化工学报 ›› 2019, Vol. 70 ›› Issue (6): 2259-2268.DOI: 10.11949/j.issn.0438-1157.20181497
收稿日期:2018-12-21
修回日期:2019-03-05
出版日期:2019-06-05
发布日期:2019-06-05
通讯作者:
盛昌栋
作者简介:<named-content content-type="corresp-name">郑传杰</named-content>(1994—),男,硕士研究生,<email>920343372@qq.com</email>
基金资助:
Chuanjie ZHENG( ),Changdong SHENG(
),Changdong SHENG( )
)
Received:2018-12-21
Revised:2019-03-05
Online:2019-06-05
Published:2019-06-05
Contact:
Changdong SHENG
摘要:
针对生物质燃烧时采用吸附剂控制烟气中含K气体成分行为的性能描述,采用一维柱塞流反应器模型探究高岭土和煤灰捕集KOH(K2CO3)、KCl和K2SO4的性能及影响因素。通过模型计算与文献实验值比较检验模型合理性和确定动力学参数,并比较高岭土和煤灰捕集不同含K成分的性能差异。结果表明,采用表观动力学模型和单一的动力学参数可预测广泛反应条件下高岭土和煤灰捕集K的量,所确定的动力学参数是合理的;在给定的K浓度和烟气温度范围内,单位质量高岭土和煤灰捕集K的规律相似,均随烟气温度的升高而增大,随K浓度的增大而增大,且高岭土捕集能力受K浓度影响更明显;高岭土和煤灰捕集K的能力均为KOH强于KCl,强于K2SO4,且高岭土捕集能力明显强于煤灰。
中图分类号:
郑传杰, 盛昌栋. 高温烟气中吸附剂捕集K的模型及其反应动力学研究[J]. 化工学报, 2019, 70(6): 2259-2268.
Chuanjie ZHENG, Changdong SHENG. Modeling and reaction kinetics study on K capture by adsorbents in high temperature flue gas[J]. CIESC Journal, 2019, 70(6): 2259-2268.
| 高岭土 | 煤灰 | ||||||
|---|---|---|---|---|---|---|---|
| KOH | K2CO3 | KCl | K2SO4 | KOH | KCl | K2SO4 | |
| k 0×10-8/(m3 gas/((m3 pore)·s)) | 3.8 | 3.8 | 2.3 | 1.57 | 0.21 | 0.11 | 0.094 |
| E/(kJ/mol) | 25.08 | 25.08 | 25.08 | 25.08 | 25.08 | 25.08 | 25.08 |
表1 高岭土和煤灰吸附不同含K气体成分的反应动力学参数
Table 1 Kinetic parameters of kaolin and coal fly ash adsorbing different K-containing gas species
| 高岭土 | 煤灰 | ||||||
|---|---|---|---|---|---|---|---|
| KOH | K2CO3 | KCl | K2SO4 | KOH | KCl | K2SO4 | |
| k 0×10-8/(m3 gas/((m3 pore)·s)) | 3.8 | 3.8 | 2.3 | 1.57 | 0.21 | 0.11 | 0.094 |
| E/(kJ/mol) | 25.08 | 25.08 | 25.08 | 25.08 | 25.08 | 25.08 | 25.08 |
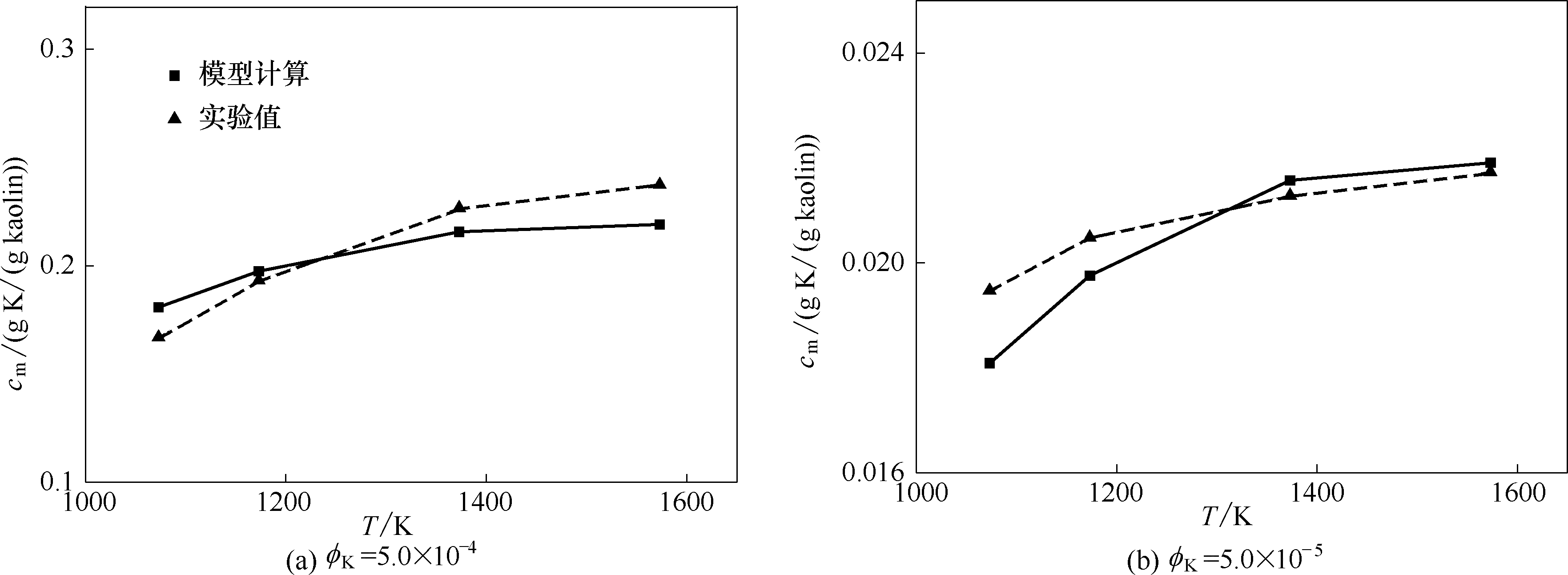
图1 不同温度和KOH浓度下高岭土捕集KOH的模型计算与实验结果的比较
Fig.1 Comparison of model calculations and experimental measurements of KOH adsorption by kaolin at different temperatures and KOH concentrations
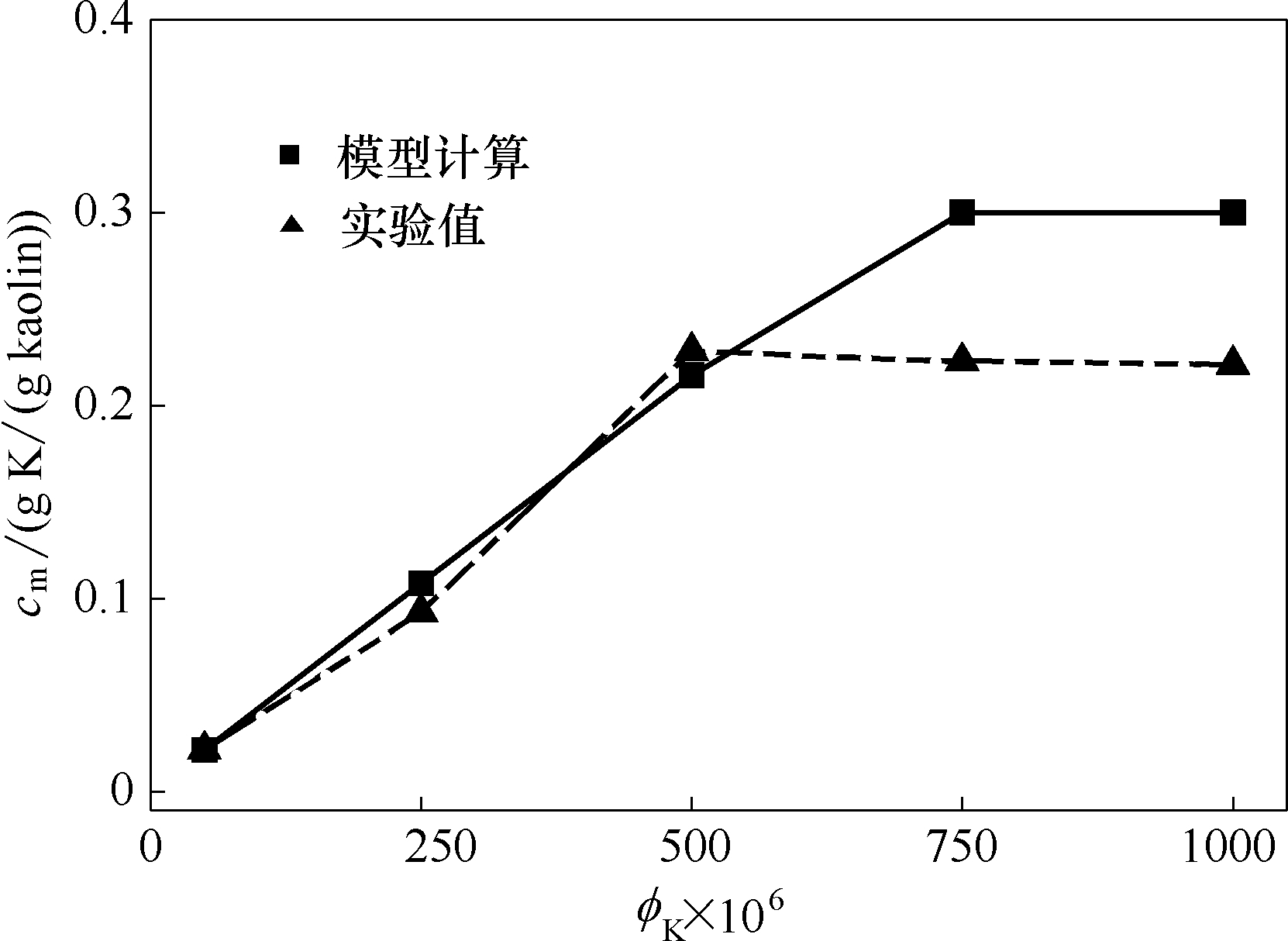
图2 不同KOH浓度下高岭土捕集K的模型计算与实验值比较(温度为1373 K,停留时间1.2 s)
Fig.2 Comparison of model calculated and experiment measured K capture by kaolin at different initial KOH concentrations (at temperature of 1373 K and residence time of 1.2 s)
| 含K成分 | ? K×106 | 烟气温度T/K | 停留时间t/s |
|---|---|---|---|
| K2CO3 | 50 | 1373 | 1.2 |
| 250 | 1373 | 1.2 | |
| 500 | 1073、1173、1373、1573 | 1.2 | |
| 750 | 1373 | 1.2 | |
| 1000 | 1373 | 1.2 | |
| KCl | 50 | 1573 | 1.0 |
| 250 | 1573 | 1.0 | |
| 500 | 1073、1173、1373、1573 | 1.2(1573 K时1.0) | |
| 750 | 1573 | 1.0 | |
| 1000 | 1573 | 1.0 | |
| K2SO4 | 50 | 1373 | 1.2 |
| 250 | 1373 | 1.2 | |
| 500 | 1073、1173、1373、1573 | 1.2 | |
| 750 | 1373 | 1.2 |
表2 高岭土捕集K2CO3、KCl和K2SO4的模型计算条件
Table 2 Model calculating conditions for kaolin adsorbing K2CO3, KCl and K2SO4
| 含K成分 | ? K×106 | 烟气温度T/K | 停留时间t/s |
|---|---|---|---|
| K2CO3 | 50 | 1373 | 1.2 |
| 250 | 1373 | 1.2 | |
| 500 | 1073、1173、1373、1573 | 1.2 | |
| 750 | 1373 | 1.2 | |
| 1000 | 1373 | 1.2 | |
| KCl | 50 | 1573 | 1.0 |
| 250 | 1573 | 1.0 | |
| 500 | 1073、1173、1373、1573 | 1.2(1573 K时1.0) | |
| 750 | 1573 | 1.0 | |
| 1000 | 1573 | 1.0 | |
| K2SO4 | 50 | 1373 | 1.2 |
| 250 | 1373 | 1.2 | |
| 500 | 1073、1173、1373、1573 | 1.2 | |
| 750 | 1373 | 1.2 |
| 平均粒径/μm | BET表面积/(m2/g) | 含量/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SO3 | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | P2O5 | ||
| 10.2 | 8.04 | 0.52 | 37.98 | 42.61 | 6.68 | 5.08 | 1.31 | 0.59 | 1.69 | 1.18 | 2.36 |
表3 煤灰物理化学特性[31]
Table 3 Physical and chemical properties of coal fly ash[31]
| 平均粒径/μm | BET表面积/(m2/g) | 含量/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SO3 | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | P2O5 | ||
| 10.2 | 8.04 | 0.52 | 37.98 | 42.61 | 6.68 | 5.08 | 1.31 | 0.59 | 1.69 | 1.18 | 2.36 |
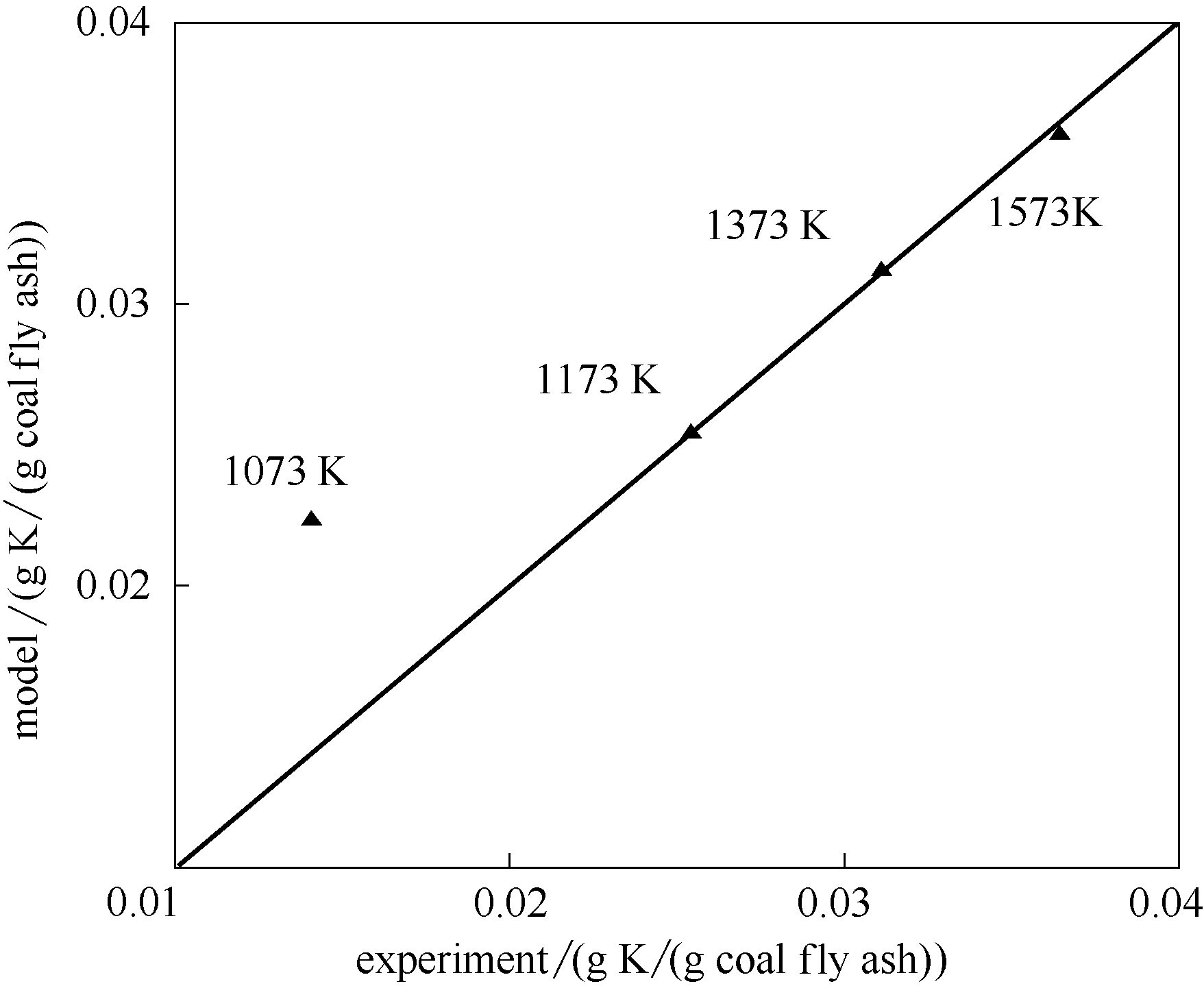
图5 煤灰捕集K2SO4模型计算值与实验值比较(? K=5.0×10-4,停留时间1.2 s)
Fig.5 Comparison of model calculations and experiment measurements of K2SO4 adsorption by coal fly ash at ? K of 5.0×10-4 and residence time of 1.2 s
| 1 | 斯俊平 . 燃煤过程中钠对焦特性及细颗粒物控制的影响[D]. 武汉: 华中科技大学, 2014. |
| Si J P . Effects of sodium on char characteristics and fine particulate matters control during coal combustion[D]. Wuhan: Huazhong University of Science and Technology, 2014. | |
| 2 | 黄忠友 . 试析生物质发电发展现状及前景[J]. 科技风, 2019, (2): 185. |
| Huang Z Y . Analysis of current situation and prospect of biomass power generation[J]. Technology Wind, 2019, (2): 185. | |
| 3 | 徐林林 . 高岭土对高钠煤燃烧过程中碱金属的脱除效果研究[D]. 天津: 天津大学, 2015. |
| Xu L L . Effect of kaolin on removal of alkali metals during combustion of high sodium coal[D]. Tianjin: Tianjin University, 2015. | |
| 4 | Mcnallan M J , Yurek G J , Elliott J F . The formation of inorganic particulates by homogeneous nucleation in gases produced by the combustion of coal[J]. Combustion & Flame, 1981, 42(81): 45-60. |
| 5 | 兰泽全, 曹欣玉, 周俊虎, 等 . 锅炉受热面沾污结渣的危害及其防治措施[J]. 电站系统工程, 2003, 19(1): 31-33. |
| Lan Z Q , Cao X Y , Zhou J H , et al . Hazards and prevention measures of fouling and slagging on heating surfaces of boilers[J]. Power Plant System Engineering, 2003, 19(1): 31-33. | |
| 6 | 董雪玲 . 大气可吸入颗粒物对环境和人体健康的危害[J]. 资源·产业, 2004, (5): 52-55. |
| Dong X L . Hazards of inhalable particulates to environment and human health[J]. Resources·Industry, 2004, (5): 52-55. | |
| 7 | Zhan Z , Fry A R , Wendt J O L . Relationship between submicron ash aerosol characteristics and ash deposit compositions and formation rates during air- and oxy-coal combustion[J]. Fuel, 2016, 181: 1214-1223. |
| 8 | Waindich A , Müller M . Alkali removal at 1400℃ under gasification conditions[J]. Fuel, 2014, 116: 889-893. |
| 9 | Gibbs A R , Pooley F D . Analysis and interpretation of inorganic mineral particles in "lung" tissues[J]. Thorax, 1996, 51(3): 327-334. |
| 10 | 周科 . 燃煤细微颗粒物生成特性与炉内控制的研究[D]. 武汉: 华中科技大学, 2011. |
| Zhou K . Study on the generation characteristics and control of fine particles in coal combustion[D]. Wuhan: Huazhong University of Science and Technology, 2011. | |
| 11 | 温昶, 徐明厚, 于敦喜, 等 . 煤粉O2/CO2燃烧时PM2. 5及其Fe、S的生成特性[J]. 化工学报, 2011, 62(4): 1062-1069. |
| Wen C , Xu M H , Yu D X , et al . Formation characteristics of PM2. 5 including Fe, S during O2/CO2 combustion of pulverized coal[J]. CIESC Journal, 2011, 62(4): 1062-1069. | |
| 12 | Quann R J , Sarofim A F . Vaporization of refractory oxides during pulverized coal combustion[J]. Symposium on Combustion, 1982, 19(1): 1429-1440. |
| 13 | Si J P , Liu X W , Xu M H , et al . Effect of kaolin additive on PM2. 5, reduction during pulverized coal combustion: importance of sodium and its occurrence in coal[J]. Applied Energy, 2014, 114(2): 434-444. |
| 14 | Yan L , Gupta R P , Wall T F . The implication of mineral coalescence behaviour on ash formation and ash deposition during pulverised coal combustion[J]. Fuel, 2001, 80(9): 1333-1340. |
| 15 | 徐明厚, 于敦喜, 刘小伟 . 燃煤可吸入颗粒物的形成与排放[M]. 北京: 科学出版社, 2009. |
| Xu M H , Yu D X , Liu X W . Formation and Emission of Inhalable Particles from Coal Combustion[M]. Beijing: Science Press, 2009. | |
| 16 | 刘建忠, 张光学, 周俊虎, 等 . 燃煤细灰的形成及微观形态特征[J]. 化工学报, 2006, 57(12): 2976-2980. |
| Liu J Z , Zhang G X , Zhou J H , et al . Formation and micromorphology characteristics of fine particles generated during coal combustion [J]. Journal of Chemical Industry and Engineering(China), 2006, 57(12): 2976-2980. | |
| 17 | 刘勇, 赵汶, 刘瑞, 等 . 化学团聚促进电除尘脱除PM2. 5的实验研究[J]. 化工学报, 2014, 65(9): 3609-3616. |
| Liu Y , Zhao W , Liu R , et al . Improving removal of PM2.5 by electrostatic precipitator with chemical agglomeration[J]. CIESC Journal, 2014, 65(9): 3609-3616. | |
| 18 | Quann R J . Ash vaporization under simulated pulverized coal combustion conditions[D]. Massachusetts: Massachusetts Institute of Technology, 1982. |
| 19 | Li Y , Raj G A , Wall T . Fragmentation behavior of pyrite and calcite during high-temperature processing and mathematical simulation[J]. Energy & Fuels, 2001, 15(2): 389-394. |
| 20 | Quann R J , Neville M , Janghorbani M , et al . Mineral matter and trace-element vaporization in a laboratory-pulverized coal combustion system[J]. Environmental Science & Technology, 1982, 16(11): 776. |
| 21 | Helble J J . Mechanisms of ash particle formation and growth during pulverized coal combustion[D]. Massachusetts: MIT Library in America, 1987. |
| 22 | 孙伟, 刘小伟, 徐义书, 等 . 两种改性高岭土减排超细颗粒物的对比分析[J]. 化工学报, 2016, 67(4): 1179-1185. |
| Xun W , Liu X W , Xu Y S , et al . Contrastive analysis of reducing ultrafine particulate matters emission by two modified kaolin[J]. CIESC Journal, 2016, 67(4): 1179-1185. | |
| 23 | Mwabe P O , Wendt J O L . Mechanisms governing trace sodium capture by kaolinite in a downflow combustor[J]. Symposium on Combustion, 1996, 26(2): 2447-2453. |
| 24 | Wang G L , Jensen P A , Wu H , et al . Potassium capture by kaolin (1): KOH[J]. Energy & Fuels, 2018, 32(2): 1851-1862. |
| 25 | Schürmann H , Unterberger S , Hein K R , et al . The influence of fuel additives on the behaviour of gaseous alkali-metal compounds during pulverised coal combustion[J]. Faraday Discussions, 2001, 119(119): 433-444. |
| 26 | Gale T , Wendt J L . Mechanisms and models describing sodium and lead scavenging by a kaolinite aerosol at high temperatures[J]. Aerosol Science & Technology, 2003, 37(11): 865-876. |
| 27 | Wang G L , Jensen P A , Wu H , et al . Potassium capture by kaolin (2): K2CO3, KCl, and K2SO4 [J]. Energy & Fuels, 2018, 32(3): 3566-3578. |
| 28 | Damoe A J , Wu H , Frandsen F J , et al . Impact of coal fly ash addition on combustion aerosols (PM2. 5) from full-scale suspension-firing of pulverized wood[J]. Energy & Fuels, 2014, 28(5): 3217-3223. |
| 29 | Scandrett L A , Clift R . The thermodynamics of alkali removal from coal derived gases[J]. Journal of the Institute of Energy, 1984, 57 (433): 391-397. |
| 30 | Li Y , Li J , Jin Y , et al . Study on alkali-metal vapor removal for high-temperature cleaning of coal gas[J]. Energy & Fuels, 2005, 19(4): 1606-1610. |
| 31 | Wang G L . Potassium capture by kaolin and coal fly ash[D]. Kongens Lyngby: Technical University of Denmark, 2018. |
| [1] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [2] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [3] | 肖明堃, 杨光, 黄永华, 吴静怡. 浸没孔液氧气泡动力学数值研究[J]. 化工学报, 2023, 74(S1): 87-95. |
| [4] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [5] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [6] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [7] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [8] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [9] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [10] | 杨越, 张丹, 郑巨淦, 涂茂萍, 杨庆忠. NaCl水溶液喷射闪蒸-掺混蒸发的实验研究[J]. 化工学报, 2023, 74(8): 3279-3291. |
| [11] | 曾如宾, 沈中杰, 梁钦锋, 许建良, 代正华, 刘海峰. 基于分子动力学模拟的Fe2O3纳米颗粒烧结机制研究[J]. 化工学报, 2023, 74(8): 3353-3365. |
| [12] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [13] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [14] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [15] | 何宣志, 何永清, 闻桂叶, 焦凤. 磁液液滴颈部自相似破裂行为[J]. 化工学报, 2023, 74(7): 2889-2897. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号