化工学报 ›› 2020, Vol. 71 ›› Issue (6): 2457-2465.DOI: 10.11949/0438-1157.20191530
杨乐1( ),余金河1,付蓉1,谢远洋1,于畅1(
),余金河1,付蓉1,谢远洋1,于畅1( ),邱介山2(
),邱介山2( )
)
收稿日期:2019-12-17
修回日期:2020-01-17
出版日期:2020-06-05
发布日期:2020-06-05
通讯作者:
于畅,邱介山
作者简介:杨乐(1995—),男,硕士研究生,基金资助:
Le YANG1( ),Jinhe YU1,Rong FU1,Yuanyang XIE1,Chang YU1(
),Jinhe YU1,Rong FU1,Yuanyang XIE1,Chang YU1( ),Jieshan QIU2(
),Jieshan QIU2( )
)
Received:2019-12-17
Revised:2020-01-17
Online:2020-06-05
Published:2020-06-05
Contact:
Chang YU,Jieshan QIU
摘要:
Solvent-in-salt (SIS)型电解液作为一类新型超浓缩电解液,主要由水或者有机溶剂和易溶盐组成,具有溶液溶剂化程度小、自由溶剂分子少、电化学窗口宽、电化学稳定性高等特点,在超级电容器中显示了独特的优势并展现了良好的应用前景。本文重点综述了SIS型电解液的原理和优势,梳理了近年来SIS作为超级电容器电解液的研究进展,总结了其存在的问题,同时展望了SIS型电解液未来的发展方向。
中图分类号:
杨乐, 余金河, 付蓉, 谢远洋, 于畅, 邱介山. 超级电容器用solvent-in-salt型电解液的研究进展[J]. 化工学报, 2020, 71(6): 2457-2465.
Le YANG, Jinhe YU, Rong FU, Yuanyang XIE, Chang YU, Jieshan QIU. Research progress of solvent-in-salt electrolyte for supercapacitor[J]. CIESC Journal, 2020, 71(6): 2457-2465.
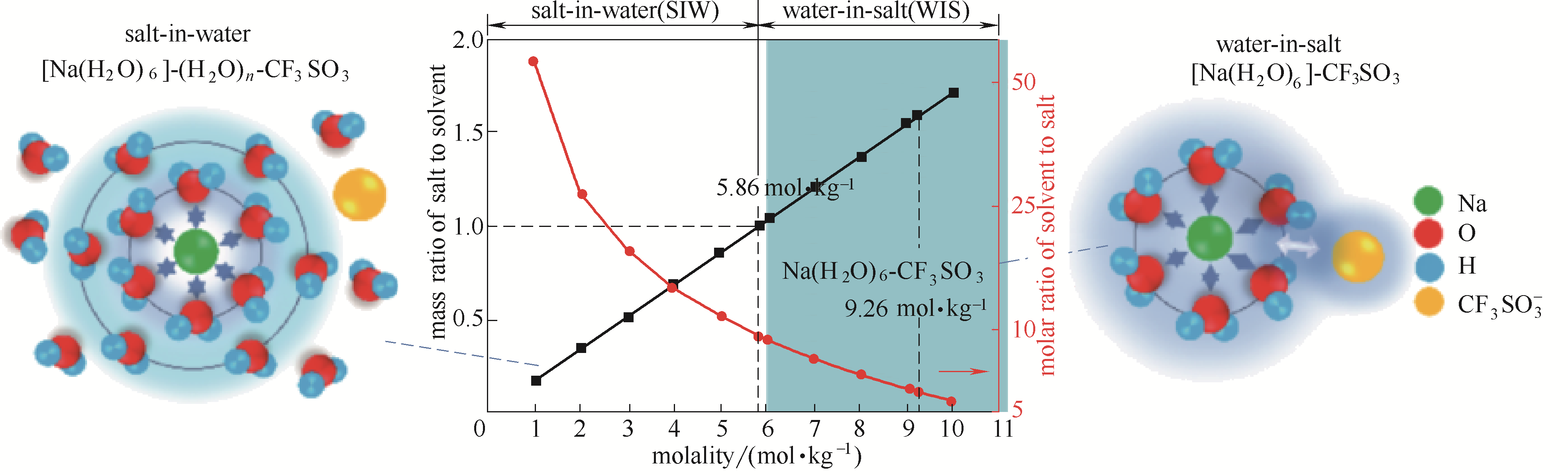
图3 电解质与溶剂的摩尔或质量比对电解液微观性质的影响过程示意图(NaOTF-H2O二元体系)[59]
Fig.3 Schematic diagram of process at different molar or mass ratios of electrolytes to solvent in NaOTF-H2O binary system[59]
| 1 | Yang J, Yu C, Fan X. et al. Electroactive edge site-enriched nickel-cobalt sulfide into graphene frameworks for high-performance asymmetric supercapacitors[J]. Energy & Environmental Science, 2016, 9(4): 1299-1307. |
| 2 | Lin T, Chen I W, Liu F, et al. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage[J]. Science, 2015, 350(6267): 1508-1513. |
| 3 | Guo W, Yu C, Li S F, et al. A phase transformation-resistant electrode enabled by a MnO2-confined effect for enhanced energy storage[J]. Advanced Functional Materials, 2019, 29(27): 1901342. |
| 4 | Li S F, Yu C, Yang Y, et al. Phosphate species up to 70% mass ratio for enhanced pseudocapacitive properties[J]. Small, 2018, 14(50): 1803811. |
| 5 | Li J, Xiong D, Wang L, et al. High-performance self-assembly MnCo2O4 nanosheets for asymmetric supercapacitors[J]. Journal of Energy Chemistry, 2019, 37: 66-72. |
| 6 | Zhu C, He Y, Liu Y, et al. ZnO@MOF@pani core-shell nanoarrays on carbon cloth for high-performance supercapacitor electrodes[J]. Journal of Energy Chemistry, 2019, 35: 124-131. |
| 7 | Sun Z H, Yuan A B. Electrochemical performance of nickel hydroxide/activated carbon supercapacitors using a modified polyvinyl alcohol based alkaline polymer electrolyte[J]. Chinese Journal of Chemical Engineering, 2009, 17(1): 150-155. |
| 8 | Smolin Y Y, Lau K K S, Soroush M. First-principles modeling for optimal design, operation, and integration of energy conversion and storage systems[J]. AIChE Journal, 2018, 65(7): 16482. |
| 9 | Guo W, Yu C, Li S F, et al. A universal converse voltage process for triggering transition metal hybrids in situ phase restruction toward ultrahigh-rate supercapacitors[J]. Advanced Materials, 2019, 31: 1901241. |
| 10 | Li S F, Yu C, Yang J, et al. A superhydrophilic “nanoglue” for stabilizing metal hydroxides onto carbon materials for high-energy and ultralong-life asymmetric supercapacitors[J]. Energy & Environmental Science, 2017, 10(9): 1958-1965. |
| 11 | Li P, Zhang D, Xu Y, et al. Nitrogen-doped hierarchical porous carbon from polyaniline/silica self-aggregates for supercapacitor[J]. Chinese Journal of Chemical Engineering, 2019, 27(3): 709-716. |
| 12 | Burke A, Miller M. The power capability of ultracapacitors and lithium batteries for electric and hybrid vehicle applications[J]. Journal of Power Sources, 2011, 196(1): 514-522. |
| 13 | Korenblit Y, Kajdos A, West W C, et al. In situ studies of ion transport in microporous supercapacitor electrodes at ultralow temperatures[J]. Advanced Functional Materials, 2012, 22(8): 1655-1662. |
| 14 | Kurzweil P, Chwistek M. Electrochemical stability of organic electrolytes in supercapacitors: spectroscopy and gas analysis of decomposition products[J]. Journal of Power Sources, 2008, 176(2): 555-567. |
| 15 | Lewandowski A, Olejniczak A, Galinski M, et al. Performance of carbon–carbon supercapacitors based on organic, aqueous and ionic liquid electrolytes[J]. Journal of Power Sources, 2010, 195(17): 5814-5819. |
| 16 | Lai Y, Chen X, Zhang Z, et al. Tetraethylammonium difluoro(oxalato)borate as electrolyte salt for electrochemical double-layer capacitors[J]. Electrochimica Acta, 2011, 56(18): 6426-6430. |
| 17 | Ishimoto S, Asakawa Y, Shinya M, et al. Degradation responses of activated-carbon-based EDLs for higher voltage operation and their factors[J]. Journal of the Electrochemical Society, 2009, 156(7): A563-A571. |
| 18 | Jung N, Kwon S, Lee D, et al. Synthesis of chemically bonded graphene/carbon nanotube composites and their application in large volumetric capacitance supercapacitors[J]. Advanced Materials, 2013, 25(47): 6854-6858. |
| 19 | Yu X, Ruan D, Wu C,et al. Spiro-(1, 1')-bipyrrolidinium tetrafluoroborate salt as high voltage electrolyte for electric double layer capacitors[J]. Journal of Power Sources, 2014, 265: 309-316. |
| 20 | Armand M, Endres F, MacFarlane D R, et al. Ionic-liquid materials for the electrochemical challenges of the future[J]. Nature Materials, 2009, 8(8): 621-629. |
| 21 | Lewandowski A, Galinski M. Practical and theoretical limits for electrochemical double-layer capacitors[J]. Journal of Power Sources, 2007, 173(2): 822-828. |
| 22 | Lewandowski A, Galiński M. Carbon-ionic liquid double-layer capacitors[J]. Journal of Physics and Chemistry of Solids, 2004, 65(2/3): 281-286. |
| 23 | Lewandowski A, Świderska A. Electrochemical capacitors with polymer electrolytes based on ionic liquids[J]. Solid State Ionics, 2003, 161(3/4): 243-249. |
| 24 | Coadou E, Goodrich P, Neale A R, et al. Synthesis and thermophysical properties of ether-functionalized sulfonium ionic liquids as potential electrolytes for electrochemical applications[J]. ChemPhysChem, 2016, 17(23): 3992-4002. |
| 25 | Lu X, Yu M, Wang G, et al. Flexible solid-state supercapacitors: design, fabrication and applications[J]. Energy & Environmental Science, 2014, 7(7): 2160-2181. |
| 26 | Li S F, Yu C, Yang J, et al. Ultrathin nitrogen-enriched hybrid carbon nanosheets for supercapacitors with ultrahigh rate performance and high energy density[J]. ChemElectrochem, 2017, 4(2): 369-375. |
| 27 | Yu J H, Yu C, Guo W, et al. Decoupling and correlating the ion transport by engineering 2D carbon nanosheets for enhanced charge storage[J]. Nano Energy, 2019, 64: 103921. |
| 28 | Zeng Z, Murugesan V, Han K S, et al. Non-flammable electrolytes with high salt-to-solvent ratios for Li-ion and Li-metal batteries[J]. Nature Energy, 2018, 3(8): 674-681. |
| 29 | Azov V A, Egorova K S, Seitkalieva M M, et al. “Solvent-in-salt” systems for design of new materials in chemistry, biology and energy research[J]. Chemical Society Reviews, 2018, 47(4): 1250-1284. |
| 30 | Wang J, Yamada Y, Sodeyama K, et al. Superconcentrated electrolytes for a high-voltage lithium-ion battery[J]. Nature Communications, 2016, 7: 12032. |
| 31 | Bu X, Su L, Dou Q, et al. A low-cost “water-in-salt” electrolyte for a 2.3 V high-rate carbon-based supercapacitor[J]. Journal of Materials Chemistry A, 2019, 7(13): 7541-7547. |
| 32 | Krummacher J, Hess L H, Balducci A. Al(TFSI)3 in acetonitrile as electrolytes for electrochemical double layer capacitors[J]. Journal of the Electrochemical Society, 2019, 166(10): A1763-A1768. |
| 33 | Liu Q, Zhou J, Song C, et al. 2.2 V high performance symmetrical fiber-shaped aqueous supercapacitors enabled by “water-in-salt” gel electrolyte and N-doped graphene fiber[J]. Energy Storage Materials, 2020, 24: 495-503. |
| 34 | Suo L, Hu Y S, Li H, et al. A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries[J]. Nature Communications, 2013, 4: 1481. |
| 35 | Yang C, Chen J, Qing T, et al. 4.0 V aqueous Li-ion batteries[J]. Joule, 2017, 1(1): 122-132. |
| 36 | Suo L, Borodin O, Sun W, et al. Advanced high-voltage aqueous lithium-ion battery enabled by “water-in-bisalt” electrolyte[J]. Angewandte Chemie International Edition, 2016, 55(25): 7136-7141. |
| 37 | Suo L, Borodin O, Gao T, et al. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries[J]. Science, 2015, 350(6263): 938-943. |
| 38 | Tian Z, Deng W, Wang X, et al. Superconcentrated aqueous electrolyte to enhance energy density for advanced supercapacitors[J]. Functional Materials Letters, 2017, 10(6): 1750081. |
| 39 | Dou Q, Lei S, Wang D W, et al. Safe and high-rate supercapacitors based on an “acetonitrile/water in salt” hybrid electrolyte[J]. Energy & Environmental Science, 2018, 11(11): 3212-3219. |
| 40 | Vatamanu J, Borodin O. Ramifications of water-in-salt interfacial structure at charged electrodes for electrolyte electrochemical stability[J]. The Journal of Physical Chemistry Letters, 2017, 8(18): 4362-4367. |
| 41 | Borodin O, Suo L, Gobet M, et al. Liquid structure with nano-heterogeneity promotes cationic transport in concentrated electrolytes[J]. ACS Nano, 2017, 11(10): 10462-10471. |
| 42 | Hasegawa G, Kanamori K, Kiyomura T, et al. Hierarchically porous carbon monoliths comprising ordered mesoporous nanorod assemblies for high-voltage aqueous supercapacitors[J]. Chemistry of Materials, 2016, 28(11): 3944-3950. |
| 43 | Yin J, Zheng C, Qi L, et al. Concentrated NaClO4 aqueous solutions as promising electrolytes for electric double-layer capacitors[J]. Journal of Power Sources, 2011, 196(8): 4080-4087. |
| 44 | Fan X, Chen L, Borodin O, et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries[J]. Nature Nanotechnology, 2018, 13(8): 715-722. |
| 45 | Zheng J, Lochala J A, Kwok A, et al. Research progress towards understanding the unique interfaces between concentrated electrolytes and electrodes for energy storage applications[J]. Advanced Science, 2017, 4(8): 1700032. |
| 46 | Gambou-Bosca A, Bélanger D. Electrochemical characterization of MnO2-based composite in the presence of salt-in-water and water-in-salt electrolytes as electrode for electrochemical capacitors[J]. Journal of Power Sources, 2016, 326: 595-603. |
| 47 | Simon P, Gogotsi Y, Dunn B. Where do batteries end and supercapacitors begin?[J]. Science, 2014, 343(6176): 1210-1211. |
| 48 | Xiao D, Wu Q, Liu X, et al. Aqueous symmetric supercapacitors with carbon nanorod electrodes and water-in-salt electrolyte[J]. ChemElectrochem, 2019, 6(2): 439-443. |
| 49 | Lannelongue P, Bouchal R, Mourad E, et al. “Water-in-salt” for supercapacitors: a compromise between voltage, power density, energy density and stability[J]. Journal of the Electrochemical Society, 2018, 165(3): A657-A663. |
| 50 | Zhang M, Makino S, Mochizuki D, et al. High-performance hybrid supercapacitors enabled by protected lithium negative electrode and “water-in-salt” electrolyte[J]. Journal of Power Sources, 2018, 396: 498-505. |
| 51 | Tomiyasu H, Shikata H, Takao K, et al. An aqueous electrolyte of the widest potential window and its superior capability for capacitors[J]. Scientific Reports, 2017, 7: 45048. |
| 52 | Fic K, Lota G, Meller M, et al. Novel insight into neutral medium as electrolyte for high-voltage supercapacitors[J]. Energy & Environmental Science, 2012, 5(2): 5842-5850. |
| 53 | Suo L, Oh D, Lin Y, et al. How solid-electrolyte interphase forms in aqueous electrolytes[J]. Journal of the American Chemical Society, 2017, 139(51): 18670-18680. |
| 54 | Smith L, Dunn B. Opening the window for aqueous electrolytes[J]. Science, 2015, 350(6263): 918-918. |
| 55 | Kuhnel R S, Reber D, Remhof A, et al. “Water-in-salt” electrolytes enable the use of cost-effective aluminum current collectors for aqueous high-voltage batteries[J]. Chemical Communications, 2016, 52(68): 10435-10438. |
| 56 | Coustan L, Zaghib K, Bélanger D. New insight in the electrochemical behaviour of stainless steel electrode in water-in-salt electrolyte[J]. Journal of Power Sources, 2018, 399: 299-303. |
| 57 | Suo L, Han F, Fan X, et al. “Water-in-salt” electrolytes enable green and safe Li-ion batteries for large scale electric energy storage applications[J]. Journal of Materials Chemistry A, 2016, 4(17): 6639-6644. |
| 58 | Fan X, Chen L, Ji X, et al. Highly fluorinated interphases enable high-voltage Li-metal batteries[J]. Chemistry, 2018, 4(1): 174-185. |
| 59 | Suo L, Borodin O, Wang Y, et al. “Water-in-salt” electrolyte makes aqueous sodium-ion battery safe, green, and long-lasting[J]. Advanced Energy Materials, 2017, 7(21): 1701189. |
| 60 | Dou Q, Lu Y, Su L, et al. A sodium perchlorate-based hybrid electrolyte with high salt-to-water molar ratio for safe 2.5 V carbon-based supercapacitor[J]. Energy Storage Materials, 2019, 63: 603-609. |
| 61 | Yamada Y, Furukawa K, Sodeyama K, et al. Unusual stability of acetonitrile-based superconcentrated electrolytes for fast-charging lithium-ion batteries[J]. Journal of the American Chemical Society, 2014, 136(13): 5039-5046. |
| 62 | Chen S, Zheng J, Mei D, et al. High-voltage lithium-metal batteries enabled by localized high-concentration electrolytes[J]. Advanced Materials, 2018, 30(21): 1706102. |
| 63 | Lee J, Lee Y, Lee J, et al. Ultraconcentrated sodium bis(fluorosulfonyl)imide-based electrolytes for high-performance sodium metal batteries[J]. ACS Applied Materials & Interfaces, 2017, 9(4): 3723-3732. |
| 64 | Messaggi F, Ruggeri I, Genovese D, et al. Oxygen redox reaction in lithium-based electrolytes: from salt-in-solvent to solvent-in-salt[J]. Electrochimica Acta, 2017, 245: 296-302. |
| 65 | Taggougui M, Diaw M, Carré B, et al. Solvents in salt electrolyte: benefits and possible use as electrolyte for lithium-ion battery[J]. Electrochimica Acta, 2008, 53(17): 5496-5502. |
| 66 | Ding M S, Von C A, Xu K. Conductivity, viscosity, and their correlation of a super-concentrated aqueous electrolyte[J]. The Journal of Physical Chemistry C, 2017, 121(4): 2149-2153. |
| 67 | Yang C, Suo L, Borodin O, et al. Unique aqueous Li-ion/sulfur chemistry with high energy density and reversibility[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(24): 6197-6202. |
| 68 | Yamada Y, Usui K, Sodeyama K, et al. Hydrate-melt electrolytes for high-energy-density aqueous batteries[J]. Nature Energy, 2016, 1(10): 16129. |
| 69 | Hu P, Yan M, Zhu T, et al. Zn/V2O5 aqueous hybrid-ion battery with high voltage platform and long cycle life[J]. ACS Applied Materials & Interfaces, 2017, 9(49): 42717-42722. |
| 70 | Reber D, Kühnel R S, Battaglia C. High-voltage aqueous supercapacitors based on NaTFSI [J]. Sustainable Energy & Fuels, 2017, 1(10): 2155-2161. |
| [1] | 江河, 袁俊飞, 王林, 邢谷雨. 均流腔结构对微细通道内相变流动特性影响的实验研究[J]. 化工学报, 2023, 74(S1): 235-244. |
| [2] | 郑玉圆, 葛志伟, 韩翔宇, 王亮, 陈海生. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192. |
| [3] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [4] | 蔡斌, 张效林, 罗倩, 党江涛, 左栗源, 刘欣梅. 导电薄膜材料的研究进展[J]. 化工学报, 2023, 74(6): 2308-2321. |
| [5] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [6] | 李靖, 沈聪浩, 郭大亮, 李静, 沙力争, 童欣. 木质素基碳纤维复合材料在储能元件中的应用研究进展[J]. 化工学报, 2023, 74(6): 2322-2334. |
| [7] | 徐文超, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂E-1310对HCFC-141b水合物生成的影响[J]. 化工学报, 2023, 74(5): 2179-2185. |
| [8] | 王子健, 柯明, 李佳涵, 李舒婷, 孙巾茹, 童燕兵, 赵治平, 刘加英, 任璐. 短b轴ZSM-5分子筛制备方法及应用研究进展[J]. 化工学报, 2023, 74(4): 1457-1473. |
| [9] | 徐东, 田杜, 陈龙, 张禹, 尤庆亮, 胡成龙, 陈韶云, 陈建. 聚苯胺/二氧化锰/聚吡咯复合纳米球的制备及其电化学储能性[J]. 化工学报, 2023, 74(3): 1379-1389. |
| [10] | 刘润竹, 储甜甜, 张孝阿, 王成忠, 张军营. α,ω-端羟基亚苯基氟硅聚合物的合成及性能[J]. 化工学报, 2023, 74(3): 1360-1369. |
| [11] | 陈向上, 马振杰, 任希华, 贾悦, 吕晓龙, 陈华艳. 三维网络萃取膜的制备及传质效率研究[J]. 化工学报, 2023, 74(3): 1126-1133. |
| [12] | 陈健鑫, 朱瑞杰, 盛楠, 朱春宇, 饶中浩. 纤维素基生物质多孔炭的制备及其超级电容器性能研究[J]. 化工学报, 2022, 73(9): 4194-4206. |
| [13] | 杨宏欣, 李兴亚, 葛亮, 徐铜文. 含哌啶阳离子侧长链型一/二价阴离子选择性分离膜的制备[J]. 化工学报, 2022, 73(8): 3739-3748. |
| [14] | 顾仁杰, 张加威, 靳雪阳, 文利雄. 微撞击流反应器制备镍钴复合氢氧化物超级电容器材料及其性能研究[J]. 化工学报, 2022, 73(8): 3749-3757. |
| [15] | 宋健斐, 孙立强, 解明, 魏耀东. 旋风分离器内气相旋转流不稳定性的实验研究[J]. 化工学报, 2022, 73(7): 2858-2864. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号