化工学报 ›› 2020, Vol. 71 ›› Issue (11): 4971-4980.DOI: 10.11949/0438-1157.20200804
收稿日期:2020-06-22
修回日期:2020-09-15
出版日期:2020-11-05
发布日期:2020-11-05
通讯作者:
吴有庭
作者简介:涂卓恒(1994—),男,博士研究生,基金资助:
Zhuoheng TU( ),Mingzhen SHI,Xiaomin ZHANG,Youting WU(
),Mingzhen SHI,Xiaomin ZHANG,Youting WU( ),Xingbang HU
),Xingbang HU
Received:2020-06-22
Revised:2020-09-15
Online:2020-11-05
Published:2020-11-05
Contact:
Youting WU
摘要:
鉴于目前离子液体交联反应所用交联剂存在的高成本、难制备的问题,提出以廉价易得的环氧氯丙烷交联二胺质子型离子液体制备离子液体凝胶的设想。基于此,通过监测反应体系动力学信息证明了反应的可能性,同时研究了与酸中和顺序、反应温度、交联比例对环氧基团和氯基团转化率的影响。探究了环氧氯丙烷-二胺交联体系的凝胶转变性能以及可能的反应机理。并且初步测试了交联产物的CO2分离性能。
中图分类号:
涂卓恒,史名珍,张效敏,吴有庭,胡兴邦. 环氧氯丙烷与离子液体的交联过程研究[J]. 化工学报, 2020, 71(11): 4971-4980.
Zhuoheng TU,Mingzhen SHI,Xiaomin ZHANG,Youting WU,Xingbang HU. Research on crosslinking of epichlorohydrin and ionic liquids[J]. CIESC Journal, 2020, 71(11): 4971-4980.

图3 不同温度条件下ECH与[DMAPAH][MOAc]等摩尔反应时体系的pH(a)和氯基团转化率(b)随时间的变化曲线
Fig.3 Plots of pH (a) and conversion of chloride group (b) in the equimolar reaction system of ECH with [DMAPAH][MOAc] as a function of time at different temperatures

图4 25℃条件下ECH与DMAPA按不同摩尔比反应时体系的pH(a)、氯基团转化率(b)和环氧基团转化率(c)随时间的变化曲线
Fig.4 Plots of pH (a), conversion of chloride group (b) and conversion of epoxy group (c) in the reaction systems with different ECH-to-DMAPA molar ratio as a function of time at 25℃
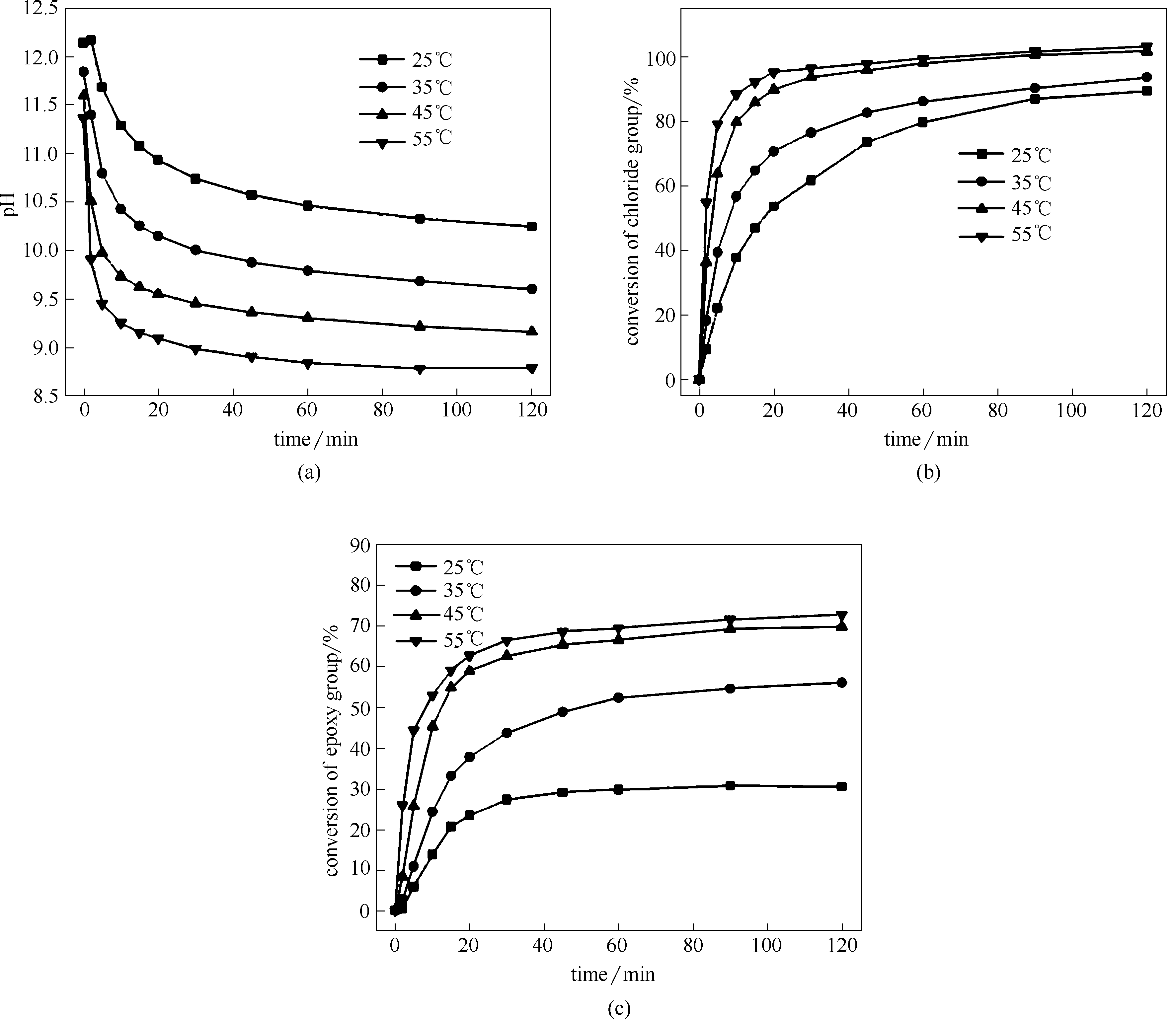
图5 不同温度条件下ECH与DMAPA等摩尔反应时体系的pH(a)、氯基团转化率(b)和环氧基团转化率(c)随时间的变化曲线
Fig.5 Plots of pH (a), conversion of chloride group (b) and conversion of epoxy group (c) in the equimolar reaction systems of ECH with DMAPA as a function of time at different temperatures
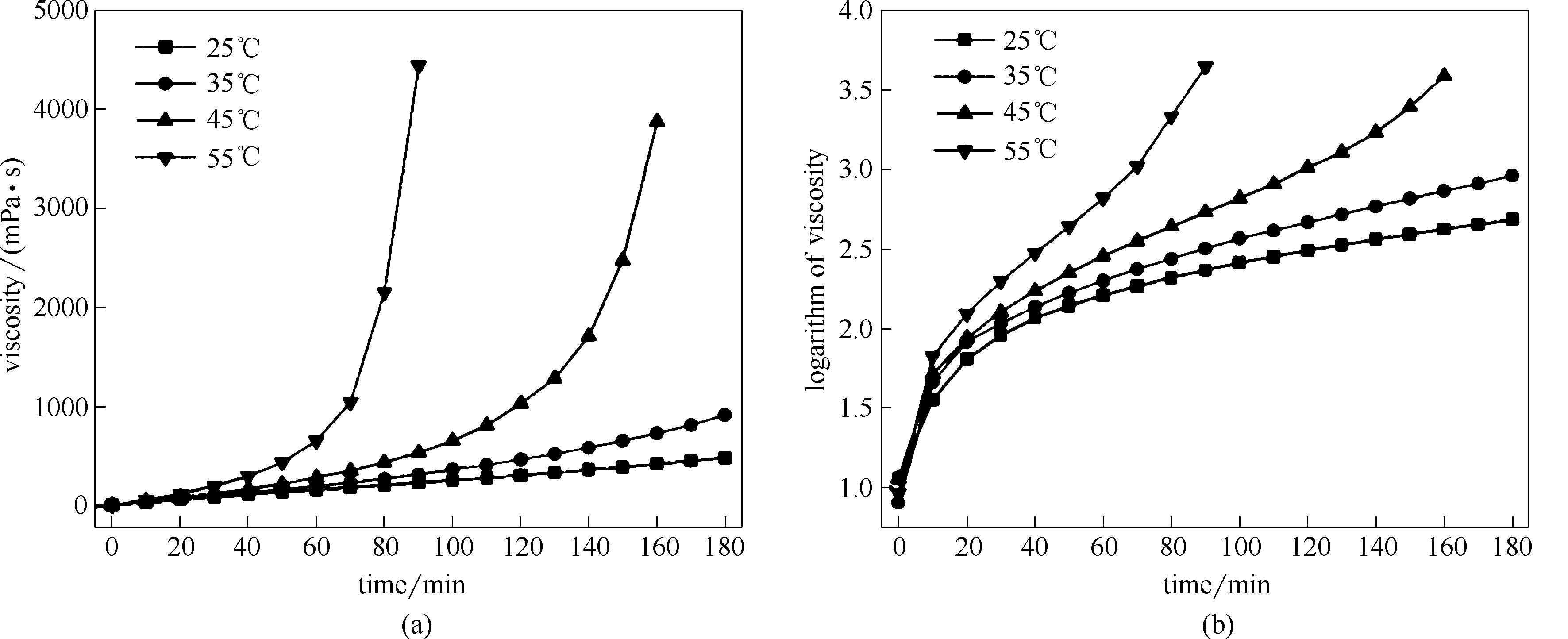
图6 ECH与DMAPA摩尔比1∶1时不同温度条件下反应体系黏度(a)、黏度对数(b)随时间变化曲线
Fig.6 Plots of viscosities(a), logarithms of viscosity (b) in reaction systems with 1.0 of ECH-to-DMAPA molar ratio as a function of time at different temperatures

图8 不同ECH与DMAPA摩尔比的ECHn-DMAPA交联产物FT-IR谱图(反应温度55℃,反应时间2 h)
Fig.8 FT-IR spectra of ECHn-DMAPA with different ECH-to-DMAPA molar ratio (reaction temperature = 55℃, reaction time =2 h)
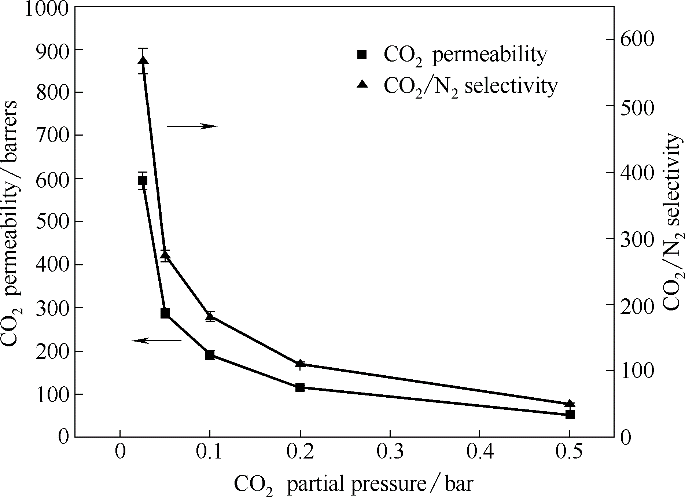
图10 40°C含水条件下ECH0.5-[DMAPAH][MOAc] [含水20%(质量)]中CO2的渗透系数及CO2/N2选择性随跨膜压差的变化关系
Fig.10 Plots of permeability of CO2 and CO2/N2 selectivity in ECH0.5-[DMAPAH][MOAc] of 20%(mass) water as a function of transmembrane pressure difference under humidified condition at 40℃
| 1 | Chestnut D H. Ten years of experience with accidental dural puncture and post-dural puncture headache in a tertiary obstetric anaesthesia department[J]. Yearbook of Anesthesiology and Pain Management, 2009, 2009: 252-253. |
| 2 | Ramdin M, de Loos T W, Vlugt T J H. State-of-the-art of CO2 capture with ionic liquids[J]. Industrial & Engineering Chemistry Research, 2012, 51(24): 8149-8177. |
| 3 | Liu A H, Ma R, Song C, et al. Equimolar CO2 capture by N-substituted amino acid salts and subsequent conversion[J]. Angewandte Chemie International Edition, 2012, 51(45): 11306-11310. |
| 4 | Rochelle G T. Amine scrubbing for CO2 capture [J]. Science, 2009, 325(5948): 1652-1654. |
| 5 | Bernardo P, Drioli E, Golemme G. Membrane gas separation: a review/state of the art [J]. Industrial & Engineering Chemistry Research, 2009, 48(10): 4638-4663. |
| 6 | D􀆳Alessandro D M, Smit B, Long J R. Carbon dioxide capture: prospects for new materials [J]. Angewandte Chemie International Edition,2010, 49(35): 6058-6082. |
| 7 | Blanchard L A, Hancu D, Beckman E J, et al. Green processing using ionic liquids and CO2[J]. Nature, 1999, 399(6731): 28-29. |
| 8 | Aki S N V K, Mellein B R, Saurer E M, et al. High-pressure phase behavior of carbon dioxide with imidazolium-based ionic liquids [J]. Journal of Physical Chemistry B, 2004, 108(52): 20355-20365. |
| 9 | Bates E D, Mayton R D, Ntai I, et al. CO2 capture by a task-specific ionic liquid [J]. Journal of the American Chemical Society, 2002, 124(6): 926-927. |
| 10 | Xue Z, Zhang Z, Han J, et al. Carbon dioxide capture by a dual amino ionic liquid with amino-functionalized imidazolium cation and taurine anion [J]. International Journal of Greenhouse Gas Control, 2011, 5(4): 628-633. |
| 11 | Luo X Y, Fan X, Shi G L, et al. Decreasing the viscosity in CO2 capture by amino-functionalized ionic liquids through the formation of intramolecular hydrogen bond [J]. Journal of Physical Chemistry B, 2016, 120(10): 2807-2813. |
| 12 | Hu X B, Li Y X, Huang K, et al. Impact of α-d-glucose pentaacetate on the selective separation of CO2 and SO2 in supported ionic liquid membranes [J]. Green Chemistry, 2012, 14(5): 1440-1446. |
| 13 | Huang K, Lu J F, Wu Y T, et al. Absorption of SO2 in aqueous solutions of mixed hydroxylammonium dicarboxylate ionic liquids [J]. Chemical Engineering Journal, 2013, 215/216: 36-44. |
| 14 | Huang K, Chen Y L, Zhang X M, et al. Experimental study and thermodynamical modelling of the solubilities of SO2, H2S and CO2 in N-dodecylimidazole and 1,1'-[oxybis(2,1-ethanediyloxy-2,1-ethanediyl)]bis(imidazole): an evaluation of their potential application in the separation of acidic gases [J]. Fluid Phase Equilibria, 2014, 378: 21-33. |
| 15 | Huang K, Xia S, Zhang X M, et al. Comparative study of the solubilities of SO2 in five low volatile organic solvents (sulfolane, ethylene glycol, propylene carbonate, N-methylimidazole, and N-methylpyrrolidone) [J]. Journal of Chemical & Engineering Data, 2014, 59(4): 1202-1212. |
| 16 | Zhao T X, Liang J, Zhang Y Y, et al. Unexpectedly efficient SO2 capture and conversion to sulfur in novel imidazole-based deep eutectic solvents [J]. Chemical Communication, 2018, 54(65): 8964-8967. |
| 17 | Huang K, Cai D N, Chen Y L, et al. Thermodynamic validation of 1-alkyl-3-methylimidazolium carboxylates as task-specific ionic liquids for H2S absorption [J]. AIChE Journal, 2013, 59(6): 2227-2235. |
| 18 | Zhang X M, Tu Z H, Li H, et al. Selective separation of H2S and CO2 from CH4 by supported ionic liquid membranes [J]. Journal of Membrane Science, 2017, 543: 282-287. |
| 19 | Zheng W T, Wu D S, Feng X, et al. Low viscous protic ionic liquids functionalized with multiple Lewis base for highly efficient capture of H2S [J]. Journal of Molecular Liquids, 2018, 263: 209-217. |
| 20 | Tao D J, Chen F F, Tian Z Q, et al. Highly efficient carbon monoxide capture by carbanion-functionalized ionic liquids through C-site interactions [J]. Angewandte Chemie International Edition, 2017, 56(24): 6843-6847. |
| 21 | Liu Y M, Tian Z, Qu F, et al. Tuning ion-pair interaction in cuprous-based protic ionic liquids for significantly improved CO capture [J]. ACS Sustainable Chemistry & Engineering, 2019, 7(13): 11894-11900. |
| 22 | Tu Z H, Zhang Y Y, Wu Y T, et al. Self-enhancement of CO reversible absorption accompanied by phase transition in protic chlorocuprate ionic liquids for effective CO separation from N2 [J]. Chemical Communication, 2019, 55(23): 3390-3393. |
| 23 | Jiang Y Y, Zhou Z, Jiao Z, et al. SO2 gas separation using supported ionic liquid membranes [J]. Journal of Physical Chemistry B, 2007, 111(19): 5058-5061. |
| 24 | Bara J E, Gabriel C J, Carlisle T K, et al. Gas separations in fluoroalkyl-functionalized room-temperature ionic liquids using supported liquid membranes [J]. Chemical Engineering Journal, 2009, 147(1): 43-50. |
| 25 | Scovazzo P. Determination of the upper limits, benchmarks, and critical properties for gas separations using stabilized room temperature ionic liquid membranes (SILMs) for the purpose of guiding future research [J]. Journal of Membrane Science, 2009, 343(1/2): 199-211. |
| 26 | Hanioka S, Maruyama T, Sotani T, et al. CO2 separation facilitated by task-specific ionic liquids using a supported liquid membrane [J]. Journal of Membrane Science, 2008, 314(1/2): 1-4. |
| 27 | Zhang X M, Tu Z H, Li H, et al. Supported protic-ionic-liquid membranes with facilitated transport mechanism for the selective separation of CO2 [J]. Journal of Membrane Science, 2017, 527: 60-67. |
| 28 | Kasahara S, Kamio E, Ishigami T, et al. Amino acid ionic liquid-based facilitated transport membranes for CO2 separation [J]. Chemical Communication, 2012, 48(55): 6903-6905. |
| 29 | Zhang X M, Xiong W J, Tu Z H, et al. Supported ionic liquid membranes with dual-site interaction mechanism for efficient separation of CO2 [J]. ACS Sustainable Chemistry & Engineering, 2019, 7(12): 10792-10799. |
| 30 | Huang K, Zhang X M, Li Y X, et al. Facilitated separation of CO2 and SO2 through supported liquid membranes using carboxylate-based ionic liquids [J]. Journal of Membrane Science, 2014, 471: 227-236. |
| 31 | Zeng S J, Zhang X P, Bai L, et al. Ionic-liquid-based CO2 capture systems: structure, interaction and process [J]. Chemical Reviews, 2017, 117(14): 9625-9673. |
| 32 | Bara J E, Lessmann S, Gabriel C J, et al. Synthesis and performance of polymerizable room-temperature ionic liquids as gas separation membranes [J]. Industrial & Engineering Chemistry Research, 2007, 46(16): 5397-5404. |
| 33 | Carlisle T K, Wiesenauer E F, Nicodemus G D, et al. Ideal CO2/light gas separation performance of poly(vinylimidazolium) membranes and poly(vinylimidazolium)-ionic liquid composite films [J]. Industrial & Engineering Chemistry Research, 2012, 52(3): 1023-1032. |
| 34 | Li P, Coleman M R. Synthesis of room temperature ionic liquids based random copolyimides for gas separation applications [J]. European Polymer Journal, 2013, 49(2): 482-491. |
| 35 | Chen H Z, Li P, Chung T-S. PVDF/ionic liquid polymer blends with superior separation performance for removing CO2 from hydrogen and flue gas [J]. International Journal of Hydrogen Energy, 2012, 37(16): 11796-11804. |
| 36 | Voss B A, Bara J E, Gin D L, et al. Physically gelled ionic liquids: solid membrane materials with liquidlike CO2 gas transport [J]. Chemistry of Materials, 2009, 21(14): 3027-3029. |
| 37 | Nguyen P T, Voss B A, Wiesenauer E F, et al. Physically gelled room-temperature ionic liquid-based composite membranes for CO2/N2 separation: effect of composition and thickness on membrane properties and performance [J]. Industrial & Engineering Chemistry Research, 2012, 52(26): 8812-8821. |
| 38 | Zhang X M, Kar M, Mendes T C, et al. Supported ionic liquid gel membrane electrolytes for flexible supercapacitors [J]. Advanced Energy Materials, 2018, 8(15): 1702702. |
| 39 | McDanel W M, Cowan M G, Chisholm N O, et al. Fixed-site-carrier facilitated transport of carbon dioxide through ionic-liquid-based epoxy-amine ion gel membranes [J]. Journal of Membrane Science, 2015, 492: 303-311. |
| 40 | Dai Z, Ansaloni L, Gin D L, et al. Facile fabrication of CO2 separation membranes by cross-linking of poly(ethylene glycol) diglycidyl ether with a diamine and a polyamine-based ionic liquid [J]. Journal of Membrane Science, 2017, 523: 551-560. |
| [1] | 邵苛苛, 宋孟杰, 江正勇, 张旋, 张龙, 高润淼, 甄泽康. 水平方向上冰中受陷气泡形成和分布实验研究[J]. 化工学报, 2023, 74(S1): 161-164. |
| [2] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [3] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [4] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [5] | 吴延鹏, 李晓宇, 钟乔洋. 静电纺丝纳米纤维双疏膜油性细颗粒物过滤性能实验分析[J]. 化工学报, 2023, 74(S1): 259-264. |
| [6] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [7] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [8] | 肖明堃, 杨光, 黄永华, 吴静怡. 浸没孔液氧气泡动力学数值研究[J]. 化工学报, 2023, 74(S1): 87-95. |
| [9] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [10] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [11] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [12] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [13] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [14] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [15] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号