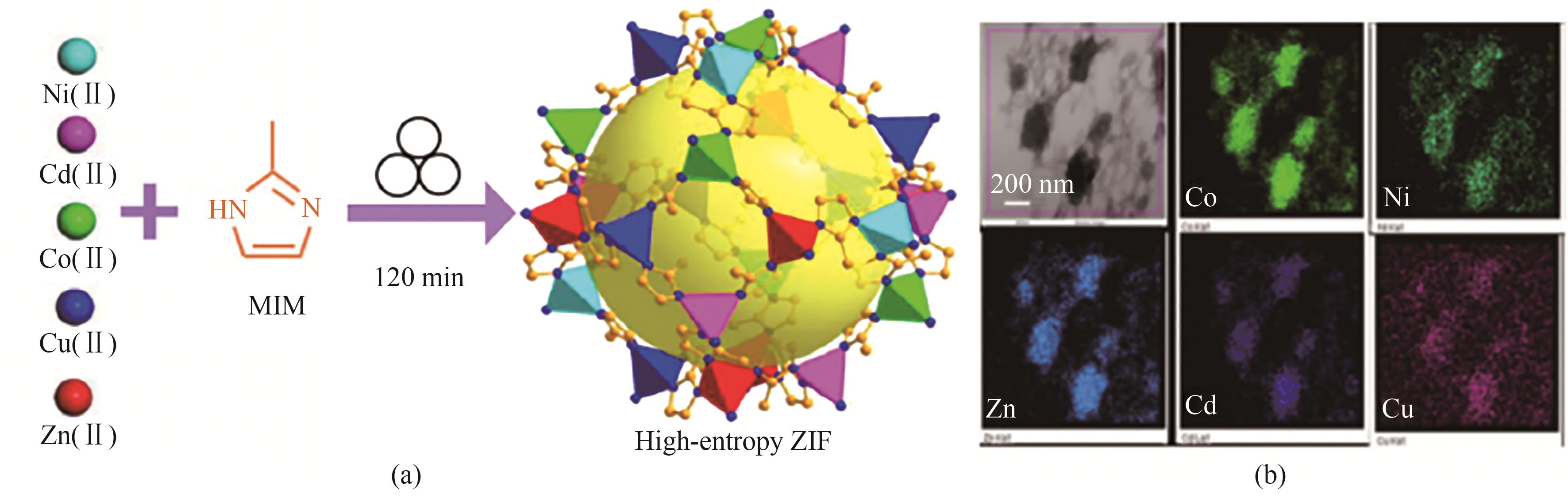
图6 (a)高熵-ZIF的机械化学合成示意图;(b)高熵-ZIF的TEM图像和EDS元素分布图[39]
Fig.6 (a) Schematic of mechanochemical synthesis of high-entropy ZIFs; (b) TEM image and EDS elemental distribution of high-entropy ZIFs[39]
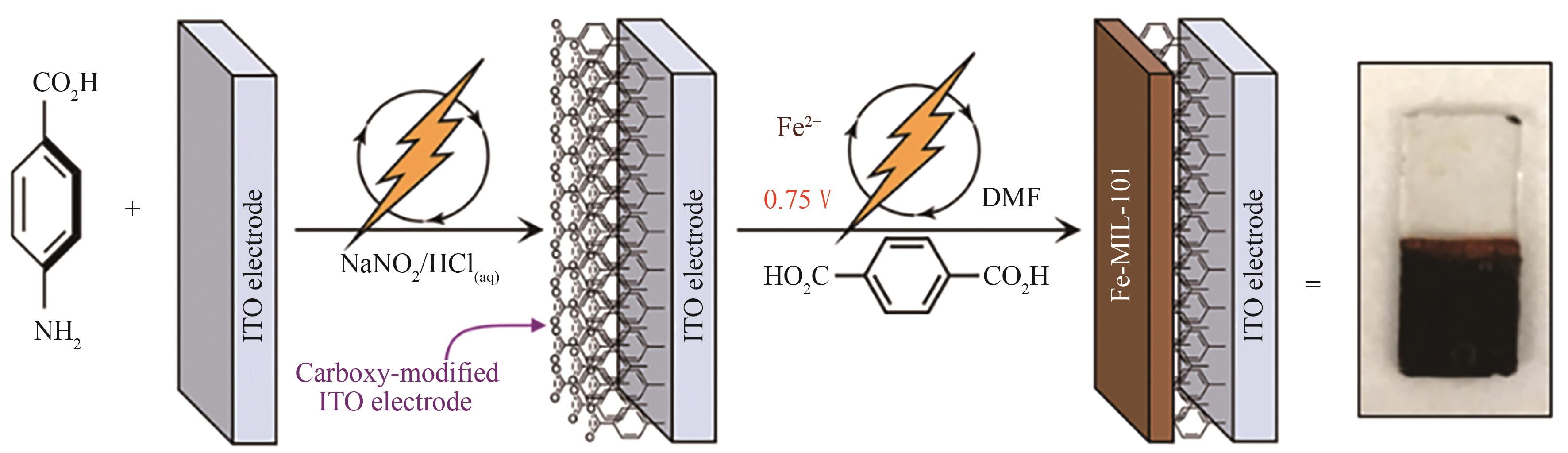
图7 在羧基功能化的ITO电极表面直接电化学合成MIL-101(Fe)薄膜[45]
Fig.7 Surface functionalization of ITO electrodes with carboxylic acid groups enables direct electrosynthesis of MIL-101(Fe) films on the inert conducting support[45]
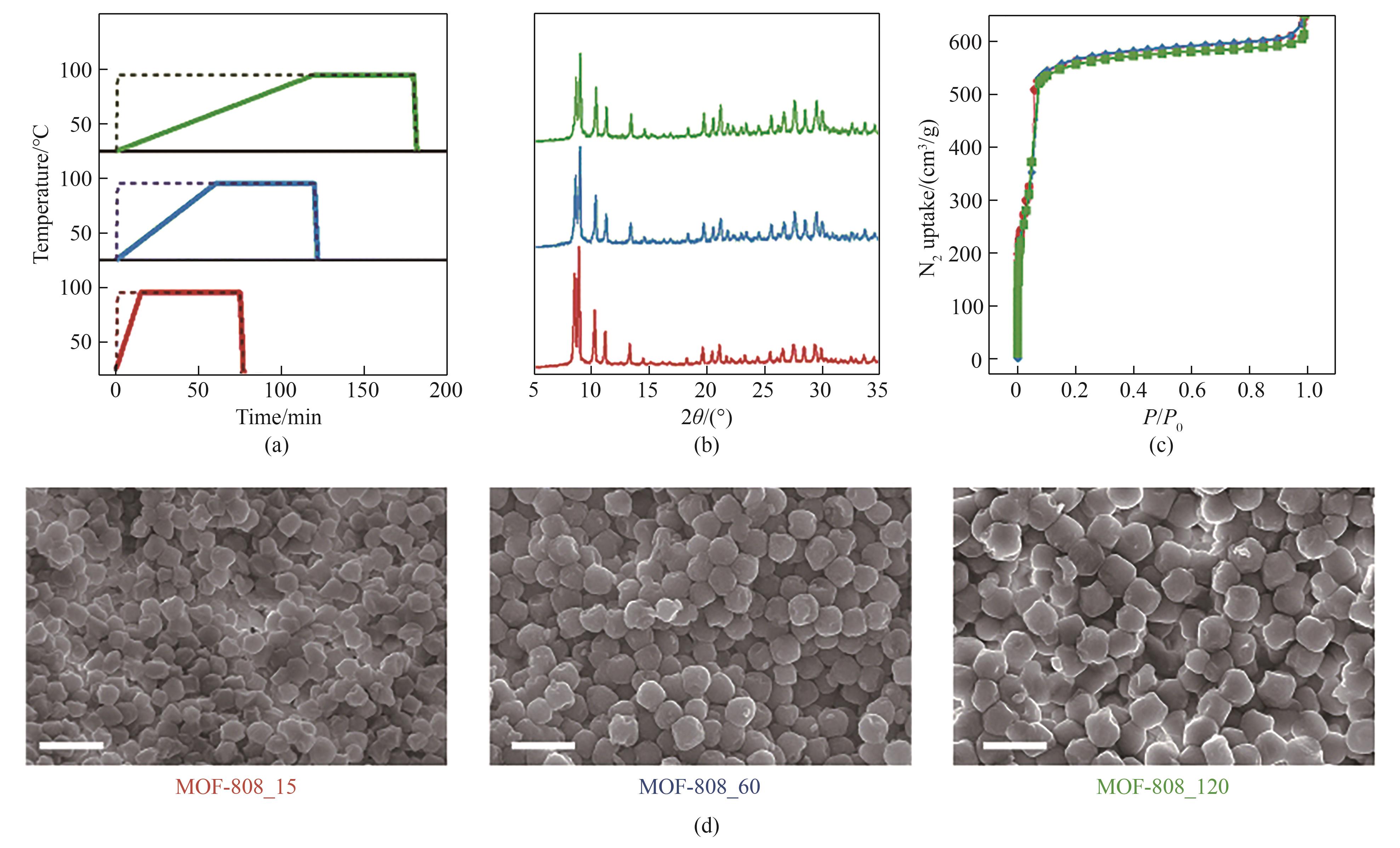
图8 MOF-808_15(红色)、MOF-808_60(蓝色)和 MOF-808_120(绿色)的:(a)用于制备MOF-808(实线)和对照材料(虚线)的受控加热斜率;(b) 粉末X射线衍射图;(c) 77 K下的N2吸附曲线;(d) SEM照片(比例尺:1 μm)[59]
Fig.8 MOF-808_15 (red), MOF-808_60 (blue) and MOF-808_120 (green): (a) controlled-heating ramp used in the preparation of MOF-808 (continuous lines) and control materials (dashed lines); (b) powder X-ray diffractograms; (c) N2 adsorption at 77 K; (d) SEM images (scale bar: 1 μm)[59]
| 宏量制备方法 | 典型MOF | 温度/℃ | 时间 | 溶剂 | 金属源 | 时空产率/ (kg/(m3·d)) | 文献 |
|---|---|---|---|---|---|---|---|
| 加速老化法 | Cd-MOF | 45 | 4 d | — | CdO | — | [ |
| 水热法/溶剂热法 | UiO-66 | 120 | 24 h | DMF | ZrCl4 | — | [ |
| HKUST-1 | 90 | 12 min | DMF、乙醇、水 | Cu(NO3)2·H2O | 5.8 | [ | |
| MOF-5 | 120 | 12 min | DMF | Zn(NO3)2·6H2O | — | ||
| IRMOF-3 | 120 | 12 min | DMF | Zn(NO3)2·6H2O | — | ||
| UiO-66 | 140 | 15 min | DMF | ZrCl4 | — | ||
| 常温快速合成法/ 溶剂回流法 | UiO-66-(COOH)2 | 100 | 16 h | 水 | ZrCl4 | 96 | [ |
| MIP-202(Zr) | 120 | 1 h | 水 | ZrCl4 | 7030 | [ | |
| MOF-801(Zr) | 室温 | 5.5 h | 水 | ZrOCl2·8H2O | 168 | [ | |
| NKMOF-8-Br | 室温 | 1 min | 乙腈 | CuI | — | [ | |
| Cu-AD-SA | 80 | 24 h | DMF、水 | Cu(NO3)2·3H2O | 160 | [ | |
| 碱辅助法 | MOF-5 | 室温 | 24 h | DMF | Zn(NO3)2·6H2O | — | [ |
| MIL-53(Al) | 室温 | 7 d | 水 | Al(NO3)3·9H2O | — | ||
| MOF-74 | 室温 | 20 h | 水 | Zn(CH3COO)2·2H2O | — | ||
| MOF-303 | 120 | 6 h | 水 | AlCl3·6H2O | 179.5 | [ | |
| 机械化学法 | ZIF-8 | 室温 | 96 h | — | ZnO | — | [ |
| ZIF-8 | 室温 | 2 h | 水 | Zn(CH3COO)2·2H2O | — | [ | |
| MOF-74(Zn) | 室温 | 70 min | 水 | ZnO | — | [ | |
| Fe-MIL-88A | 室温 | 10 min | — | FeCl3·6H2O | — | [ | |
| HKUST-1 | 室温 | — | 乙醇 | Cu(OH)2 | 144000 | [ | |
| ZIF-8 | 200 | — | — | [ZnCO3]2[Zn(OH)2]3 | 144000 | ||
| Al-Fum | 室温 | — | — | Al2(SO4)3·18H2O | 27000 | ||
| UiO-66 | 室温 | 90 min | 水 | 异丙醇锆 | — | [ | |
| 电化学法 | Fe-MIL-101 | 室温 | 14 h | DMF | FeCl2 | — | [ |
| Fe-MIL-101-NH2 | 室温 | 8 h | DMF | FeCl2 | — | ||
| Fe-MIL-100 | 室温 | 18 h | DMF | FeCl2 | — | ||
| Fe-MIL-88B-NH2 | 室温 | 2 h | DMF | FeCl2 | — | ||
| 微波辅助法 | MOF-74 (Ni) | 125 | 60 min | DMF、乙醇、水 | Ni(NO3)2·6H2O | — | [ |
| MOF-808 | 95 | 1 h | 水 | ZrOCl2·8H2O | — | [ | |
| 超声波辅助法 | CPO-27-Co | 70 | 75 min | DMF、水 | Co(NO3)2·6H2O | — | [ |
| MIL-53(Fe) | — | 7 min | DMF | FeCl3·6H2O | — | [ | |
| 喷雾干燥法 | UiO-66 | 220 | — | DMF | 乙酰丙酮锆 | — | [ |
| CID-1 | 220 | — | DMF | Zn(NO3)2·4H2O | — | ||
| 干凝胶转换法 | MIL-100(Fe) | 室温 | 30 min | 水 | Fe(NO3)3·9H2O | — | [ |
表1 部分MOF宏量制备工作主要参数
Table 1 Some key parameters related to the scale-up synthesis of MOFs
| 宏量制备方法 | 典型MOF | 温度/℃ | 时间 | 溶剂 | 金属源 | 时空产率/ (kg/(m3·d)) | 文献 |
|---|---|---|---|---|---|---|---|
| 加速老化法 | Cd-MOF | 45 | 4 d | — | CdO | — | [ |
| 水热法/溶剂热法 | UiO-66 | 120 | 24 h | DMF | ZrCl4 | — | [ |
| HKUST-1 | 90 | 12 min | DMF、乙醇、水 | Cu(NO3)2·H2O | 5.8 | [ | |
| MOF-5 | 120 | 12 min | DMF | Zn(NO3)2·6H2O | — | ||
| IRMOF-3 | 120 | 12 min | DMF | Zn(NO3)2·6H2O | — | ||
| UiO-66 | 140 | 15 min | DMF | ZrCl4 | — | ||
| 常温快速合成法/ 溶剂回流法 | UiO-66-(COOH)2 | 100 | 16 h | 水 | ZrCl4 | 96 | [ |
| MIP-202(Zr) | 120 | 1 h | 水 | ZrCl4 | 7030 | [ | |
| MOF-801(Zr) | 室温 | 5.5 h | 水 | ZrOCl2·8H2O | 168 | [ | |
| NKMOF-8-Br | 室温 | 1 min | 乙腈 | CuI | — | [ | |
| Cu-AD-SA | 80 | 24 h | DMF、水 | Cu(NO3)2·3H2O | 160 | [ | |
| 碱辅助法 | MOF-5 | 室温 | 24 h | DMF | Zn(NO3)2·6H2O | — | [ |
| MIL-53(Al) | 室温 | 7 d | 水 | Al(NO3)3·9H2O | — | ||
| MOF-74 | 室温 | 20 h | 水 | Zn(CH3COO)2·2H2O | — | ||
| MOF-303 | 120 | 6 h | 水 | AlCl3·6H2O | 179.5 | [ | |
| 机械化学法 | ZIF-8 | 室温 | 96 h | — | ZnO | — | [ |
| ZIF-8 | 室温 | 2 h | 水 | Zn(CH3COO)2·2H2O | — | [ | |
| MOF-74(Zn) | 室温 | 70 min | 水 | ZnO | — | [ | |
| Fe-MIL-88A | 室温 | 10 min | — | FeCl3·6H2O | — | [ | |
| HKUST-1 | 室温 | — | 乙醇 | Cu(OH)2 | 144000 | [ | |
| ZIF-8 | 200 | — | — | [ZnCO3]2[Zn(OH)2]3 | 144000 | ||
| Al-Fum | 室温 | — | — | Al2(SO4)3·18H2O | 27000 | ||
| UiO-66 | 室温 | 90 min | 水 | 异丙醇锆 | — | [ | |
| 电化学法 | Fe-MIL-101 | 室温 | 14 h | DMF | FeCl2 | — | [ |
| Fe-MIL-101-NH2 | 室温 | 8 h | DMF | FeCl2 | — | ||
| Fe-MIL-100 | 室温 | 18 h | DMF | FeCl2 | — | ||
| Fe-MIL-88B-NH2 | 室温 | 2 h | DMF | FeCl2 | — | ||
| 微波辅助法 | MOF-74 (Ni) | 125 | 60 min | DMF、乙醇、水 | Ni(NO3)2·6H2O | — | [ |
| MOF-808 | 95 | 1 h | 水 | ZrOCl2·8H2O | — | [ | |
| 超声波辅助法 | CPO-27-Co | 70 | 75 min | DMF、水 | Co(NO3)2·6H2O | — | [ |
| MIL-53(Fe) | — | 7 min | DMF | FeCl3·6H2O | — | [ | |
| 喷雾干燥法 | UiO-66 | 220 | — | DMF | 乙酰丙酮锆 | — | [ |
| CID-1 | 220 | — | DMF | Zn(NO3)2·4H2O | — | ||
| 干凝胶转换法 | MIL-100(Fe) | 室温 | 30 min | 水 | Fe(NO3)3·9H2O | — | [ |
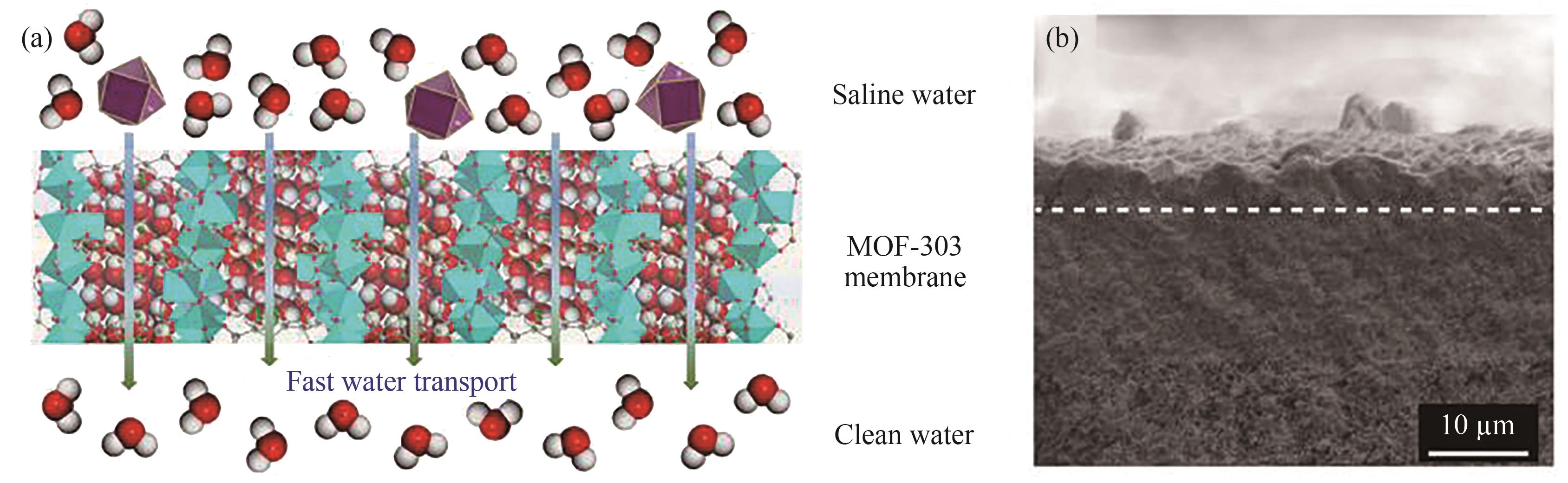
图9 (a)用于海水淡化的MOF-303膜;(b)在α-Al2O3表面上生长的MOF-303膜横截面SEM图[78]
Fig. 9 (a) MOF-303 membrane for seawater desalination; (b) SEM image of the cross-section of MOF-303 membrane grown on α-Al2O3 surface[78]
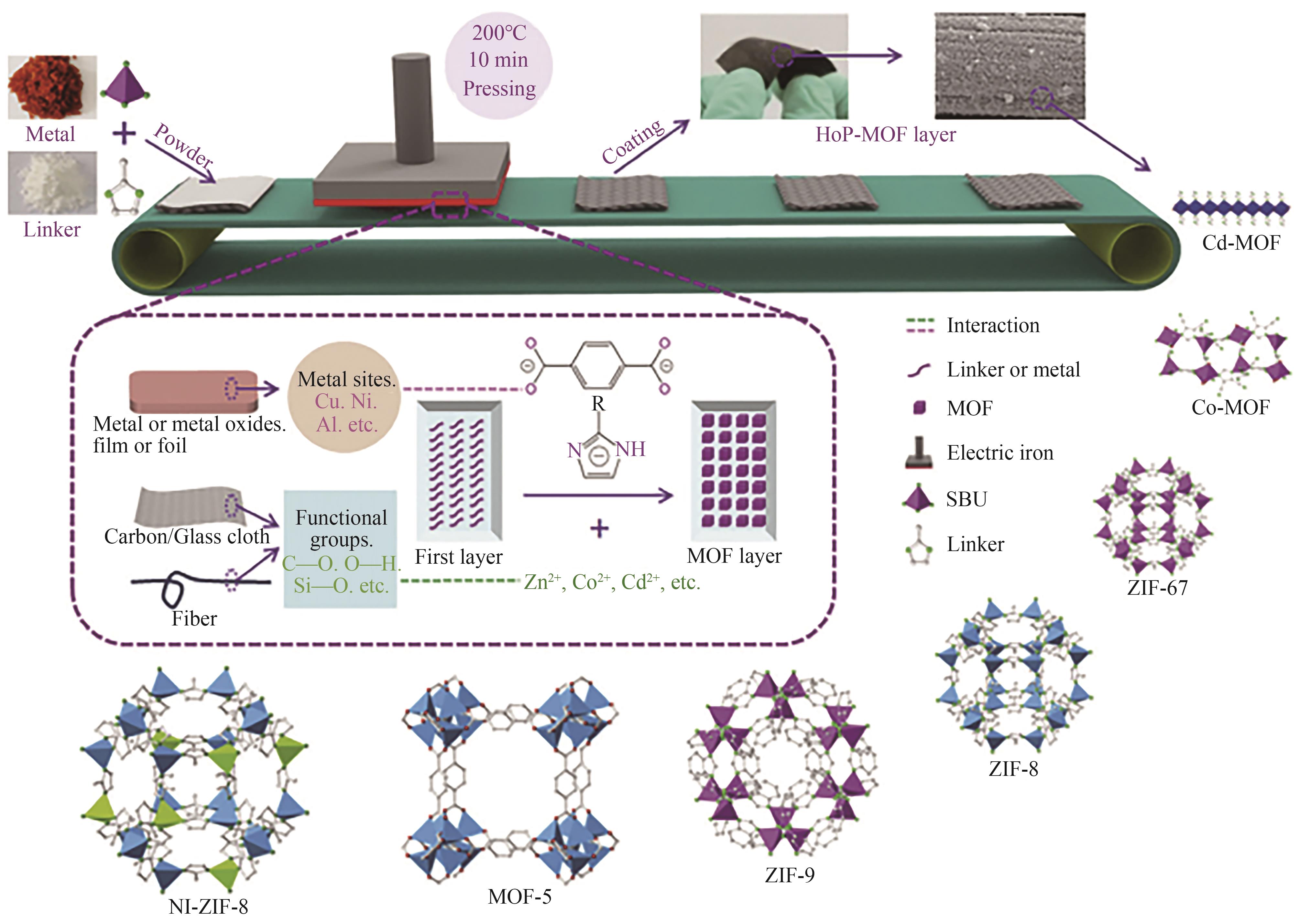
图10 热压法生产MOF涂层示意图(SBU为次级构建单元)[79]
Fig.10 Schematic presentation of the hot-pressing (HoP) method for MOF coating (SBU is the secondary building unit)[79]
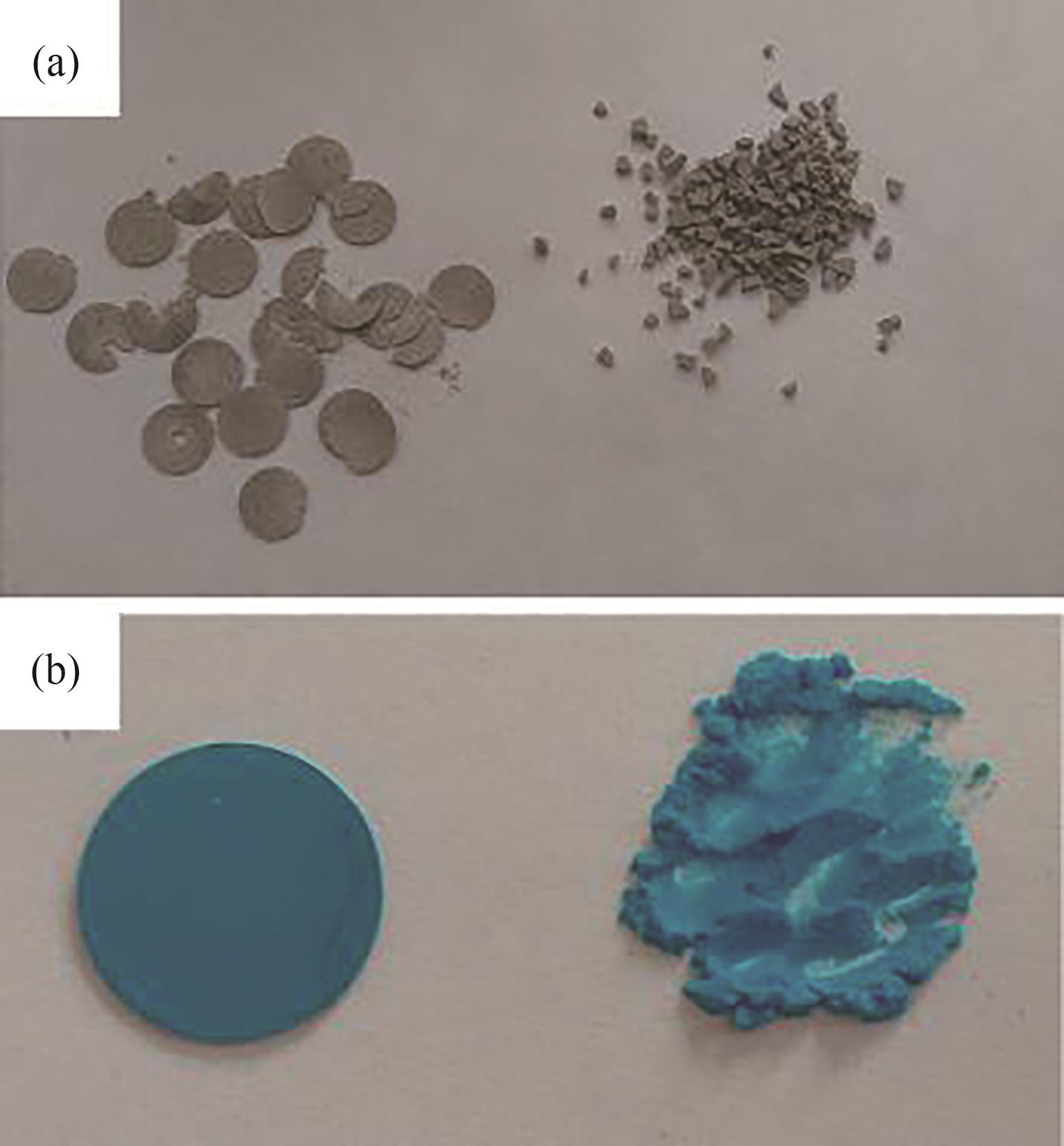
图12 粉末和压片成型的(a) MIL-53(Al)(压片、碎片),(b) Cu3(BTC)2(压片、粉末)[84]
Fig.12 Powder and pelletized (a) MIL-53(Al) (tablets, crushed tablets), (b) Cu3(BTC)2 (tablet, powder)[84]

图13 使用不同黏合剂制备的ZUL-300颗粒:(a) ZUL-300(左)和ZUL-300@HPC(右),(b) ZUL-300(左)和ZUL-300@PVB(右),(c) ZUL-300(左)和ZUL-300@PVA(右)[87]
Fig.13 ZUL-300 pellets prepared with different binders: (a) ZUL-300 (left) and ZUL-300@HPC (right), (b) ZUL-300 (left) and ZUL-300@PVB (right), (c) ZUL-300 (left) and ZUL-300@PVA (right)[87]
| 21 | Yang Q Y, Vaesen S, Ragon F, et al. A water stable metal-organic framework with optimal features for CO2 capture[J]. Angewandte Chemie International Edition, 2013, 52(39): 10316-10320. |
| 22 | Wang S J, Wahiduzzaman M, Davis L, et al. A robust zirconium amino acid metal-organic framework for proton conduction[J]. Nature Communications, 2018, 9(1): 4937. |
| 23 | Dai S, Nouar F, Zhang S J, et al. One-step room-temperature synthesis of metal (Ⅳ) carboxylate metal-organic frameworks[J]. Angewandte Chemie International Edition, 2021, 133(8): 4328-4334. |
| 24 | Geng S B, Lin E, Li X, et al. Scalable room-temperature synthesis of highly robust ethane-selective metal-organic frameworks for efficient ethylene purification[J]. Journal of the American Chemical Society, 2021, 143(23): 8654-8660. |
| 25 | Wang L, Wang K C, An H T, et al. A hydrolytically stable Cu(Ⅱ)-based metal-organic framework with easily accessible ligands for water harvesting[J]. ACS Applied Materials & Interfaces, 2021, 13(41): 49509-49518. |
| 26 | Sánchez-Sánchez M, Getachew N, Díaz K, et al. Synthesis of metal-organic frameworks in water at room temperature: salts as linker sources[J]. Green Chemistry, 2015, 17(3): 1500-1509. |
| 27 | Fathieh F, Kalmutzki M J, Kapustin E A, et al. Practical water production from desert air[J]. Science Advances, 2018, 4(6): eaat3198. |
| 28 | Zheng Z L, Nguyen H L, Hanikel N, et al. High-yield, green and scalable methods for producing MOF-303 for water harvesting from desert air[J]. Nature Protocols, 2023, 18(1): 136-156. |
| 29 | An H T, Zhang X, Dong C, et al. Seed-aided green synthesis of metal-organic frameworks in water[J]. Green Chemical Engineering, 2023, 4(1): 64-72. |
| 30 | Martinez V, Stolar T, Karadeniz B, et al. Advancing mechanochemical synthesis by combining milling with different energy sources[J]. Nature Reviews Chemistry, 2023, 7(1): 51-65. |
| 31 | Friščić T. New opportunities for materials synthesis using mechanochemistry[J]. Journal of Materials Chemistry, 2010, 20(36): 7599-7605. |
| 32 | Głowniak S, Szczęśniak B, Choma J, et al. Mechanochemistry: toward green synthesis of metal-organic frameworks[J]. Materials Today, 2021, 46: 109-124. |
| 33 | Tanaka S, Kida K, Nagaoka T, et al. Mechanochemical dry conversion of zinc oxide to zeolitic imidazolate framework[J]. Chemical Communications, 2013, 49(72): 7884-7886. |
| 34 | Taheri M, Enge T G, Tsuzuki T. Water stability of cobalt doped ZIF-8: a quantitative study using optical analyses[J]. Materials Today Chemistry, 2020, 16: 100231. |
| 35 | Julien P A, Užarević K, Katsenis A D, et al. In situ monitoring and mechanism of the mechanochemical formation of a microporous MOF-74 framework[J]. Journal of the American Chemical Society, 2016, 138(9): 2929-2932. |
| 36 | Jeong H, Lee J. 3D-superstructured networks comprising Fe-MIL-88A metal-organic frameworks under mechanochemical conditions[J]. European Journal of Inorganic Chemistry, 2019, 2019(42): 4597-4600. |
| 37 | Crawford D, Casaban J, Haydon R, et al. Synthesis by extrusion: continuous, large-scale preparation of MOFs using little or no solvent[J]. Chemical Science, 2015, 6(3): 1645-1649. |
| 38 | Karadeniz B, Howarth A J, Stolar T, et al. Benign by design: green and scalable synthesis of zirconium UiO-metal-organic frameworks by water-assisted mechanochemistry[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 15841-15849. |
| 39 | Xu W, Chen H, Jie K C, et al. Entropy-driven mechanochemical synthesis of polymetallic zeolitic imidazolate frameworks for CO2 fixation[J]. Angewandte Chemie International Edition, 2019, 58(15): 5018-5022. |
| 40 | Stolle A, Szuppa T, Leonhardt S E S, et al. Ball milling in organic synthesis: solutions and challenges[J]. Chemical Society Reviews, 2011, 40(5): 2317-2329. |
| 41 | James S L, Adams C J, Bolm C, et al. Mechanochemistry: opportunities for new and cleaner synthesis[J]. Chemical Society Reviews, 2012, 41(1): 413-447. |
| 42 | Friščić T, Mottillo C, Titi H M. Mechanochemistry for synthesis[J]. Angewandte Chemie International Edition, 2020, 59(3): 1018-1029. |
| 43 | Mueller U, Schubert M, Teich F, et al. Metal-organic frameworks—Prospective industrial applications[J]. Journal of Materials Chemistry, 2006, 16(7): 626-636. |
| 44 | Schäfer P, van der Veen M A, Domke K F. Unraveling a two-step oxidation mechanism in electrochemical Cu-MOF synthesis[J]. Chemical Communications, 2016, 52(25): 4722-4725. |
| 45 | Wu W B, Decker G E, Weaver A E, et al. Facile and rapid room-temperature electrosynthesis and controlled surface growth of Fe-MIL-101 and Fe-MIL-101-NH2 [J]. ACS Central Science, 2021, 7(8): 1427-1433. |
| 46 | Phan P T, Hong J, Tran N, et al. The properties of microwave-assisted synthesis of metal-organic frameworks and their applications[J]. Nanomaterials, 2023, 13(2): 352. |
| 47 | Khan N A, Jhung S H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: rapid reaction, phase-selectivity, and size reduction[J]. Coordination Chemistry Reviews, 2015, 285: 11-23. |
| 48 | He Q Q, Zhan F Y, Wang H Y, et al. Recent progress of industrial preparation of metal-organic frameworks: synthesis strategies and outlook[J]. Materials Today Sustainability, 2022, 17: 100104. |
| 49 | Ryu U J, Jee S H, Rao P C, et al. Recent advances in process engineering and upcoming applications of metal-organic frameworks[J]. Coordination Chemistry Reviews, 2021, 426: 213544. |
| 50 | Thomas-Hillman I, Laybourn A, Dodds C, et al. Realising the environmental benefits of metal-organic frameworks: recent advances in microwave synthesis[J]. Journal of Materials Chemistry A, 2018, 6(25): 11564-11581. |
| 51 | Lai L S, Yeong Y F, Lau K K, et al. Effect of synthesis parameters on the formation of ZIF-8 under microwave-assisted solvothermal[J]. Procedia Engineering, 2016, 148: 35-42. |
| 52 | Choi J S, Son W J, Kim J, et al. Metal-organic framework MOF-5 prepared by microwave heating: factors to be considered[J]. Microporous and Mesoporous Materials, 2008, 116(1/2/3): 727-731. |
| 53 | Seo Y K, Hundal G, Jang I T, et al. Microwave synthesis of hybrid inorganic-organic materials including porous Cu3(BTC)2 from Cu(Ⅱ)-trimesate mixture[J]. Microporous and Mesoporous Materials, 2009, 119(1/2/3): 331-337. |
| 54 | Couzon N, Ferreira M, Duval S, et al. Microwave-assisted synthesis of porous composites MOF-textile for the protection against chemical and nuclear hazards[J]. ACS Applied Materials & Interfaces, 2022, 14(18): 21497-21508. |
| 55 | Zhao Z X, Li X M, Huang S S, et al. Adsorption and diffusion of benzene on chromium-based metal organic framework MIL-101 synthesized by microwave irradiation[J]. Industrial & Engineering Chemistry Research, 2011, 50(4): 2254-2261. |
| 56 | Albuquerque G H, Fitzmorris R C, Ahmadi M, et al. Gas-liquid segmented flow microwave-assisted synthesis of MOF-74(Ni) under moderate pressures[J]. CrystEngComm, 2015, 17(29): 5502-5510. |
| 57 | Chen C W, Feng X B, Zhu Q, et al. Microwave-assisted rapid synthesis of well-shaped MOF-74 (Ni) for CO2 efficient capture[J]. Inorganic Chemistry, 2019, 58(4): 2717-2728. |
| 58 | Cho H Y, Yang D A, Kim J, et al. CO2 adsorption and catalytic application of Co-MOF-74 synthesized by microwave heating[J]. Catalysis Today, 2012, 185(1): 35-40. |
| 59 | González L, Gil-San-Millán R, Navarro J A R, et al. Green synthesis of zirconium MOF-808 for simultaneous phosphate recovery and organophosphorus pesticide detoxification in wastewater[J]. Journal of Materials Chemistry A, 2022, 10(37): 19606-19611. |
| 60 | Bergamelli F, Iannelli M, Marafie J A, et al. A commercial continuous flow microwave reactor evaluated for scale-up[J]. Organic Process Research & Development, 2010, 14(4): 926-930. |
| 61 | Suslick K S, Choe S B, Cichowlas A A, et al. Sonochemical synthesis of amorphous iron[J]. Nature, 1991, 353: 414-416. |
| 62 | Gedanken A. Using sonochemistry for the fabrication of nanomaterials[J]. Ultrasonics Sonochemistry, 2004, 11(2): 47-55. |
| 63 | Suslick K S, Hammerton D A, Cline R E. Sonochemical hot spot[J]. Journal of the American Chemical Society, 1986, 108(18): 5641-5642. |
| 64 | Haque E, Khan N A, Park J H, et al. Synthesis of a metal-organic framework material, iron terephthalate, by ultrasound, microwave, and conventional electric heating: a kinetic study[J]. Chemistry, 2010, 16(3): 1046-1052. |
| 65 | Haque E, Jhung S H. Synthesis of isostructural metal-organic frameworks, CPO-27s, with ultrasound, microwave, and conventional heating: effect of synthesis methods and metal ions[J]. Chemical Engineering Journal, 2011, 173(3): 866-872. |
| 66 | Gordon J, Kazemian H, Rohani S. Rapid and efficient crystallization of MIL-53(Fe) by ultrasound and microwave irradiation[J]. Microporous and Mesoporous Materials, 2012, 162: 36-43. |
| 67 | Samborska K, Poozesh S, Barańska A, et al. Innovations in spray drying process for food and pharma industries[J]. Journal of Food Engineering, 2022, 321: 110960. |
| 68 | Barbosa J, Teixeira P. Development of probiotic fruit juice powders by spray-drying: a review[J]. Food Reviews International, 2017, 33(4): 335-358. |
| 69 | Carné-Sánchez A, Imaz I, Cano-Sarabia M, et al. A spray-drying strategy for synthesis of nanoscale metal-organic frameworks and their assembly into hollow superstructures[J]. Nature Chemistry, 2013, 5(3): 203-211. |
| 70 | Mitsuka Y, Nagashima K, Kobayashi H, et al. A seed-mediated spray-drying method for facile syntheses of Zr-MOF and a pillared-layer-type MOF[J]. Chemistry Letters, 2016, 45(11): 1313-1315. |
| 71 | Xu W Y, Dong J X, Li J P, et al. A novel method for the preparation of zeolite ZSM-5[J]. Journal of the Chemical Society, Chemical Communications, 1990(10): 755-756. |
| 72 | Koekkoek A J J, Degirmenci V, Hensen E J M. Dry gel conversion of organosilane templated mesoporous silica: from amorphous to crystalline catalysts for benzeneoxidation[J]. Journal of Materials Chemistry, 2011, 21(25): 9279-9289. |
| 73 | Luo Y S, Tan B Q, Liang X H, et al. Dry gel conversion synthesis and performance of glass-fiber MIL-100(Fe) composite desiccant material for dehumidification[J]. Microporous and Mesoporous Materials, 2020, 297: 110034. |
| 74 | Echigo T, Kimata M. Crystal chemistry and genesis of organic minerals: a review of oxalate and polycyclic aromatic hydrocarbon minerals[J]. The Canadian Mineralogist, 2010, 48(6): 1329-1357. |
| 75 | Mottillo C, Lu Y N, Pham M H, et al. Mineral neogenesis as an inspiration for mild, solvent-free synthesis of bulk microporous metal-organic frameworks from metal (Zn, Co) oxides[J]. Green Chemistry, 2013, 15(8): 2121-2131. |
| 76 | O’Keefe C A, Mottillo C, Vainauskas J, et al. NMR-enhanced crystallography aids open metal-organic framework discovery using solvent-free accelerated aging[J]. Chemistry of Materials, 2020, 32(10): 4273-4281. |
| 77 | Czaja A U, Trukhan N, Müller U. Industrial applications of metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1284-1293. |
| 78 | Cong S Z, Yuan Y, Wang J X, et al. Highly water-permeable metal-organic framework MOF-303 membranes for desalination[J]. Journal of the American Chemical Society, 2021, 143(48): 20055-20058. |
| 79 | Chen Y F, Li S Q, Pei X K, et al. A solvent-free hot-pressing method for preparing metal-organic-framework coatings[J]. Angewandte Chemie International Edition, 2016, 55(10): 3419-3423. |
| 80 | Li W J, Tu M, Cao R, et al. Metal-organic framework thin films: electrochemical fabrication techniques and corresponding applications & perspectives[J]. Journal of Materials Chemistry A, 2016, 4(32): 12356-12369. |
| 81 | Cheng P, Kim M, Lim H, et al. A general approach to shaped MOF-containing aerogels toward practical water treatment application[J]. Advanced Sustainable Systems, 2020, 4(8): 2000060. |
| 82 | Lim G J H, Wu Y, Shah B B, et al. 3D-printing of pure metal-organic framework monoliths[J]. ACS Materials Letters, 2019, 1(1): 147-153. |
| 83 | Dhainaut J, Avci-Camur C, Troyano J, et al. Systematic study of the impact of MOF densification into tablets on textural and mechanical properties[J]. CrystEngComm, 2017, 19(29): 4211-4218. |
| 84 | Majchrzak-Kucęba I, Ściubidło A. Shaping metal-organic framework (MOF) powder materials for CO2 capture applications—A thermogravimetric study[J]. Journal of Thermal Analysis and Calorimetry, 2019, 138(6): 4139-4144. |
| 85 | Lv D F, Chen J Y, Yang K X, et al. Ultrahigh CO2/CH4 and CO2/N2 adsorption selectivities on a cost-effectively L-aspartic acid based metal-organic framework[J]. Chemical Engineering Journal, 2019, 375: 122074. |
| 86 | Park J, Chae Y S, Kang D W, et al. Shaping of a metal-organic framework-polymer composite and its CO2 adsorption performances from humid indoor air[J]. ACS Applied Materials & Interfaces, 2021, 13(21): 25421-25427. |
| 87 | Song Y F, Ke T, Shen J, et al. Shaped layered two-dimensional fluorinated metal-organic frameworks for highly efficient acetylene/ethylene separation[J]. Separation and Purification Technology, 2023, 323: 124377. |
| 88 | Zhang Y Y, Yuan S, Feng X, et al. Preparation of nanofibrous metal-organic framework filters for efficient air pollution control[J]. Journal of the American Chemical Society, 2016, 138(18): 5785-5788. |
| 1 | Feng L, Wang K Y, Lv X L, et al. Modular total synthesis in reticular chemistry[J]. Journal of the American Chemical Society, 2020, 142(6): 3069-3076. |
| 2 | Mukherjee S, Sensharma D, Chen K J, et al. Crystal engineering of porous coordination networks to enable separation of C2 hydrocarbons[J]. Chemical Communications, 2020, 56(72): 10419-10441. |
| 3 | Xu Y Y, Deng Y, Liu W, et al. Research progress of hydrogen energy and metal hydrogen storage materials[J]. Sustainable Energy Technologies and Assessments, 2023, 55: 102974. |
| 4 | Garg A, Almáši M, Saini R, et al. A highly stable terbium (Ⅲ) metal-organic framework MOF-76 (Tb) for hydrogen storage and humidity sensing[J]. Environmental Science and Pollution Research, 2023, 30(44): 98548-98562. |
| 5 | He T, Kong X J, Bian Z X, et al. Trace removal of benzene vapour using double-walled metal-dipyrazolate frameworks[J]. Nature Materials, 2022, 21(6): 689-695. |
| 6 | 李沐紫, 贾国伟, 赵砚珑, 等. 金属有机框架材料对非二氧化碳温室气体捕捉研究进展[J]. 化工学报, 2023, 74(1): 365-379. |
| Li M Z, Jia G W, Zhao Y L, et al. The progress of metal-organic frameworks for non-CO2 greenhouse gases capture[J]. CIESC Journal, 2023, 74(1): 365-379. | |
| 7 | 文一如, 付佳, 刘大欢. 基于机器学习的MOFs材料研究进展:能源气体吸附分离[J]. 化工学报, 2024, 75(4): 1370-1381. |
| Wen Y R, Fu J, Liu D H. Advances in machine learning-based materials research for MOFs: energy gas adsorption separation[J]. CIESC Journal, 2024, 75(4): 1370-1381. | |
| 8 | Abdul Hamid M R, Qian Y T, Wei R C, et al. Polycrystalline metal-organic framework (MOF) membranes for molecular separations: engineering prospects and challenges[J]. Journal of Membrane Science, 2021, 640: 119802. |
| 9 | Baniani A, Rivera M P, Lively R P, et al. Quantifying diffusion of organic liquids in a MOF component of MOF/polymer mixed-matrix membranes by high field NMR[J]. Journal of Membrane Science, 2021, 640: 119786. |
| 10 | Li D, Gao C Y, Zhang Y, et al. Unraveling the transition of ZIF-67-on-ZIF-8 core-shell structured dodecahedron formed by an oriented attachment mechanism from type Ⅱ to step-scheme for enhanced photocatalytic degradation of oxytetracycline hydrochloride[J]. Chemical Engineering Science, 2024, 300: 120668. |
| 11 | Zhou P Y, Lv J J, Huang X B, et al. Strategies for enhancing the catalytic activity and electronic conductivity of MOFs-based electrocatalysts[J]. Coordination Chemistry Reviews, 2023, 478: 214969. |
| 12 | Deng Q C, Zhang X D, Chang L, et al. The MOF/LDH derived heterostructured Co3O4/MnCo2O4 composite for enhanced degradation of levofloxacin by peroxymonosulfate activation[J]. Separation and Purification Technology, 2022, 294: 121182. |
| 13 | Li H Y, Wei Y L, Dong X Y, et al. Novel Tb-MOF embedded with viologen species for multi-photofunctionality: photochromism, photomodulated fluorescence, and luminescent pH sensing[J]. Chemistry of Materials, 2015, 27(4): 1327-1331. |
| 14 | Zhao Y L, Chen Q, Lv J, et al. Specific sensing of antibiotics with metal-organic frameworks based dual sensor system[J]. Nano Research, 2022, 15(7): 6430-6437. |
| 15 | Guan H Y, LeBlanc R J, Xie S Y, et al. Recent progress in the syntheses of mesoporous metal-organic framework materials[J]. Coordination Chemistry Reviews, 2018, 369: 76-90. |
| 16 | Kong X J, Li J R. An overview of metal-organic frameworks for green chemical engineering[J]. Engineering, 2021, 7(8): 1115-1139. |
| 17 | Kamal K, Bustam M A, Ismail M, et al. Optimization of washing processes in solvothermal synthesis of nickel-based MOF-74[J]. Materials, 2020, 13(12): 2741. |
| 18 | Seoane B, Castellanos S, Dikhtiarenko A, et al. Multi-scale crystal engineering of metal organic frameworks[J]. Coordination Chemistry Reviews, 2016, 307: 147-187. |
| 19 | Kim S N, Lee Y R, Hong S H, et al. Pilot-scale synthesis of a zirconium-benzenedicarboxylate UiO-66 for CO2 adsorption and catalysis[J]. Catalysis Today, 2015, 245: 54-60. |
| 20 | Faustini M, Kim J, Jeong G Y, et al. Microfluidic approach toward continuous and ultrafast synthesis of metal-organic framework crystals and hetero structures in confined microdroplets[J]. Journal of the American Chemical Society, 2013, 135(39): 14619-14626. |
| [1] | 王金月, 谢恩泽, 马翰泽, 袁晟, 何光伟, 姜忠义. 单原子层分离膜:进展与展望[J]. 化工学报, 2025, 76(5): 1943-1959. |
| [2] | 张耀辉, 班宇杰, 杨维慎. 以蒸气加工法制备和修饰金属-有机框架膜[J]. 化工学报, 2025, 76(5): 2070-2086. |
| [3] | 杨紫博, 王有发, 岳寒松, 远双杰, 耿付江, 李晴晴, 奥德, 李斌, 叶茂, 顾振杰, 乔志华. MOF玻璃基气体分离膜的研究进展[J]. 化工学报, 2025, 76(5): 2158-2168. |
| [4] | 朱迪, 高守建, 方望熹, 靳健. 水蒸气诱导相分离构筑海绵孔结构超亲水聚醚砜膜及其油/水乳液分离性能研究[J]. 化工学报, 2025, 76(5): 2397-2409. |
| [5] | 霍军良, 唐治国, 邱宗君, 冯玉华, 蒋旭, 王乐怡, 杨宇, 乔帆帆, 赫一凡, 喻健良. 节流作用下CO2管道放空过程的冻堵风险实验研究[J]. 化工学报, 2025, 76(4): 1898-1908. |
| [6] | 张新梅, 张傲, 邱德华, 刘晓爽, 陈晨. 基于热响应机理的罐区池火灾动态多米诺效应评估方法[J]. 化工学报, 2025, 76(4): 1885-1897. |
| [7] | 徐东亮, 赵彬彬, 孙逸玫, 刘婷婷, 刘筱然, 陈明功. 基于修正多孔介质模型的RPB模拟与流场特性研究[J]. 化工学报, 2025, 76(4): 1569-1582. |
| [8] | 蔡本安, 张建新, 龙城君, 杜乔琛, 车勋建, 张义迎, 蔡伟华. 喷雾闪蒸制备微纳米颗粒[J]. 化工学报, 2025, 76(3): 1334-1345. |
| [9] | 张恒, 魁殿禄, 常虹, 詹志刚. 机械应力对气体扩散层界面传输特性影响[J]. 化工学报, 2025, 76(2): 637-644. |
| [10] | 王绍吉, 郝矿荣, 陈磊. 基于联邦学习的聚酯纤维酯化过程温度预测研究[J]. 化工学报, 2025, 76(1): 283-295. |
| [11] | 张闯德, 陈黎. 优势通道对多孔介质中多相反应输运过程影响的孔隙尺度研究[J]. 化工学报, 2025, 76(1): 161-172. |
| [12] | 李雨霜, 王兴成, 温伯尧, 骆政园, 白博峰. 多孔介质中乳状液驱油的两相流动过程及其影响因素[J]. 化工学报, 2024, 75(S1): 56-66. |
| [13] | 代艳辉, 熊启钊, 房强, 杨东晓, 王毅, 陈杨, 李晋平, 李立博. 原位蒸汽辅助法用于一步制备多级孔Cu-BTC[J]. 化工学报, 2024, 75(9): 3329-3337. |
| [14] | 裴蓓, 郝治斌, 徐天祥, 钟子琪, 李瑞, 贾冲, 段玉龙. 表面活性剂对含盐双流体细水雾灭火效能的影响[J]. 化工学报, 2024, 75(9): 3369-3378. |
| [15] | 胡术刚, 田国庆, 刘文娟, 徐广飞, 刘华清, 张建, 王艳龙. 纳米零价铁的制备及氧化还原技术的应用进展[J]. 化工学报, 2024, 75(9): 3041-3055. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号